Published online Nov 7, 2017. doi: 10.3748/wjg.v23.i41.7478
Peer-review started: August 1, 2017
First decision: August 31, 2017
Revised: September 13, 2017
Accepted: September 26, 2017
Article in press: September 26, 2017
Published online: November 7, 2017
Processing time: 96 Days and 10 Hours
Chemotherapy has limited efficacy in the treatment of advanced and metastatic pancreatic cancer (PC), and has serious side effects. The development of novel effective agents, especially targeted therapy, is essential for patients with PC. We present a 58-year-old Chinese woman initially diagnosed with locally advanced PC. As the disease progressed to Stage IV, the patient was unable to tolerate chemotherapy after the fourth-line treatment. She was then treated with apatinib, a novel and highly selective tyrosine kinase inhibitor of vascular endothelial growth factor receptor-2 and achieved a progression-free-survival of 7 mo. All drug-related side effects were well controlled with medication. To the best of our knowledge, this is the first case of PC which responded to apatinib. Considering this remarkable response, apatinib may be a promising agent in the treatment of PC. We also reviewed the literature on chemotherapy and targeted therapy, especially the anti-angiogenesis therapy for patients with PC, and investigated the effect of apatinib in other solid tumors as well.
Core tip: As chemotherapy has limited efficacy in the treatment of advanced and metastatic pancreatic cancer, targeted therapy is becoming increasingly important in patients with pancreatic cancer. The case reported herein suggests that apatinib, a novel and highly selective tyrosine kinase inhibitor of vascular endothelial growth factor receptor-2, may be a promising and useful agent in the treatment of pancreatic cancer.
- Citation: Li CM, Liu ZC, Bao YT, Sun XD, Wang LL. Extraordinary response of metastatic pancreatic cancer to apatinib after failed chemotherapy: A case report and literature review. World J Gastroenterol 2017; 23(41): 7478-7488
- URL: https://www.wjgnet.com/1007-9327/full/v23/i41/7478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i41.7478
Pancreatic cancer (PC) is a malignant tumor with a poor prognosis, and is the seventh most common cancer worldwide[1]. Chemotherapy or chemoradiation are recommended as primary therapy according to the National Comprehensive Cancer Network (NCCN) guideline for advanced and metastatic PC[2]. However, the therapeutic outcomes are unsatisfactory, with 1-year survival rates of only 17%-23%[3-5]. Given that there is limited evidence that further systemic therapy provides meaningful benefit for patients who have progressed on chemotherapy, the development of novel effective agents, including targeted therapy, to improve patient outcome is required.
Tumor angiogenesis has been found to be essential in the proliferation, invasion and metastasis of PC[6-9]. Vascular endothelial growth factor receptor-2 (VEGFR-2) is a major element involved in pancreatic tumor angiogenesis[10-13]. Therefore, interference with the VEGFR-2 signaling pathway may have therapeutic efficacy in the treatment of PC by preventing angiogenesis.
Apatinib (Hengrui Pharmaceutical Co., Ltd., Shanghai, China), also known as YN968D1, is a multiple kinase inhibitor with in vitro activity against VEGFR-2, PDGFR-beta, c-Kit, and c-src[14,15]. It was shown to have a survival benefit in gastric cancer in a Phase II[16] and III[17] trial and is currently being studied in multiple solid tumor types, such as colon and breast cancers[18-20]. Because of its easy administration, better compliance, reduced toxicity and improved outcomes, apatinib has demonstrated substantial potential as a new therapeutic option in a variety of tumor types[17,21].
Here, we report a patient with PC who was treated with apatinib following failure of the fourth-line therapy and achieved a progression-free survival (PFS) of 7 mo, demonstrating the potential of apatinib in the treatment of PC. To the best of our knowledge, this is the first case of PC which responded to apatinib.
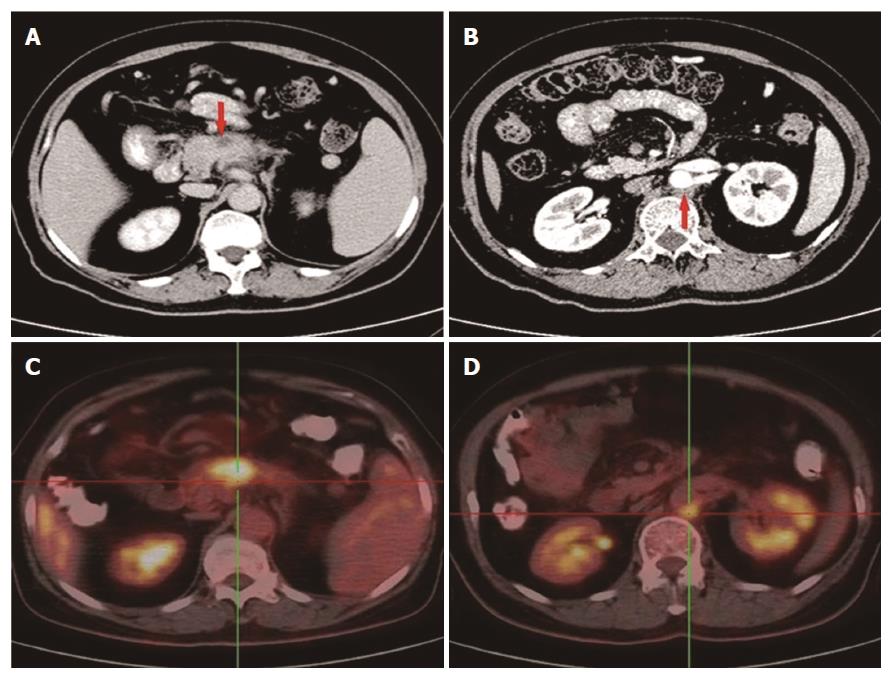
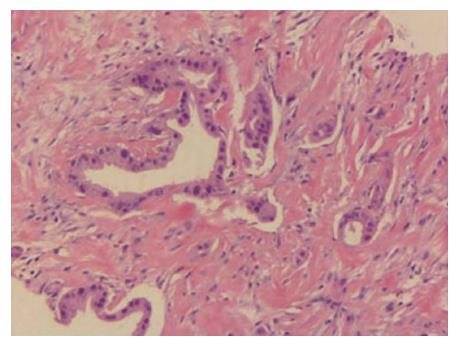
In November 2014, a 58-year-old woman attended our hospital complaining of persistent pain in the upper abdomen and back, following dyspepsia for approximately 4 d. Physical examination suggested tenderness in the upper abdomen but without rebound pain. The serum carbohydrate antigen 19-9 (CA19-9) level was 148 U/mL (normal range, 0-39 U/mL). An upper abdominal contrast-enhanced computed tomography (CT) scan revealed a 3.1 cm × 1.7 cm mass at the body of the pancreas (Figure 1A), with the mass having an intimate connection to the splenic artery and vein. An enlarged lymph node was detected behind the aorta abdominalis (Figure 1B). An 18F-FDG positron emission tomography scan also displayed a mass in the body of the pancreas with an SUV of 6.2 and an enlarged lymph node with an SUV of 4.8 (Figure 1C and D). Subsequently, an endoscopic biopsy of the mass showed a moderately differentiated adenocarcinoma (Figure 2). The patient was diagnosed with locally advanced, unresectable PC (cT4N1M0, Stage III).
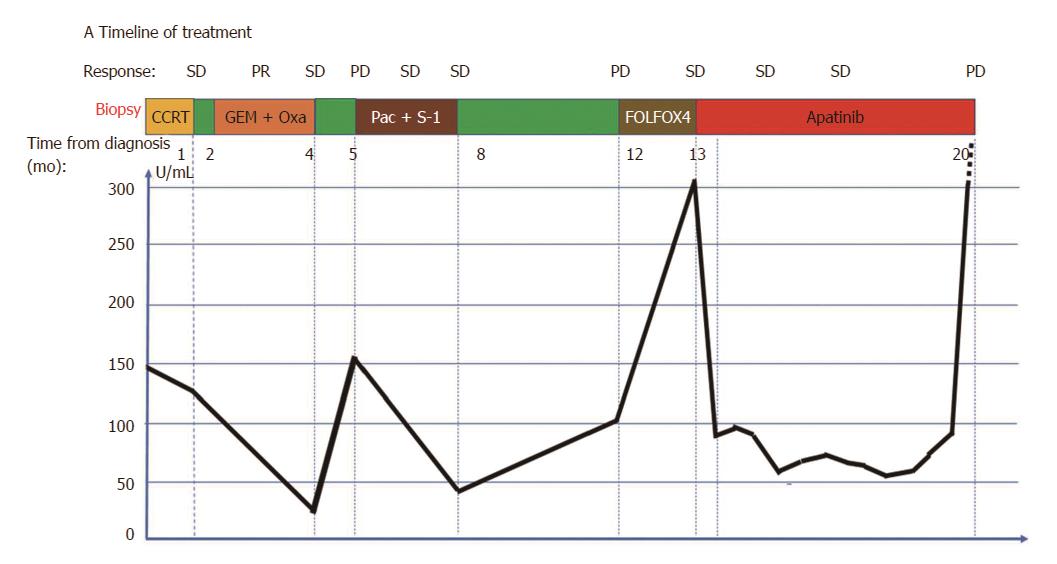
Concurrent chemoradiotherapy (CCRT) with gemcitabine (GEM) weekly and 30 fractions of radiotherapy were administered from November 7 to December 18, 2014. When CCRT was completed, the tumor response was considered stable disease (SD) on a repeat abdominal CT according to the modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria. The CA19-9 level gradually decreased to 125.6 U/mL. GEM with oxaliplatin was then administered for 4 cycles. An abdominal CT scan revealed a partial response, while serum CA19-9 level gradually decreased to a normal value (24.4 U/mL). The timeline of treatment and trend in CA19-9 during treatment are displayed in Figure 3.
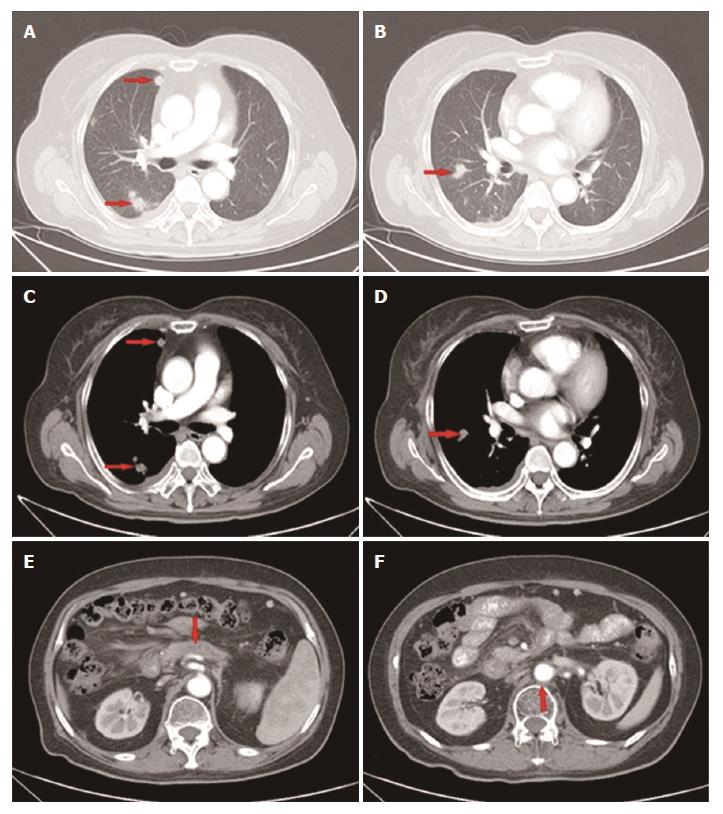
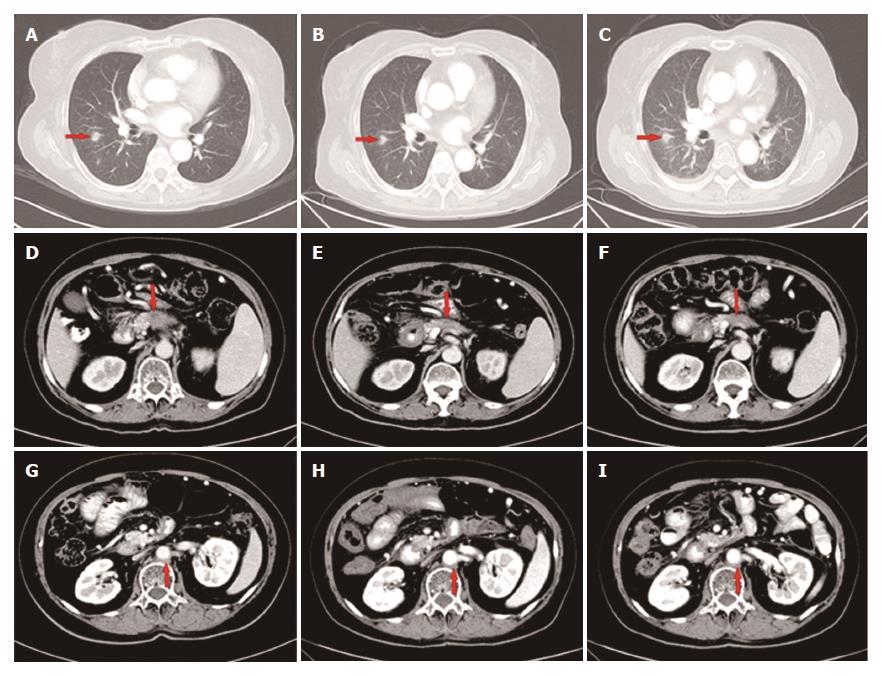
However, 1 mo later, the patient attended our hospital again because of recurrence of upper abdominal pain. The CT scan showed metastatic lesions in the right pleura and lungs (Figure 4A-D), while the lesion in the pancreas and enlarged lymph node remained stable (Figure 4E and F). Clinical restaging showed cT4N1M1, Stage IV. The CA19-9 level increased to 150.8 U/mL. From June 27, 2015, 4 cycles of paclitaxel-albumin with S-1 were administered. The response after 2 courses and 4 courses was SD. The CA19-9 level gradually decreased to 46.87 U/mL, which was almost a normal value (Figure 3).
Approximately 4 mo later, a CT scan showed that the primary as well as the metastatic lesions had progressed. The CA19-9 level was 107.6 U/mL. As the patient had good performance status (PS), the fourth-line therapy with FOLFOX4 was administered. After 3 cycles, the CT scan showed that response to treatment was SD. However, the CA19-9 level had gradually increased from 107.6 U/mL to 302.3 U/mL. The patient was unable to tolerate this regimen due to gastrointestinal toxicity, and refused further chemotherapy. From June 3, 2016, the patient received apatinib (500 mg p.o. qd) as the fifth-line treatment. The CA19-9 level after 15 d of apatinib treatment decreased sharply from 302.3 U/mL to 88.8 U/mL. The CA19-9 level was tested every 2 wk and a CT scan was performed every 8 wk during the follow-up. The CA19-9 level was maintained between 56.8 U/mL and 92.4 U/mL (Figure 3) and the primary mass decreased from 1.7 cm to 1.2 cm, and then to 1.0 cm (Figure 5D-F) while other lesions showed no obvious change (Figure 5A-C, G-I). The response to treatment with apatinib was SD.
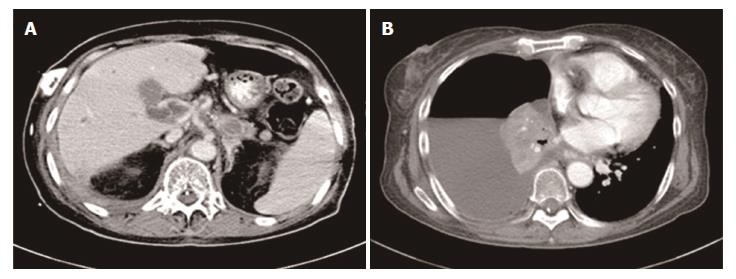
On January 13, 2017, the patient attended our hospital complaining of difficulty in breathing and the recurrence of upper abdominal pain, a CT scan revealed several metastatic lesions in the liver and pleural effusion (Figure 6). In addition, the CA19-9 level had markedly increased to 1978.0 U/mL. Her PS diminished rapidly with a score of 3, and the disease had progressed. The patient finally died of multiple organ dysfunction resulting from pulmonary infection.
During apatinib treatment, this patient developed the primary side effects of hypertension (grade 2), dental ulcer (grade 2) and a higher serum alanine transaminase level (grade 1) according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 criteria. All side effects were well controlled with drug treatment and she had a PS score of 2.
This study was approved by the Institutional Review Board of Shandong Cancer Hospital Affiliated to Shandong University. The husband of the patient provided written informed consent.
To date, there has been no effective therapy for improving the survival of a PC patient due to its aggressive nature. In this case, we administered CCRT as first-line therapy and the tumor response was SD. GEM and oxaliplatin were administered as second-line therapy and PFS was only 3 mo. Paclitaxel-albumin with S-1 was then administered as third-line therapy and FOLFOX4 regimen as the fourth-line therapy. The tumor response was SD but the CA19-9 level gradually increased. Due to chemotherapy intolerance, apatinib was then given as the fifth-line therapy to this patient. PFS following apatinib therapy was 7 mo. In addition, the patient tolerated apatinib well, with satisfactory quality of life.
With current chemotherapy regimens for PC, including GEM, paclitaxel-albumin, S-1, oxaliplatin, 5-FU, leucovorin and irinotecan, the median survival for patients with unresectable or metastatic PC is 9-11 mo[4,22,23]. In recent years, an increasing number of targeted drugs for PC have been studied. Erlotinib is the only targeted drug approved by the Federal Drug Administration to treat PC. In a randomized Phase III trial[3], the median survival and 1-year survival rate both increased in patients treated with GEM plus erlotinib, compared to those treated with only GEM. Although these results seem positive, the median overall survival (OS) was only prolonged by 9.9 d (6.24 mo vs 5.91 mo, P = 0.038) and the objective response rates (ORR) were not significantly different between the two treatment arms (57.5% vs 49.2%, P = 0.07). Furthermore, a higher incidence of some adverse events was observed with erlotinib plus GEM. In a small-sample study, sorafenib plus erlotinib also did not improve either survival or PFS rate as compared to a historical control[24]. The ViP trial, a Phase II double-blind, multicenter, randomized placebo-controlled trial, showed that vandetanib combined with GEM in patients with advanced PC did not improve OS (8.83 mo vs 8.95 mo, P = 0.303)[25]. In addition, a meta-analysis showed that there was no statistically significant improvement in survival when PC patients were treated with erlotinib or cetuximab[26]. Other trials where one[27-30] or a combination of two targeted agents[31-33] were administered for advanced or metastatic PC also did not show significant positive results (Table 1).
| Target medicine | Mechanism | Phase | Stage | n | Arm | PFS, mo | OS, mo | ORR |
| Cetuximab[27] | EGFR | III | Locally advanced/metastatic | 746 | A: GEM + cetuximab | 3.4 vs 3.0 | 6.3 vs 5.9 | 49% vs 44% |
| B: GEM | P = 0.18 | P = 0.23 | P = 0.59 | |||||
| Nimotuzumab[28] | EGFR | III | Locally advanced/metastatic | 18 | GEM + nimotuzumab | 3.71 | 9.29 | 55.50% |
| Lapatinib[29] | EGFR + Her-2 | II | Metastatic | 17 | Lapatinib + capecitabine | 2.6 | 5.2 | - |
| Cixutumumab[30] | IGF-1R | Ib/II | Metastatic | 116 | A: Erlotinib + cixutumumab + GEM | 3.6 vs 3.6 | 7.0 vs 6.7 | 12.28% vs 15.25% |
| B: Erlotinib + GEM | P = 0.97 | P = 0.64 | ||||||
| Cetuximab +Everolimus[31] | EGFR + mTOR | II | Locally advanced/metastatic | 31 | Everolimus+ cetuximab + capecitabine | - | 5.0 | 22.60% |
| Cetuximab + trastuzumab[32] | EGFR + Her-2 | I-II | Metastatic | 33 | Cetuximab + trastuzumab | 1.8 | 4.6 | - |
| Erlotinib + Selumetinib[33] | EGFR + MEK1/2 | II | Locally advanced/metastatic | 46 | Erlotinib + selumetinib | 1.9 | 7.3 | - |
Angiogenesis is an essential and significant step in tumor growth as it supplies necessary oxygen, growth factors and nutrients, and is generally considered an attractive target in cancer therapy[34-37]. Some studies have confirmed that PC is indeed angiogenesis-dependent[6-9]. Bevacizumab, an anti-angiogenesis agent, is currently the most frequently studied drug for PC in clinical trials. A double-blind phase III trial of bevacizumab in combination with GEM and erlotinib for metastatic PC showed that the addition of bevacizumab led to a statistically significant improvement in PFS (P = 0.0002). However, there was no significant improvement in OS (7.1 mo vs 6.0 mo, P = 0.2087)[38]. Moreover, in the CALGB 80303 trial[39], median PFS and ORR were similar in the two treatment arms (for PFS, 3.8 mo vs 2.9 mo, P = 0.07; for ORR, 13% vs 10%, respectively). However, in the subgroup analysis, the median survival was 7.9 mo in PS 0 patients, 4.8 mo in PS 1 patients and only 2.4 mo in PS 2 patients. These findings suggested that bevacizumab is much more effective in PS 0 and 1 patients. In another Phase II study[40] of bevacizumab combined with chemotherapy, median PFS was 5.9 mo and median OS was 7.4 mo. Partial response and stable disease occurred in 30% and 45% of patients, respectively, which met the study’s primary endpoint. These studies[39-41] demonstrated that further efforts should be focused on identifying subsets of PC patients who are more likely to benefit from bevacizumab. These findings indicate that anti-angiogenesis treatment has great potential in PC.
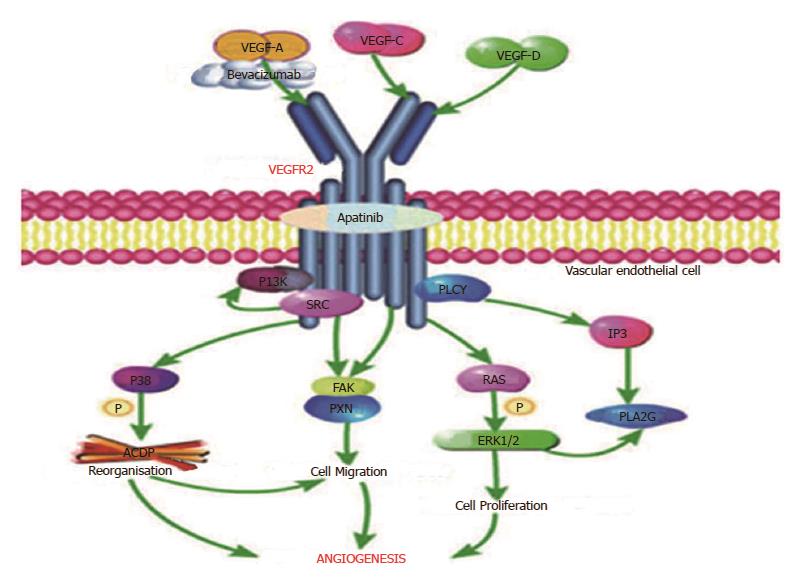
Tumors produce various angiogenic factors and cytokines to induce angiogenesis, which is essential for tumor growth. Among these tumor-derived factors, the VEGF family including VEGF-A to -D was initially identified as endothelial cell-specific mitogens with the ability to induce physiologic and pathologic angiogenesis[42-46]. It has been reported that VEGF displays these broad vascular functions by the binding and activation of VEGFR[46-49], especially VEGFR-2[50-52], mainly expressed in vascular endothelial cells. As described in Figure 7, bevacizumab, a humanized monoclonal antibody that only targets VEGF-A to prevent its interaction with VEGFR-2, was the first targeted antiangiogenic agent approved for use in oncology[53]. Apatinib, the first generation of oral anti-angiogenesis drugs, mainly targets VEGFR-2 through the intracellular ATP-binding site that inhibits all VEGF-stimulated endothelial cell migration and proliferation, decreases tumor microvascular density and promotes apoptosis[11,54,55]. Therefore, it seems that apatinib may have more potential in anti-angiogenesis than bevacizumab by affecting the VEGFR-2 pathway of angiogenesis.
Apatinib shows antitumor efficacy and good tolerance in mice when administered alone or in combination with chemotherapeutic drugs against a broad range of human tumor xenografts[15]. In patients with advanced gastric or gastroesophageal junction adenocarcinoma, a Phase II study[16] and a Phase III study[20] showed that both OS and median PFS were significantly improved in the apatinib group. Furthermore, other clinical trials[17,56] concluded that apatinib has substantial clinical activity without significant additional toxicity in patients with advanced non-squamous and non-small cell lung cancer and hepatocellular carcinoma. Whether apatinib has an important role in the treatment of PC is unknown.
The reason for the use of apatinib in our case were unsatisfactory treatment efficacy after the fourth-line chemotherapy, but the patient still wished to continue the treatment. According to the general condition of the patient who had a PS 2, she was treated with apatinib at a daily dose of 500 mg. After 15 d of apatinib treatment, the CA19-9 level decreased sharply from 302.3 U/mL to 88.8 U/mL. Following a period of treatment, the primary mass was reduced in size and other metastatic diseases were well controlled for 7 mo. Although this is an individual case, apatinib did demonstrate its curative effect in PC. As an anti-angiogenesis therapy, it seems that apatinib may be effective in the treatment of PC.
Here, we report the first case of PC which responded to apatinib. It seems that apatinib may provide an additional option for the targeted treatment of PC. Nevertheless, further large-scale prospective studies on apatinib are required to verify its efficacy in the treatment of PC.
A 58-year-old woman with no significant medical history attended our hospital complaining of persistent pain in the upper abdomen and back, following dyspepsia for approximately 4 d.
Physical examination suggested tenderness in her upper abdomen but without rebound pain.
Pancreatitis, pancreatic neuroendocrine tumor, cholecystitis, ampullary carcinoma.
The serum carbohydrate antigen 19-9 level was 148 U/mL when diagnosed.
Computed tomography revealed a 3.1 cm × 1.7 cm mass at the body of the pancreas, and an enlarged lymph node was detected behind the aorta abdominalis. 18F-FDG positron emission tomography displayed a mass in the body of the pancreas with an SUV of 6.2 and an enlarged lymph node with an SUV of 4.8.
Moderately differentiated adenocarcinoma.
Chemotherapy, radiotherapy, and targeted therapy.
Recently, many targeted drugs for pancreatic cancer have been studied. Erlotinib is the only targeted drug approved by the Federal Drug Administration to treat pancreatic cancer. Apatinib, the first generation of oral anti-angiogenesis drugs, mainly targets vascular endothelial growth factor receptor-2 (VEGFR-2). In patients with advanced gastric or gastroesophageal junction adenocarcinoma, a Phase II study and a Phase III study showed that both overall survival and median progression-free survival were significantly improved in the apatinib group. Furthermore, other clinical trials concluded that apatinib has substantial clinical activity without significant additional toxicity in patients with advanced non-squamous and non-small cell lung cancer and hepatocellular carcinoma. Therefore, apatinib seems have potential in anti-angiogenesis by affecting the VEGFR-2 pathway of angiogenesis.
Apatinib, also known as YN968D1, is a multiple kinase inhibitor with in vitro activity against VEGFR-2, PDGFR-beta, c-Kit, and c-src.
Apatinib may provide an additional option for the targeted treatment of pancreatic cancer, and further large-scale prospective studies on apatinib are required to verify its efficacy in the treatment of pancreatic cancer.
In this study, it showed that apatinib, a first-generation anti-angiogenesis drug targeting VEGFR-2, indeed improved the life of a patient with metastatic pancreatic cancer.
We thank our colleagues from Shandong Cancer Hospital for the treatment of this case, and the patient and her family members who agreed to publication of this case.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Barreto S, Chowdhury P S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20512] [Article Influence: 2051.2] [Reference Citation Analysis (20)] |
| 2. | Tempero MA, Malafa MP, Behrman SW, Benson AB 3rd, Casper ES, Chiorean EG, Chung V, Cohen SJ, Czito B, Engebretson A, Feng M, Hawkins WG, Herman J, Hoffman JP, Ko A, Komanduri S, Koong A, Lowy AM, Ma WW, Merchant NB, Mulvihill SJ, Muscarella P 2nd, Nakakura EK, Obando J, Pitman MB, Reddy S, Sasson AR, Thayer SP, Weekes CD, Wolff RA, Wolpin BM, Burns JL, Freedman-Cass DA. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2014;12:1083-1093. [PubMed] |
| 3. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2776] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 4. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5637] [Article Influence: 402.6] [Reference Citation Analysis (1)] |
| 5. | Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4351] [Cited by in RCA: 4177] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 6. | Kuwahara K, Sasaki T, Kuwada Y, Murakami M, Yamasaki S, Chayama K. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003;26:344-349. [PubMed] |
| 7. | Kisker O, Onizuka S, Banyard J, Komiyama T, Becker CM, Achilles EG, Barnes CM, O’Reilly MS, Folkman J, Pirie-Shepherd SR. Generation of multiple angiogenesis inhibitors by human pancreatic cancer. Cancer Res. 2001;61:7298-7304. [PubMed] |
| 8. | Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 237] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Chung GG, Yoon HH, Zerkowski MP, Ghosh S, Thomas L, Harigopal M, Charette LA, Salem RR, Camp RL, Rimm DL. Vascular endothelial growth factor, FLT-1, and FLK-1 analysis in a pancreatic cancer tissue microarray. Cancer. 2006;106:1677-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Solorzano CC, Baker CH, Bruns CJ, Killion JJ, Ellis LM, Wood J, Fidler IJ. Inhibition of growth and metastasis of human pancreatic cancer growing in nude mice by PTK 787/ZK222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Cancer Biother Radiopharm. 2001;16:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Büchler P, Reber HA, Ullrich A, Shiroiki M, Roth M, Büchler MW, Lavey RS, Friess H, Hines OJ. Pancreatic cancer growth is inhibited by blockade of VEGF-RII. Surgery. 2003;134:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, Cavenee WK, Cheng SY. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 254] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Yamagishi S, Yonekura H, Yamamoto Y, Fujimori H, Sakurai S, Tanaka N, Yamamoto H. Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Lab Invest. 1999;79:501-509. [PubMed] |
| 14. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2966] [Cited by in RCA: 3147] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 15. | Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 16. | Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 17. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 732] [Article Influence: 81.3] [Reference Citation Analysis (1)] |
| 18. | Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc). 2015;51:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L, Tong Z, Wang S, Li J, Wang Z. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer. 2014;14:820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, Zhang Q, Wang X, Feng J, Pang D. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 209] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 21. | Liu L, Yu H, Huang L, Shao F, Bai J, Lou D, Chen F. Progression-free survival as a surrogate endpoint for overall survival in patients with third-line or later-line chemotherapy for advanced gastric cancer. Onco Targets Ther. 2015;8:921-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Vaccaro V, Sperduti I, Milella M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;365:768-769; author reply 769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4886] [Article Influence: 407.2] [Reference Citation Analysis (0)] |
| 24. | Cardin DB, Goff L, Li CI, Shyr Y, Winkler C, DeVore R, Schlabach L, Holloway M, McClanahan P, Meyer K. Phase II trial of sorafenib and erlotinib in advanced pancreatic cancer. Cancer Med. 2014;3:572-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Middleton G, Palmer DH, Greenhalf W, Ghaneh P, Jackson R, Cox T, Evans A, Shaw VE, Wadsley J, Valle JW. Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial. Lancet Oncol. 2017;18:486-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Ottaiano A, Capozzi M, De Divitiis C, De Stefano A, Botti G, Avallone A, Tafuto S. Gemcitabine mono-therapy versus gemcitabine plus targeted therapy in advanced pancreatic cancer: a meta-analysis of randomized phase III trials. Acta Oncol. 2017;56:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605-3610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 28. | Su D, Jiao SC, Wang LJ, Shi WW, Long YY, Li J, Bai L. Efficacy of nimotuzumab plus gemcitabine usage as first-line treatment in patients with advanced pancreatic cancer. Tumour Biol. 2014;35:2313-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Wu Z, Gabrielson A, Hwang JJ, Pishvaian MJ, Weiner LM, Zhuang T, Ley L, Marshall JL, He AR. Phase II study of lapatinib and capecitabine in second-line treatment for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2015;76:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Philip PA, Goldman B, Ramanathan RK, Lenz HJ, Lowy AM, Whitehead RP, Wakatsuki T, Iqbal S, Gaur R, Benedetti JK. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer. 2014;120:2980-2985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Kordes S, Richel DJ, Klümpen HJ, Weterman MJ, Stevens AJ, Wilmink JW. A phase I/II, non-randomized, feasibility/safety and efficacy study of the combination of everolimus, cetuximab and capecitabine in patients with advanced pancreatic cancer. Invest New Drugs. 2013;31:85-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Assenat E, Azria D, Mollevi C, Guimbaud R, Tubiana-Mathieu N, Smith D, Delord JP, Samalin E, Portales F, Larbouret C. Dual targeting of HER1/EGFR and HER2 with cetuximab and trastuzumab in patients with metastatic pancreatic cancer after gemcitabine failure: results of the “THERAPY”phase 1-2 trial. Oncotarget. 2015;6:12796-12808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Ko AH, Bekaii-Saab T, Van Ziffle J, Mirzoeva OM, Joseph NM, Talasaz A, Kuhn P, Tempero MA, Collisson EA, Kelley RK. A Multicenter, Open-Label Phase II Clinical Trial of Combined MEK plus EGFR Inhibition for Chemotherapy-Refractory Advanced Pancreatic Adenocarcinoma. Clin Cancer Res. 2016;22:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327-336. [PubMed] |
| 35. | Schuch G, Kisker O, Atala A, Soker S. Pancreatic tumor growth is regulated by the balance between positive and negative modulators of angiogenesis. Angiogenesis. 2002;5:181-190. [PubMed] |
| 36. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. [PubMed] |
| 37. | Costache MI, Ioana M, Iordache S, Ene D, Costache CA, Săftoiu A. VEGF Expression in Pancreatic Cancer and Other Malignancies: A Review of the Literature. Rom J Intern Med. 2015;53:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 502] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 39. | Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 675] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 40. | Martin LK, Li X, Kleiber B, Ellison EC, Bloomston M, Zalupski M, Bekaii-Saab TS. VEGF remains an interesting target in advanced pancreas cancer (APCA): results of a multi-institutional phase II study of bevacizumab, gemcitabine, and infusional 5-fluorouracil in patients with APCA. Ann Oncol. 2012;23:2812-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Ko AH, Dito E, Schillinger B, Venook AP, Xu Z, Bergsland EK, Wong D, Scott J, Hwang J, Tempero MA. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Invest New Drugs. 2008;26:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6951] [Article Influence: 316.0] [Reference Citation Analysis (0)] |
| 43. | Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:re1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 44. | Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306-1309. [PubMed] |
| 45. | Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368-4380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1170] [Cited by in RCA: 1151] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 46. | Kieran MW, Kalluri R, Cho YJ. The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med. 2012;2:a006593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 47. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6488] [Article Influence: 259.5] [Reference Citation Analysis (0)] |
| 48. | Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 728] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 49. | Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3:263-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222-3230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 465] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 51. | Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J Jr, Fischer W, Lukas J, Rich JN, Bartek J. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209:507-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 322] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 52. | Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358-C1366. [PubMed] |
| 53. | Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1788] [Cited by in RCA: 1891] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 54. | Peng H, Zhang Q, Li J, Zhang N, Hua Y, Xu L, Deng Y, Lai J, Peng Z, Peng B. Apatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinoma. Oncotarget. 2016;7:17220-17229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 55. | Peng S, Zhang Y, Peng H, Ke Z, Xu L, Su T, Tsung A, Tohme S, Huang H, Zhang Q. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib. Cancer Lett. 2016;373:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 56. | Qin S. Apatinib in Chinese patients with advanced hepatocellular carcinoma: A phase II randomized, open-label trial. : Asco Meeting Abstracts 2014; . |