Published online Nov 7, 2017. doi: 10.3748/wjg.v23.i41.7433
Peer-review started: July 5, 2017
First decision: July 28, 2017
Revised: August 10, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: November 7, 2017
Processing time: 123 Days and 12.6 Hours
To establish the surgical flow for anatomic isolated caudate lobe resection.
The study was approved by the ethics committee of the Second Affiliated Hospital Zhejiang University School of Medicine (SAHZU). From April 2004 to July 2014, 20 patients were enrolled who underwent anatomic isolated caudate lobectomy at SAHZU. Clinical and postoperative pathological data were analyzed.
Of the total 20 cases, 4 received isolated complete caudate lobectomy (20%) and 16 received isolated partial caudate lobectomy (80%). There were 4 cases with the left approach (4/20, 20%), 6 cases with the right approach (6/20, 30%), 7 cases with the bilateral combined approach (7/20, 35%), 3 cases with the anterior approach (3/20, 15%), and the hanging maneuver was also combined in 2 cases. The median tumor size was 5.5 cm (2-12 cm). The median intra-operative blood loss was 600 mL (200-5700 mL). The median intra-operative blood transfusion volume was 250 mL (0-2400 mL). The median operation time was 255 min (110-510 min). The median post-operative hospital stay was 14 d (7-30 d). The 1- and 3-year survival rates for malignant tumor were 88.9% and 49.4%, respectively.
Caudate lobectomy was a challenging procedure. It was demonstrated that anatomic isolated caudate lobectomy can be done safely and effectively.
Core tip: Caudate lobe resection is still a challenging procedure for the vast majority of surgeons because of the difficult anatomical location and intraoperative bleeding. According to prior experience, six steps were established and validated on the patient. Anatomic isolated caudate lobectomy can be done safely and effectively following the surgical flow.
- Citation: Jin Y, Wang L, Yu YQ, Zhou DE, Liu DR, Yang JJ, Peng SY, Li JT. Anatomic isolated caudate lobectomy: Is it possible to establish a standard surgical flow? World J Gastroenterol 2017; 23(41): 7433-7439
- URL: https://www.wjgnet.com/1007-9327/full/v23/i41/7433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i41.7433
The caudate lobe (CL), situated in a tricky location, lies deep in the liver and is adjacent to major blood vessels. CL consists of a Spiegel lobe on the left, a caudate process on the right, and a paracaval portion between them[1]. Surgical resection is the most suitable strategy to eradicate hepatic malignant lesions in this area. In recent years, the number of caudate lobectomies has increased with the improvement of imaging[2-5]. However, CL resection remains a challenging procedure for the vast majority of surgeons because of the difficult anatomical location and intraoperative bleeding.
Overall survival (OS) can be achieved by anatomic liver resection for hepatocellular carcinoma (HCC) patients[6,7]. However, reports of anatomic resection usually refer to segment II to segment VIII, excepting CL. The implement of more CL resection is limited due to a lack of standard flow. Therefore, the aim of this study was to establish the surgical flow for anatomic isolated CL resection.
The study was approved by the ethics committee of the Second Affiliated Hospital Zhejiang University School of Medicine. Twenty patients who underwent anatomic isolated caudate lobectomy between April 2004 to July 2014, performed by our group, were enrolled in this study, including 13 males (13/20, 65%) and 7 females (7/20, 35%). The median age of patients was 55.5 years, ranging from 24-72 years. Postoperative pathological examinations included 14 cases of HCC (14/20, 70%), 2 cases of adenocarcinoma (2/20, 10%), 1 case of hepatic hemangioma (1/20, 5%), 1 case of hepatosarcoma (1/20, 5%), 1 case of angioleiomyoma (1/20, 5%), and 1 case of adenoma (1/20, 5%) (Table 1).
| n = 20 | Data |
| Sex | |
| Female | 7 (35) |
| Male | 13 (65) |
| Age in yr | 55.5 (24-72) |
| Pathological examinations | |
| Hepatocellular carcinoma | 14 (70) |
| Intrahepatic cholangia carcinoma | 2 (10) |
| Hepatic hemangioma | 1 (5) |
| Hepatosarcoma | 1 (5) |
| Angiomyolipoma | 1 (5) |
| Adenoma | 1 (5) |
| Tumor size in cm | 5.5 (2-12) |
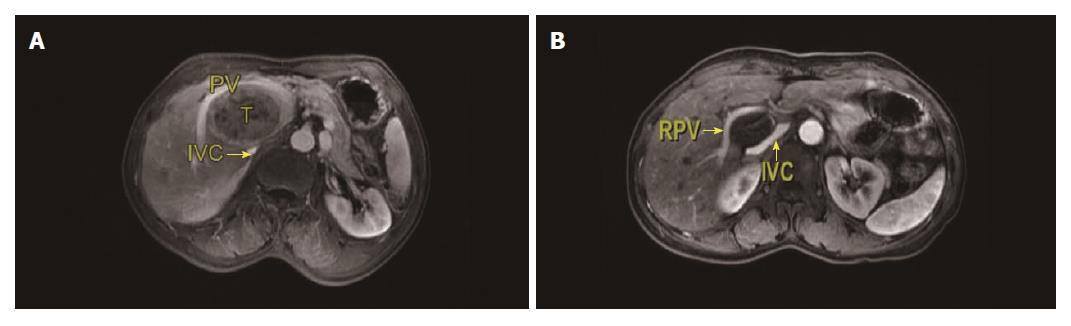
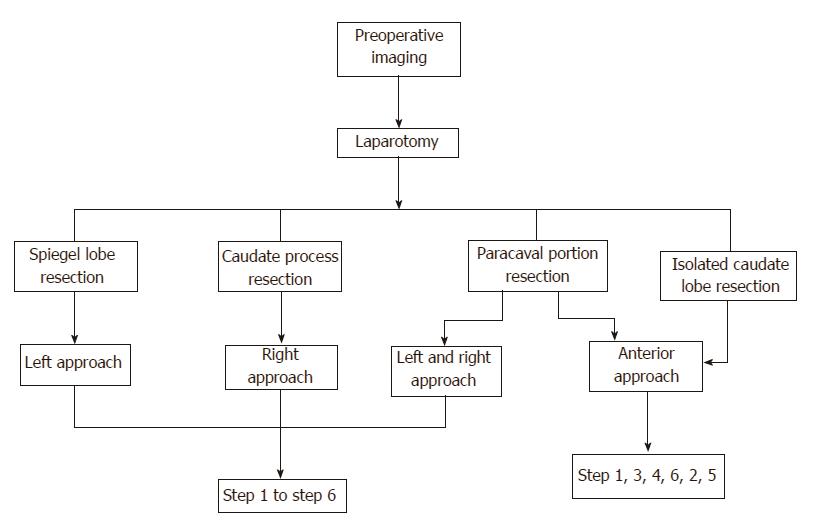
The procedures were performed according to the results of computed tomography (CT) or magnetic resonance imaging (MRI) before surgery (Figure 1). Based on the location of the tumor, four different flows were planned before surgery (Figure 2).
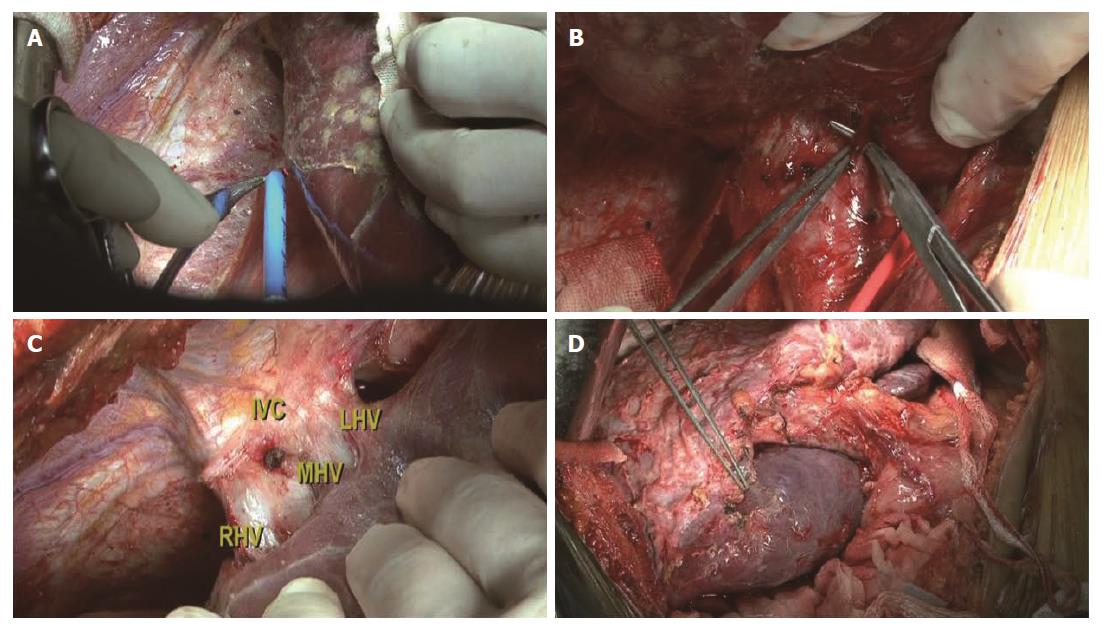
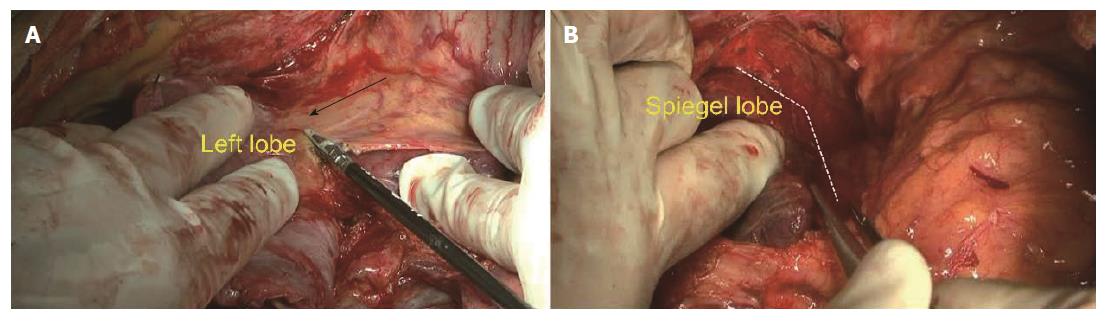
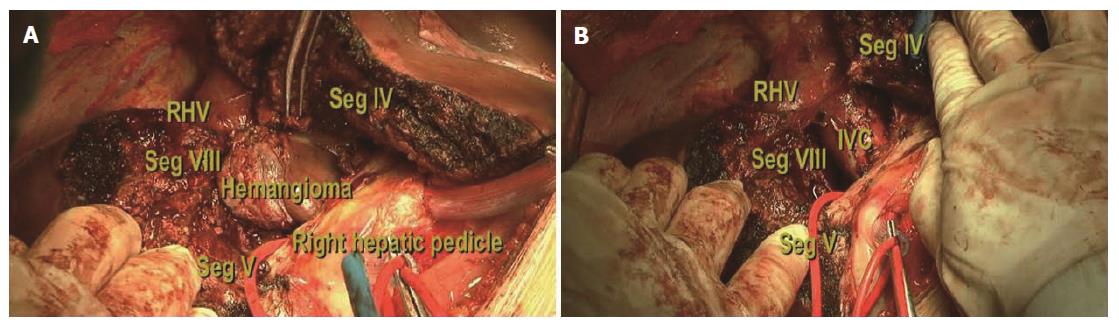
Step 1: Mobilization of the liver. For all patients, a reversed L-shaped incision was adopted from xiphoid to the tip of 12th rib. For resection of the caudate process, the right ligamentum teres, falciform ligament, coronary ligament and triangular ligament were divided (Figure 3A). The hepatorenal ligament was dissected and the right adrenal gland was separated from the right liver with a right approach. For resection of the Spiegel lobe, usually, the ligaments of the left liver were dissected, Arantius ligament was divided until the tip of the CL and the Spiegel lobe was exposed completely with a left approach (Figure 4). For complete CL resection, usually, both left and right liver were mobilized. For giant CL tumors or the paracaval portion, sometimes, the transection of the liver parenchyma along Cantilie’s line were conducted.
Step 2: Ligation of short hepatic veins. For a right approach, liver was turned to the left and the inferior vena cava (IVC) was exposed. The inferior right hepatic vein was divided firstly and short hepatic veins were ligated gradually along the IVC (Figure 3B). The caudate process was exposed and dissociated from the IVC. For a left approach, the liver was turned to the right and short hepatic veins were isolated as well as divided until the Spiegel lobe was separated from the IVC completely.
Step 3: Isolation and taping of three major hepatic veins. The liver was pushed down medially, and the fossa was exposed between the right hepatic vein (RHV) and the middle hepatic vein (MHV) (Figure 3C). A tape was positioned around the common trunk of the MHV and the left hepatic vein (LHV). The hepatocaval ligament (Makuuchi ligament[8]) was extra-hepatically dissected from the right side and another tape was positioned around the RHV carefully.
Step 4: Controlling the inflow blood supply. The hepatoduodenal ligament was exposed and a tape was encircled around the hepatic hilum to control hepatic blood inflow if necessary. The occlusion time was usually 5 min, 8 min and 10 min during the first three times and then 10 min with 2 min interruption every time. The branch of portal triads to the CL was divided and ligated. Afterwards, ischemia landmarks could be found easily (Figure 3D).
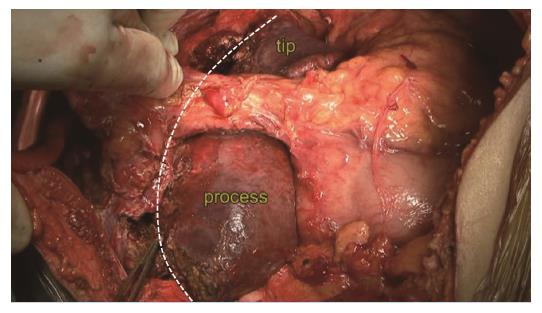
Step 5: Identification of the CL boundary. The left boundary was established as the Arantius ligament, the right boundary was described as Peng’s line from the CL tip to the caudate process[9] (Figure 5). The tip of the CL was located between the LHV and the IVC.
Step 6: Liver transection. Hepatic parenchyma was transected with the curettage and aspiration dissection technique using Peng’s multifunction operative dissector[4,10].
The anatomic isolated caudate lobectomy is presented in the accompanying video (Supplement 1).
Clinical variables were described as median or mean ± SD. Discrete and continuous variables were analyzed by chi-square test and t-test, respectively. Survival time was analyzed by the Kaplan-Meier method. All data were analyzed by SPSS 16.0 software (SPSS Inc, Chicago, IL, United States).
Among all patients, 4 cases received isolated complete caudate lobectomy (4/20, 20%), while 16 cases received isolated partial caudate lobectomy (16/20, 80%). Based on the type of dissection, there were 4 cases with the left approach (4/20, 20%), 6 cases with the right approach (6/20, 30%), 7 cases with the bilateral combined approach (7/20, 35%), 3 cases with the anterior approach (3/20, 15%) (Figure 6), and the hanging maneuver was also combined in 2 cases. The median tumor size was 5.5 cm (2-12 cm). The median intra-operative blood loss was 600 mL (200-5700 mL). The median intra-operative blood transfusion volume was 250 mL (0-2400 mL). The median operation time was 255 min (110-510 min), and the median post-operative hospital stay was 14 d (7-30 d).
There was 1 case of incision infection (1/20, 5%), 3 cases of ascites (3/20, 15%), and 2 cases of pleural effusion (2/20, 10%) (Table 2). There was no mortality and the median survival time for malignant tumor was 48 mo, with 1- and 3-year survival rates of 88.9% and 49.4%, respectively.
| Surgical feature | Data |
| Approach | |
| LSR | 4 (20) |
| RSR | 6 (30) |
| CSR | 7 (35) |
| AR | 3 (15) |
| Procedure | |
| ICCL | 4 (20) |
| IPCL | 16 (80) |
| Blood loss in mL | 600 (200-5700) |
| VIBT in mL | 250 (0-2400) |
| Operation time in min | 255 (110-510) |
| HSPO in d | 14 (7-30) |
| Postoperative complication | |
| Incision infection | 1 (5) |
| Ascites | 3 (15) |
| Pleural effusion | 2 (10) |
Due to abundant adjacent blood vessels of the CL, chemoembolization, radiofrequency ablation and other treatments are not appropriate for CL lesions[11]. Surgical resection is considered as the preferred method and anatomic resection can improve prognosis[7,12]. Hasegawa et al[6] believed that anatomic resection was one of the independent predictors of improved OS for HCC. However, the majority of anatomic resection refers to the segment II to segment VIII, excepting segment I. Caudate lobectomy was once called a ‘bloody gultch’[13]. While the resection of the tumor in the CL is not difficult today, it is still difficult to perform isolated anatomic resection for CL; the vast majority reports are from case reports. In this study, we mainly explored the possibility to establish a standard surgical flow for anatomical isolated caudate lobectomy[5,14,15].
Chaib et al[16] classified CL tumors into five types, depending on their locations, and this classification has clinical significance for small size tumors. However, in China, HCC is usually large and concurrent with cirrhosis. And, anatomic resection is necessary for longer survival. Blumgart’s group[17] performed 150 cases of caudate resection, which led to a median survival for HCC of 32 mo. In 2013, Philips et al[18] reported their experience of caudate resection, having a median OS of 21 mo. Our results showed a median survival time of 48 mo for malignant patients, though this was not a comparison study.
Like other anatomic resections, the identification of the blood supply to one part or the whole CL is a prerequisite. The key steps are preoperative assessment and intra-operative identification of the precise boundary. We called this boundary as two tips, two boundaries and one virtual plane. The confluence of Arantius ligament and IVC was the upper tip, while the converging point of the right lobe and the caudate process was the lower tip. The Arantius ligament is regarded as the left border of CL, while Peng’s line is the right border. The plane of the retro-CL is the surface of the retro-hepatic IVC. In practice, resection of the CL along the Peng’s line shows similar effects as intra-operative dye labeling, which is safe and convenient[5].
The advantages of the different surgical approaches are to perform CL resection easily. Left approach is suitable for the tumor located in the Spiegel lobe. Right approach is for resection of tumor located in the caudate process. For complete CL resection, we usually used the left and right approach. For giant CL tumors or the paracaval portion, the anterior approach should be used. The disadvantage of the anterior approach is that incidence of biliary fistula will be high because of hepatic parenchyma transection. The laparoscopic resection of the CL has been reported sporadically, and we will be working on this in the future.
During the resection, other surgical techniques can be used to reduce bleeding and increase surgical safety. These techniques include the selective control of inflow, the liver hanging maneuver and retrograde resection of the CL[15,19,20].
In conclusion, as an individual anatomic segment, caudate lobectomy remains a substantially challenging procedure. Our experience demonstrated that anatomic isolated caudate lobectomy can be done safely and effectively by establishing a surgical flow.
Caudate lobe (CL) is an important part of the liver and adjacent to the major blood vessels. Surgical resection is the preferred treatment for patients with malignant lesions in this area. However, CL resection remains a challenging procedure.
The implementation of more CL resections is limited due to a lack of standard flow. The hotspot of this study is the establishment a standard surgical flow for anatomic isolated caudate lobectomy.
In recent years, the number of caudate lobectomies has increased. However, anatomic isolated caudate lobectomy is not common because of the difficult anatomical location and intraoperative bleeding. This study presents the recommendation of a surgical flow which facilitates the performance of anatomic isolated caudate lobectomy safely.
The surgical flow in this study was established according to prior experience and validated on the patient. Anatomic isolated caudate lobectomy can be done safely and effectively based on this flow.
Peng’s line is the right boundary of CL, from the CL tip to the right border of the caudate process. Peng’s multifunction operative dissector is an electrosurgical instrument, which was used for transection hepatic parenchyma.
CL isolated resection is a rare and challenging procedure. I think that the strength of the paper is the description of the different techniques, detailed with intraoperative pictures. The authors deserve praise for having done a difficult job - the article has described a series of 20 resections in a decade. It may be expected that the authors will come up with a larger series in future. The description of operative steps is one of the strong points of this article.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bandyopadhyay SK, Garcia-Olmo D, Petrucciani N S- Editor: Qi Y L- Editor: Filipodia E- Editor: Huang Y
| 1. | Kumon M. Anatomy of the caudate lobe with special refefence to portal vein and bile duct. Acta Hepatol Jpn. 1985;26:1193-1199. |
| 2. | Dai WD, Huang JS, Hu JX. Isolated caudate lobe resection for huge hepatocellular carcinoma (10 cm or greater in diameter). Am Surg. 2014;80:159-165. [PubMed] |
| 3. | Cai X, Li Z, Yu H, Liu K, Liang X, Wang Y, Liang Y. Isolated laparoscopic resection of the hepatic caudate lobe. Chin Med J (Engl). 2014;127:3194-3195. [PubMed] |
| 4. | Yang JH, Gu J, Dong P, Chen L, Wu WG, Mu JS, Li ML, Wu XS, Zhao YL, Zhang L. Isolated complete caudate lobectomy for hepatic tumor of the anterior transhepatic approach: surgical approaches and perioperative outcomes. World J Surg Oncol. 2013;11:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Peng SY, Qian HR, Xu B, Wang YF, Wang XA. Surgical treatment of the caudate lobe diseases. J Clin Surg. 2009;17:581-583. [DOI] [Full Text] |
| 6. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [PubMed] |
| 7. | Jia CK, Weng J, Chen YK, Fu Y. Anatomic resection of liver segments 6-8 for hepatocellular carcinoma. World J Gastroenterol. 2014;20:4433-4439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Makuuchi M, Yamamoto J, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H. Extrahepatic division of the right hepatic vein in hepatectomy. Hepatogastroenterology. 1991;38:176-179. [PubMed] |
| 9. | Peng SY. Hepatic caudate lobe resection: Springer Verlag Berlin Heidelberg, 2010; 9-11. . [DOI] [Full Text] |
| 10. | Peng SY, Li JT. “Curettage and aspiration dissection technique” using PMOD for liver resection. HPB (Oxford). 2008;10:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Asahara T, Dohi K, Hino H, Nakahara H, Katayama K, Itamoto T, Ono E, Moriwaki K, Yuge O, Nakanishi T. Isolated caudate lobectomy by anterior approach for hepatocellular carcinoma originating in the paracaval portion of the caudate lobe. J Hepatobiliary Pancreat Surg. 1998;5:416-421. [PubMed] |
| 12. | Yoo PS, Enestvedt CK, Kulkarni S. Anatomic considerations in the surgical resection of hepatocellular carcinoma. J Clin Gastroenterol. 2013;47 Suppl:S11-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Colonna JO 2nd, Shaked A, Gelabert HA, Busuttil RW. Resection of the caudate lobe through “bloody gultch”. Surg Gynecol Obstet. 1993;176:401-402. [PubMed] |
| 14. | Peng SY, Li JT, Mou YP, Liu YB, Wu YL, Fang HQ, Cao LP, Chen L, Cai XJ, Peng CH. Different approaches to caudate lobectomy with “curettage and aspiration” technique using a special instrument PMOD: a report of 76 cases. World J Gastroenterol. 2003;9:2169-2173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Peng SY, Liu YB, Wang JW, Li JT, Liu FB, Xue JF, Xu B, Cao LP, Hong DF, Qian HR. Retrograde resection of caudate lobe of liver. J Am Coll Surg. 2008;206:1232-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Chaib E, Ribeiro MA Jr, Silva Fde S, Saad WA, Cecconello I. Caudate lobectomy: tumor location, topographic classification, and technique using right- and left-sided approaches to the liver. Am J Surg. 2008;196:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Hawkins WG, DeMatteo RP, Cohen MS, Jarnagin WR, Fong Y, D’Angelica M, Gonen M, Blumgart LH. Caudate hepatectomy for cancer: a single institution experience with 150 patients. J Am Coll Surg. 2005;200:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Philips P, Farmer RW, Scoggins CR, McMasters KM, Martin RC 2nd. Caudate lobe resections: a single-center experience and evaluation of factors predictive of outcomes. World J Surg Oncol. 2013;11:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kim SH, Park SJ, Lee SA, Lee WJ, Park JW, Kim CM. Isolated caudate lobectomy using the hanging maneuver. Surgery. 2006;139:847-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Li Z, Sun YM, Wu FX, Yang LQ, Lu ZJ, Yu WF. Controlled low central venous pressure reduces blood loss and transfusion requirements in hepatectomy. World J Gastroenterol. 2014;20:303-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |