Published online Oct 28, 2017. doi: 10.3748/wjg.v23.i40.7337
Peer-review started: August 9, 2017
First decision: August 30, 2017
Revised: September 13, 2017
Accepted: September 26, 2017
Article in press: September 26, 2017
Published online: October 28, 2017
Processing time: 81 Days and 18.6 Hours
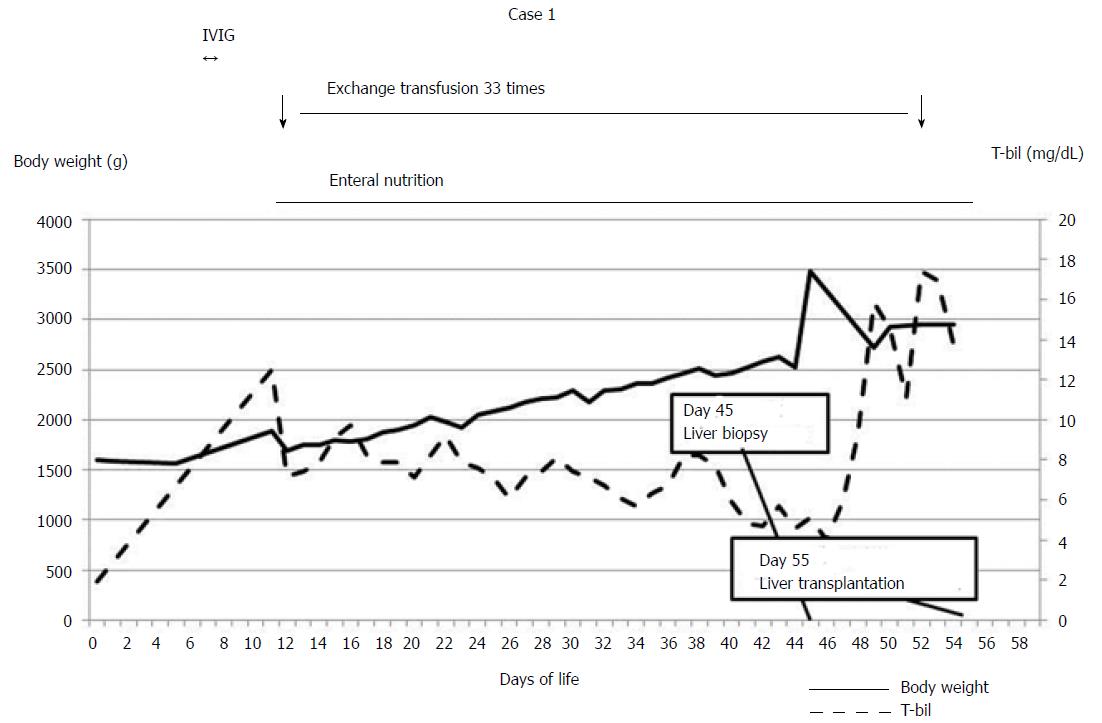
We report a case involving a rescued low birth weight infant (LBWI) with acute liver failure. Case: The patient was 1594 g and 323/7 gestational wk at birth. At the age of 11 d, she developed acute liver failure due to gestational alloimmune liver disease. Exchange transfusion and high-dose gamma globulin therapy were initiated, and body weight increased with enteral nutrition. Exchange transfusion was performed a total of 33 times prior to living donor liver transplantation (LDLT). Her liver dysfunction could not be treated by medications alone. At 55 d old and a body weight of 2946 g, she underwent LDLT using an S2 monosegment graft from her mother. Three years have passed with no reports of intellectual disability or liver dysfunction. LBWIs with acute liver failure may be rescued by LDLT after body weight has increased to over 2500 g.
Core tip: We report a case involving a rescued low birth weight infant (LBWI) with acute liver failure. The patient was 1594 g at birth. At the age of 11 d, she developed acute liver failure due to gestational alloimmune liver disease. Medications were initiated, and body weight increased with enteral nutrition. Her liver dysfunction could not be treated by medications alone. At 55 d old with a body weight of 2946 g, she underwent living-donor liver transplantation (LDLT) using an S2 monosegment graft. Conclusion: LBWIs with acute liver failure may be rescued by LDLT after body weight has increased to over 2500 g.
- Citation: Okada N, Sanada Y, Urahashi T, Ihara Y, Yamada N, Hirata Y, Katano T, Ushijima K, Otomo S, Fujita S, Mizuta K. Rescue case of low birth weight infant with acute hepatic failure. World J Gastroenterol 2017; 23(40): 7337-7342
- URL: https://www.wjgnet.com/1007-9327/full/v23/i40/7337.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i40.7337
Neonatal acute hepatic failure is a rare but serious disease[1]. Reports have indicated that the cause of neonatal acute hepatic failure is most frequently gestational alloimmune liver disease (GALD), but in rare cases, it can be metabolic disorder, viral infection, or mitochondrial disorder, among other possibilities[2]. The initial treatment for neonatal acute liver failure is apheresis and medication while the cause of hepatic failure is determined. If hepatic recovery has not been achieved with medication alone, liver transplantation is indicated[1,3]. However, the living donor liver transplantation (LDLT) procedure for neonatal recipients is challenging due to the size mismatch between the liver graft and the body of the recipient. Moreover, management during the perioperative period is also challenging[4]. LDLT is particularly difficult for low birth weight infants (LBWIs) for the reasons discussed above. In such cases, LDLT can result in recovery if the body weight of the infant can be increased by nutritional management[5]. However, few reports have discussed LDLT for neonatal recipients with low body weights[6]. Herein, we report a case involving a rescued LBWI with acute liver failure and discuss the limitation of body weight as an indicator for neonatal LDLT.
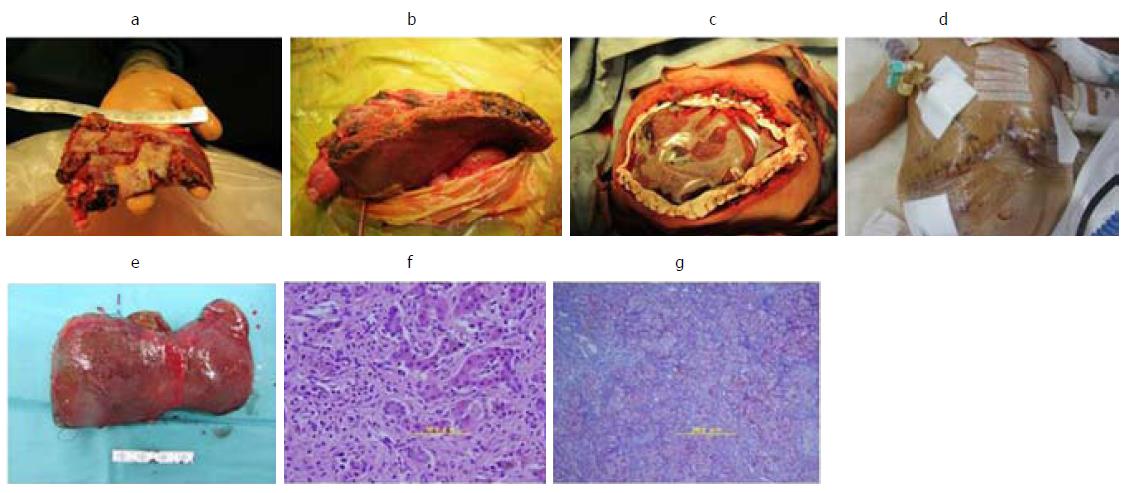
The patient was born at 323/7 gestational wk because of fetal distress. Her birth weight was 1594 g (Figures 1 and 2). Immediately after birth, hypoglycemia and hypotension appeared. A daily administration of hydrocortisone and continuous administration of dopamine hydrochloride were started at the age of 1 day. At the age of 11 d, coagulation dysfunction and an elevated serum ferritin level due to acute liver failure were observed (ferritin 2865 ng/mL, total bilirubin 12.5 mg/dL, PT-INR 4.57). The patient’s older sister was highly suspected of GALD by clinical course and pathological findings and underwent LDLT at 13 d of age. GALD was highly suspected in this case after excluding the possibility of metabolic disorder or infectious disease. The patient did not undergo magnetic resonance imaging or salivary grand biopsy for GALD diagnosis. We explained the high recurrence rate of GALD in siblings to her parents; however, the parents gave birth to a baby in another hospital without informing us. Starting at 11 d of age, exchange transfusion and medication therapy using high-dose gamma globulin and deferoxamine were initiated. The patient’s body weight had been gradually increased with enteral nutrition using commercially available nutrients, administered 8 times per day via a gastric tube. She then recovered from hypotension, and the continuous administration of dopamine hydrochloride was finished. Exchange transfusion was performed a total of 33 times prior to LDLT. The patient was transported to our hospital at 44 d of age; at that time, her body weight was 2525 g. We speculated that her target body weight for LDLT would be over 2500 g based on the estimated graft volume. Computed tomography scan revealed a markedly atrophied liver (resected liver was 78 g). Laboratory data showed repeated coagulopathy and hyperbilirubinemia with daily exchange transfusion and a pediatric end-stage liver disease (PELD) score of 15.8 (T-bil 5.09 mg/dL, PT-INR 1.91, Albumin 3.4 g/dL). A liver biopsy revealed a marked loss of hepatocytes. The remaining hepatocytes were multinucleated, and there was widespread fibrosis around Glisson’s sheath and in the parenchymal area (F3-4) (Figure 2F and G). Thus, her liver dysfunction could not be successfully treated via medications alone, and she was judged to be indicated for liver transplantation at that time. We tried to perform LDLT at 45 d of age, but before LDLT, she went into shock following hemothorax due to failure of catheter insertion with injury of the right subclavian artery. After the recovery period, she underwent LDLT at 55 d of age using an S2 monosegment graft from her mother (107 g, graft-recipient weight ratio (GRWR) 3.6%; Figure 2 A). The body weight of the patient at LDLT was 2946 g. At the time of LDLT, the PELD score of the patient was 21.9 (T-bil 13.74 mg/dL, PT-INR 2.12, Albumin 3.7 g/dL). The operation duration was 13 h and 37 min and bleeding was 700 mL [238 mL/recipient body weight (kg)]. A transverse incision was created, and the liver was resected with temporary bypass of the portal vein. The resected liver was 78 g (Figure 2E). The recipient’s right hepatic artery (2.0 mm) was anastomosed to the graft’s left hepatic artery (2.5 mm) via a dorsal position of the portal vein anastomosis using a microsurgical technique. The graft-to-recipient distance ratio (GRDR) was 2.4 (58/24). Biliary reconstruction was performed using a Roux-en-Y hepaticojejunostomy. The abdominal wound could not be closed because respiratory failure occurred due to abdominal compartment syndrome (Figure2B and C). Intraoperative water balance was +1645 mL [558 mL/recipient body weight (kg)]. After LDLT, continuous hemodiafiltration (CHDF) had been performed for systemic edema, removing water as long as blood pressure and portal vein flow remained steady. Respiratory failure due to abdominal compartment syndrome and lung edema gradually improved. Thus, on postoperative day (POD) 5, the abdominal skin of the patient was closed without closing the abdominal fascia (Figure 2D). Tacrolimus and methylprednisolone were used as the standard postoperative immunosuppression therapy regimen. Acute rejection occurred on POD 17, so steroid pulse therapy was initiated. After steroid pulse therapy, liver enzyme, PT-INR and T-bil were nearly normalized. Respiratory failure due to large-for-size graft syndrome had been prolonged; however, it gradually improved, and she was extubated on POD 81. Cytomegalovirus infection and catheter infection occurred several times and were treated using antivirus or antibiotic drugs. The patient showed difficulty eating sufficient meals and required habilitation to eating. She was discharged on POD 225. Vessel complications did not occur. Three years have passed since LDLT, with no reports of intellectual disability or liver dysfunction.
We rescued a case involving an LBWI with acute liver failure; however, few reports to date describe such a case (Table 1). To our knowledge, the lightest LDLT recipient had a body weight of 2.4 kg at the time of LDLT[7], and the youngest LDLT recipient in Japan was 9 d of age[8-10]. For deceased donor liver transplantation, the smallest reported recipient was 2 kg, and the youngest was 7 d of age[11]. No consensus has been reached regarding the safe lower limit of body weight for small recipients. A recent UNOS database analysis of infants weighting less than 5 kg revealed one-year patient and graft survival rates of 77.7% and 66.1%, respectively[12]. A recent Japanese study reported an improved survival rate of 90.1% in an infant recipient within 3 mo[7]. Recipients who were within 3 mo of age also showed a higher rate of biliary complications[7]. However, our case did not have biliary complications. The problems we experienced in the described case were related to the following: (1) large-for-size graft syndrome[4]; (2) the transplantable body weight of the recipient; and (3) vessel reconstruction difficulties[13]. Challenges in managing transplantation for LBWIs with acute liver failure are nearly always due to the low body weight and fragility of the recipient.
| Problems | Management | |
| Pre-LT | Low body weight Liver failure Donor | Enteral nutrition targeting to over 2500 g Apheresis (exchange transfusion, plasmapheresis) Informed consent |
| LT | Large-for-size graft syndrome Hepatic artery reconstruction Abdominal compartment syndrome | Monosegment graft Brunch patch, dorsal approach Skin closure Open management→secondary skin closure |
| Post-LT | Fluid overload Respiratory failure | Aggressive water removal using CHDF |
In liver transplantation, the transplantable body weight of a neonatal recipient is limited by graft size. If the GRWR is greater than 4.0%, the risk of abdominal compartment syndrome and insufficient blood supply in relation to a large-for-size graft are increased; the recipient abdomen cannot be closed, or massive hepatocyte necrosis may occur in the transplanted graft[8,10,14,15]. In pediatric LDLT, particularly in cases for which the recipient’s body weight is less than 6 kg, monosegment grafts or hyper-reduced left lateral grafts have been transplanted[4]. A total of 268 pediatric patients underwent LDLT 275 times between May 2001 and December 2015 at Jichi Medical University Hospital. These transplantation procedures involved 196 left lateral segment grafts, with a median graft weight of 230 g (range, 138-382 g). Monosegment grafts were transplanted in 13 cases (including 9, 3, and 1 cases involving S2, reduced S2, and S3 monosegment grafts, respectively); in these cases, the median graft weight was 124 g (range, 93-180 g). A hyper-reduced left lateral segment graft was transplanted in 1 case, and the graft volume was 172 g. The smallest graft that we have transplanted was 93 g, and the median GRWR was 3.6% (2.4%-4.2%). A previous study reported hyper-reduced left lateral segment graft weights of 72-189 g, with most graft weights being 100-150 g[7]. The lower limit of the smallest graft volume from a living donor is approximately 100 g[10]. Given this graft weight, transplantable recipient body weights of 2500 g or more will result in a GRWR of 4.0% or less. Based on this estimation, 2500 g is a reasonable body weight for such recipients.
In our experience, the body weights of the described patient were increased by enteral nutrition and became greater than 2500 g at 44 d of age. Another case of increased body weight by enteral nutrition was reported, and the body weight increased to greater than 2500 g at 41 d of age[5]. Despite the existence of acute liver failure, the body weights of the patients increased to over 2500 g via enteral nutrition while exchange transfusions were performed. Thus, in cases of acute liver failure, body weight may be increased with enteral nutrition if appropriate medication therapy that includes apheresis, such as exchange transfusion or plasmapheresis, is performed.
At times, it is impossible to close the abdominal fascia of the recipient during LDLT due to abdominal compartment syndrome. In this case, the abdominal fascia could not be closed during LDLT; instead, the case required secondary skin closure. In cases involving pediatric LDLT patients, apheresis and dialysis are reportedly effective[16]. Aggressive water removal using CHDF was required in this case. Thus, even if LDLT for an LBWI with acute liver failure is successfully performed after an increase in body weight, circulation and respiratory management are challenging due to volume overload. Under these circumstances, aggressive water removal using CHDF and secondary abdominal closure approaches are useful and important tools for successful postoperative management.
Vessel reconstruction in cases similar to the described case is difficult due to the high GRDR between the hepatic vein and the portal vein bifurcation and to diameter mismatch for the hepatic artery between the graft and the recipient[13]. Hepatic artery reconstruction by the dorsal approach of portal vein anastomosis has been reported to help reduce the risk of hepatic artery complications among small recipients if the GRDR is over 2.4[13]. At times, the hepatic arteries of such recipients are thin. The hepatic artery of this case was not thin; however, the GRDR was 2.4, and the hepatic artery was anastomosed by a dorsal approach. In our experience of 13 cases of LDLT using monosegment grafts, the thinnest artery among the recipients was 1.2 mm, with 3 cases involving hepatic arteries of less than 1.5 mm. The graft-to-recipient diameter ratio was 0.67: 1.67. In addition, the dorsal position was selected for hepatic artery anastomotic approaches in 7 cases (53.8%), and the branch patch technique was selected in 5 cases (38.5%). Hepatic artery complications after LDLT occurred in 3 cases, although interventional radiology was sufficient for achieving recovery in all 3 cases.
We rescued a case of LBWI with acute liver failure by performing LDLT. In cases involving LBWIs with acute liver failure, infants may be rescued by LDLT after their body weights have been increased to over 2500 g via repeated exchange transfusions and enteral nutrition.
The patient was 1594 g, 323/7 gestational wk at birth and immediately after birth, hypoglycemia and hypotension appeared.
At the age of 11 d, coagulation dysfunction and an elevated serum ferritin level due to acute liver failure were observed.
The case was highly suspected of gestational alloimmune liver disease and differential diagnosis was metabolic disorder, infectious disease and Neimann-Pick disease type C.
Laboratory data showed repeated coagulopathy and hyperbilirubinemia with daily exchange transfusion and a pediatric end-stage liver disease score of 15.8.
Computed tomography scan revealed a markedly atrophied liver.
A liver biopsy revealed a marked loss of hepatocytes and the remaining hepatocytes were multinucleated, and there was widespread fibrosis around Glisson’s sheath and in the parenchymal area (F3-4).
The case underwent living donor liver transplantation at 55 d of age using an S2 monosegment graft from her mother (107 g).
Kasahara M et al and Mizuta K et al reported the living donor liver transplantation for small recipient (Exp Clin Transplant 2014; 12 Suppl 1:1-4, Am J Transplant 2010; 10: 2547-2552).
Low birth weight infant (LBWI) is defined as a birth weight of a infant of 2499 g or less regarding of gestational age.
In cases involving LBWI with acute liver failure, infants may be rescued by living donor liver transplantation after their body weights have been increased to over 2500 g.
The authors presented an interesting case with LBWIs (1594g, 323/7 wk) with acute liver failure.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hashimoto K, Xu X, Quintero J S- Editor: Chen K L- Editor: A E- Editor: Ma YJ
| 1. | Taylor SA, Whitington PF. Neonatal acute liver failure. Liver Transpl. 2016;22:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Bitar R, Thwaites R, Davison S, Rajwal S, McClean P. Liver Failure in Early Infancy: Aetiology, Presentation, and Outcome. J Pediatr Gastroenterol Nutr. 2017;64:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Lopriore E, Mearin ML, Oepkes D, Devlieger R, Whitington PF. Neonatal hemochromatosis: management, outcome, and prevention. Prenat Diagn. 2013;33:1221-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Yamada N, Sanada Y, Hirata Y, Okada N, Wakiya T, Ihara Y, Miki A, Kaneda Y, Sasanuma H, Urahashi T. Selection of living donor liver grafts for patients weighing 6kg or less. Liver Transpl. 2015;21:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Koura U, Horikawa S, Okabe M, Kawasaki Y, Makimoto M, Mizuta K, Yoshida T. Successful treatment of hemochromatosis with renal tubular dysgenesis in a preterm infant. Clin Case Rep. 2015;3:690-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Alawi K, Mitros FA, Bishop WP, Rayhill S, Wu Y. A reduced segment II/III graft for neonatal liver failure with absence of detectable hepatocytes. A case report and literature review. Pediatr Transplant. 2011;15:e60-e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Kasahara M, Sakamoto S, Sasaki K, Uchida H, Kitajima T, Shigeta T, Narumoto S, Hirata Y, Fukuda A. Living donor liver transplantation during the first 3 months of life. Liver Transpl. 2017;23:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Kasahara M, Sakamoto S, Umeshita K, Uemoto S. Effect of graft size matching on pediatric living-donor liver transplantation in Japan. Exp Clin Transplant. 2014;12 Suppl 1:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Umeshita K, Inomata Y, Furukawa H, Kasahara M, Kawasaki S, Kobayashi E, Kokudo N, Sakisaka S, Shimada M, Tanaka E. Liver transplantation in Japan: Registry by the Japanese Liver Transplantation Society. Hepatol Res. 2016;46:1171-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Mizuta K, Yasuda Y, Egami S, Sanada Y, Wakiya T, Urahashi T, Umehara M, Hishikawa S, Hayashida M, Hyodo M. Living donor liver transplantation for neonates using segment 2 monosubsegment graft. Am J Transplant. 2010;10:2547-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Grabhorn E, Richter A, Fischer L, Ganschow R. Emergency liver transplantation in neonates with acute liver failure: long-term follow-up. Transplantation. 2008;86:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Arnon R, Annunziato R, Miloh T, Sogawa H, Nostrand KV, Florman S, Suchy F, Kerkar N. Liver transplantation in children weighing 5 kg or less: analysis of the UNOS database. Pediatr Transplant. 2011;15:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Sanada Y, Hishikawa S, Okada N, Yamada N, Katano T, Hirata Y, Ihara Y, Urahashi T, Mizuta K. Dorsal approach plus branch patch technique is the preferred method for liver transplanting small babies with monosegmental grafts. Langenbecks Arch Surg. 2017;402:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Ogawa K, Kasahara M, Sakamoto S, Ito T, Taira K, Oike F, Ueda M, Egawa H, Takada Y, Uemoto S. Living donor liver transplantation with reduced monosegments for neonates and small infants. Transplantation. 2007;83:1337-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Kasahara M, Uryuhara K, Kaihara S, Kozaki K, Fujimoto Y, Ogura Y, Ogawa K, Oike F, Ueda M, Egawa H. Monosegmental living donor liver transplantation. Transplant Proc. 2003;35:1425-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Sanada Y, Mizuta K, Urahashi T, Ihara Y, Wakiya T, Okada N, Yamada N, Koinuma T, Koyama K, Tanaka S. Role of apheresis and dialysis in pediatric living donor liver transplantation: a single center retrospective study. Ther Apher Dial. 2012;16:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |