Published online Oct 28, 2017. doi: 10.3748/wjg.v23.i40.7253
Peer-review started: May 13, 2017
First decision: June 22, 2017
Revised: July 24, 2017
Accepted: August 25, 2017
Article in press: August 25, 2017
Published online: October 28, 2017
Processing time: 170 Days and 13.3 Hours
To investigate the protective effect of prostaglandin E1 (PGE1) against endoplasmic reticulum (ER) stress-induced hepatocyte apoptosis, and to explore its underlying mechanisms.
Thapsigargin (TG) was used to induce ER stress in the human hepatic cell line L02 and hepatocarcinoma-derived cell line HepG2. To evaluate the effects of PGE1 on TG-induced apoptosis, PGE1 was used an hour prior to TG treatment. Activation of unfolded protein response signaling pathways were detected by western blotting and quantitative real-time RT-PCR. Apoptotic index and cell viability of L02 cells and HepG2 cells were determined with flow cytometry and MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay.
Pretreatment with 1 μmol/L PGE1 protected against TG-induced apoptosis in both L02 cells and HepG2 cells. PGE1 enhanced the TG-induced expression of C/EBP homologous protein (CHOP), glucose-regulated protein (GRP) 78 and spliced X box-binding protein 1 at 6 h. However, it attenuated their expressions after 24 h. PGE1 alone induced protein and mRNA expressions of GRP78; PGE1 also induced protein expression of DNA damage-inducible gene 34 and inhibited the expressions of phospho-PKR-like ER kinase, phospho-eukaryotic initiation factor 2α and CHOP. Treatment with protein kinase A (PKA)-inhibitor H89 or KT5720 blocked PGE1-induced up-regulation of GRP78. Further, the cytoprotective effect of PGE1 on hepatocytes was not observed after blockade of GRP78 expression by H89 or small interfering RNA specifically targeted against human GRP78.
Our study demonstrates that PGE1 protects against ER stress-induced hepatocyte apoptosis via PKA pathway-dependent induction of GRP78 expression.
Core tip: The mechanism underlying the hepatoprotective effect of prostaglandin E1 (PGE1) remains unclear. In this study, we found that pretreatment with PGE1 protected hepatocytes against thapsigargin-induced apoptosis. PGE1 alone induced protein and mRNA expressions of glucose-regulated protein (GRP)78. Treatment with protein kinase A (PKA)-inhibitor H89, KT5720 or small interfering (si)RNA specifically targeted against human GRP78 blocked PGE1-induced up-regulation of GRP78. The hepatoprotective effect of PGE1 was lost by blocking GRP78 expression with either H89 or siRNA. Our study demonstrates for the first time that PGE1 protects against endoplasmic reticulum stress-induced hepatocyte apoptosis via PKA pathway-dependent induction of GRP78 expression.
- Citation: Yang FW, Fu Y, Li Y, He YH, Mu MY, Liu QC, Long J, Lin SD. Prostaglandin E1 protects hepatocytes against endoplasmic reticulum stress-induced apoptosis via protein kinase A-dependent induction of glucose-regulated protein 78 expression. World J Gastroenterol 2017; 23(40): 7253-7264
- URL: https://www.wjgnet.com/1007-9327/full/v23/i40/7253.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i40.7253
Hepatocyte apoptosis can be triggered by intra- or extra-cellular signals[1]. The intracellular signals for hepatocyte apoptosis are induced by DNA damage, oxidative stress, growth factor deprivation, mitochondrial dysfunction, ATP depletion and endoplasmic reticulum (ER) stress[2,3]. A complex interaction occurs among these intracellular apoptotic signaling pathways. ER stress is known to induce hepatocyte apoptosis under various pathological conditions[4]. ER stress is implicated in the pathogenesis of various liver diseases, such as obesity-associated fatty liver disease[5,6], viral hepatitis[7], alcohol-induced liver injury[8], drug-induced liver injury[9] and ischemia/reperfusion injury of the liver[10,11]. Devising a treatment strategy to protect hepatocytes from ER stress-induced apoptosis will benefit most patients with liver diseases.
ER is a multifunctional intracellular organelle responsible for the synthesis, processing and trafficking of proteins that are essential for cell growth and survival. ER also serves as a storage organelle for calcium[12]. When the homeostasis of ER is disturbed under various pathophysiological conditions, ER stress is induced and the unfolded protein response (UPR) is activated. The UPR activates three ER transmembrane transducers: inositol-requiring enzyme (IRE) 1a, PKR-like ER kinase (PERK), and activating transcription factor (ATF) 6α[12-14]. Activation of these three UPR pathways enhances the ER’s protein folding via up-regulation of the synthesis of glucose-regulated protein (GRP) 78. UPR signals also accelerate the degradation of misfolded proteins and reduce the synthesis of new proteins. Therefore, UPR during ER stress facilitates restoration of homeostasis. However, sustained or unresolved ER stress can activate a cascade of apoptotic signals that eventually result in cell death[15,16].
In several experimental models of liver injury, prostaglandin (PG) E1 has been shown to protect against hepatocyte apoptosis[17-19]. PGE1 is also effective in the treatment of patients with fulminant hepatitis and those with primary graft non-function after liver transplantation[20,21]. Thus, PGE1 appears to protect hepatocytes against apoptosis through various mechanisms[22-24]. However, the underlying mechanism of its hepatoprotective effect is not well understood.
In two recent studies, PGE1 was shown to induce expressions of heat-shock protein (HSP) and GRP78 in animal models of liver injury caused by ischemia reperfusion and hepatectomy[25,26]. These findings suggest that modulation of UPR may mediate the hepatoprotective effects of PGE1. However, the role of PGE1 in ER stress-induced apoptosis of hepatocytes is largely unknown.
In this study, we evaluated the protective effect of PGE1 against ER stress-induced hepatocyte apoptosis in both the normal human hepatocyte cell line L02 and the hepatocarcinoma-derived cell line HepG2[27].
RPMI 1640 was obtained from Thermo-Fisher Biochemical Products Co. Ltd (Beijing, China). Fetal bovine serum (FBS), thapsigargin (TG), protein kinase A (PKA) inhibitor H89 and KT5720, 4-phenylbutyric acid (PBA) and PGE1 were purchased from Sigma (St. Louis, MO, United States). Antibodies against GRP78, PERK, eukaryotic translation initiation factor-2α (eIF-2α), phospho-PERK (p-PERK) and phospho-eIF2α (p-eIF2α), C/EBP homologous protein (CHOP), spliced X box-binding protein 1 (sXBP1), growth arrest and DNA damage-inducible gene 34 (GADD34) and β-actin were purchased from Santa Cruz Biotechnology (Dallas, TX, United States). Annexin V-FITC/propidium iodide (PI) apoptosis detection kit was purchased from Dojindo Laboratories (Kumamoto, Japan). Small interfering (si)RNA scramble control and validated human GRP78-siRNA were purchased from Santa Cruz Biotechnology. All other chemicals and reagents were obtained from Sigma, unless stated otherwise.
The human hepatocyte cell line L02 and hepatocarcinoma-derived cell line HepG2 were obtained from the cell bank of the Type Culture Collection at the Chinese Academy of Sciences (Shanghai, China). The L02 and HepG2 cells were propagated at 37 °C in 5% CO2 in RPMI 1640 medium containing 10% (v/v) FBS and 100 units/mL penicillin, and were passaged every 5-7 d. The L02 and HepG2 cells were cultured until they acquired 80%-100% confluence. Thereafter, the cells were rinsed three times with 10 mL of phosphate-buffered saline (PBS) and cultured in a medium lacking FBS for 24-36 h. To evaluate the effects of PGE1 or PBA on TG-induced apoptosis, PGE1 or PBA was used an hour prior to TG treatment and, thereafter, the medium was not changed. TG and H89 were dissolved in dimethyl sulfoxide (DMSO) at concentrations of 40 µmol/L and 20 µmol/L, respectively, as stock solution. PGE1 was dissolved in ethanol at a concentration of 2.82 mmol/L as stock solution. PBA was dissolved in RPMI 1640 medium. All control conditions included corresponding vehicles at the appropriate concentrations (ethanol for PGE1 and DMSO for TG and H89).
Apoptosis was determined using the annexin V-FITC/PI apoptosis detection kit, according to the manufacturer’s instructions. Briefly, 2 × 106 cells were harvested using 0.05% trypsin with 0.5% mmol/L EDTA. To analyze the whole apoptotic cell population, non-adherent cells present in the culture medium were added to the harvested cells. The cells were then washed twice with pre-chilled PBS and resuspended in 500 μL annexin binding buffer. Then, 5 μL of annexin V-FITC and 5 μL of PI were added to each sample and incubated in dark at room temperature for 10 min. Flow cytometry (Gallios; Beckman Coulter, Brea, CA, United States) was performed according to the manufacturer’s specifications. The apoptotic index was calculated as the percentage of annexin V+ cells divided by the total number of cells in the gated region.
Cell viability was assessed with MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] method using the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (Promega Corporation, Madison, WI, United States) according to the manufacturer’s instructions. In brief, early passage of L02 or HepG2 cells were plated in triplicate on 96-well plates (10000 cells/well) and cultured in modified RPMI 1640 for 24 h. Thereafter, the cells were rinsed three times in 200 μL of PBS and cultured in a medium lacking fetal calf serum. Cell viability was determined by replacing the medium with 20 μL of MTS. After incubation of the cells at 37 °C for 3 h, the absorbance was measured at 490 nm using a microplate reader (Bio-Rad model 680; Bio-Rad, Hercules, CA, United States). Cell viability was normalized as a percentage of control. This experiment was performed five times.
Cell lysates containing 40 μg of protein were resolved by SDS-PAGE using 4%-20% polyacrylamide gradient gel, and the fractioned proteins were subsequently transferred to nitrocellulose membranes. After blocking with Tris-based saline buffer containing 5% dry milk and 0.1% Tween 20 for 1 h, the membranes were blotted with the corresponding antibodies. The primary and secondary antibodies used were: rabbit anti-human GRP78 (1:500 dilution), PERK (1:1000 dilution), phospho (p)-PERK (1:500 dilution), eIF-2α (1:500 dilution), p-eIF-2α (1:250 dilution), sXBP1 (1:500 dilution), CHOP (1:1000 dilution), GADD34 (1:1000 dilution), mouse anti-human β-actin, goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP), and goat anti-mouse IgG conjugated with HRP. The membranes were developed using a chemiluminescence detection system and thereafter exposed to BioMax Light Film (Kodak, Rochester, NY, United States). The band intensity for each protein was measured using ImagePro Plus analysis software (MediaCybernetics, Silver Spring, MD, United States) and the expression normalized to that of β-actin.
Total RNA was isolated from L02 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. RNase-free DNase I (TaKaRa, Dalian, China) was applied before cDNA synthesis to remove any genomic contamination. A total of 1 μg RNA from each sample was used for cDNA synthesis with a reverse transcription kit (TaKaRa). Reverse transcription (final volume of 20 μL) was performed at 42 °C for 10 min, followed by 75 °C for 5 min. The real-time PCR reaction (25 μL) containing 12.5 μL of 2 × SYBR Premix ExTaq II (Tli RNaseH Plus; TaKaRa), 1 μL of each 10 μmol/L primers, 2 μL of cDNA template, and 8.5 μL RNAse/DNAse-free water was performed on a CFX96 PCR system (Bio-Rad). The reaction process was as follows: denaturation at 95 °C for 3 min, followed by 40 cycles of amplification (95 °C for 10 s and 60 °C for 30 s), ending with a melt curve ranging from 60 °C to 95 °C with a heating rate of 0.3 °C/15 s. All samples were run in triplicate. Relative expression of GRP78 was calculated using the delta-delta-Ct method with β-actin as the reference control.
Primers used in the PCR were: GRP78 forward, 5’-AAATAAGCCTCAGCGGTTTCTT-3’ and reverse, 5’-TCAAGTTCTTGCCGTTCAAGG-3’; β-actin forward, 5’- CGGGAAATCGTGCGTGAC-3’ and reverse, 5’- CAGGAAGGAAGGCTGGAAG-3’ (TaKaRa). Quantitative real-time PCR was performed according to the MIQE guidelines[28].
Briefly, cell culture plates containing 6-wells were seeded with 2 × 105 cells/well and cultured in RPMI 1640 medium containing 10% (v/v) FBS and 100 units/mL penicillin. siRNA scramble control and validated human GRP78-siRNA were used. GRP78 inhibition was performed using a commercially available siRNA kit (Santa Cruz Biotechnology). Knock-down of the target molecule, GRP78, was monitored by western blotting and real-time PCR.
Results of cell apoptosis and cell viability are expressed as mean ± SD. One-way analysis of variance (ANOVA) with Bonferroni’s post hoc analysis was performed to compare multiple groups. The Student’s t-test was used to assess between-group differences. The level of significance was set at P < 0.05.
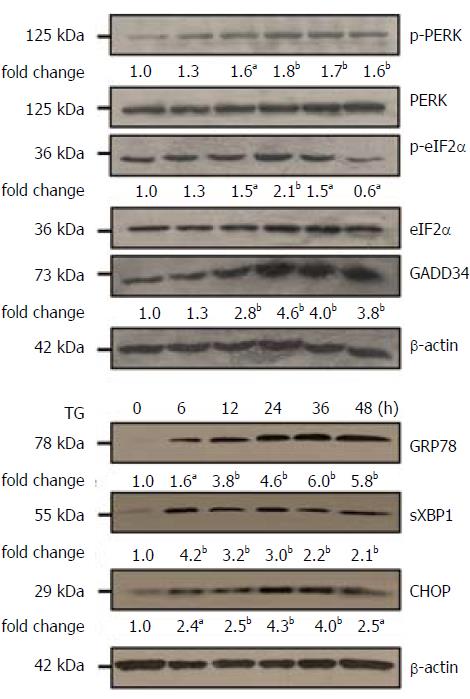
We confirmed TG-induced ER stress in L02 cells (Figure 1). At 48 h, TG (1 μmol/L) caused significant enhancement in phosphorylation of PERK and GADD34 proteins, as compared to that observed at 6 h. Further, phosphorylation of eIF2α at 24 h was also up-regulated, as compared to that at 6 h. TG also induced a significant increase in the expressions of GRP78, CHOP and sXBP1 proteins.
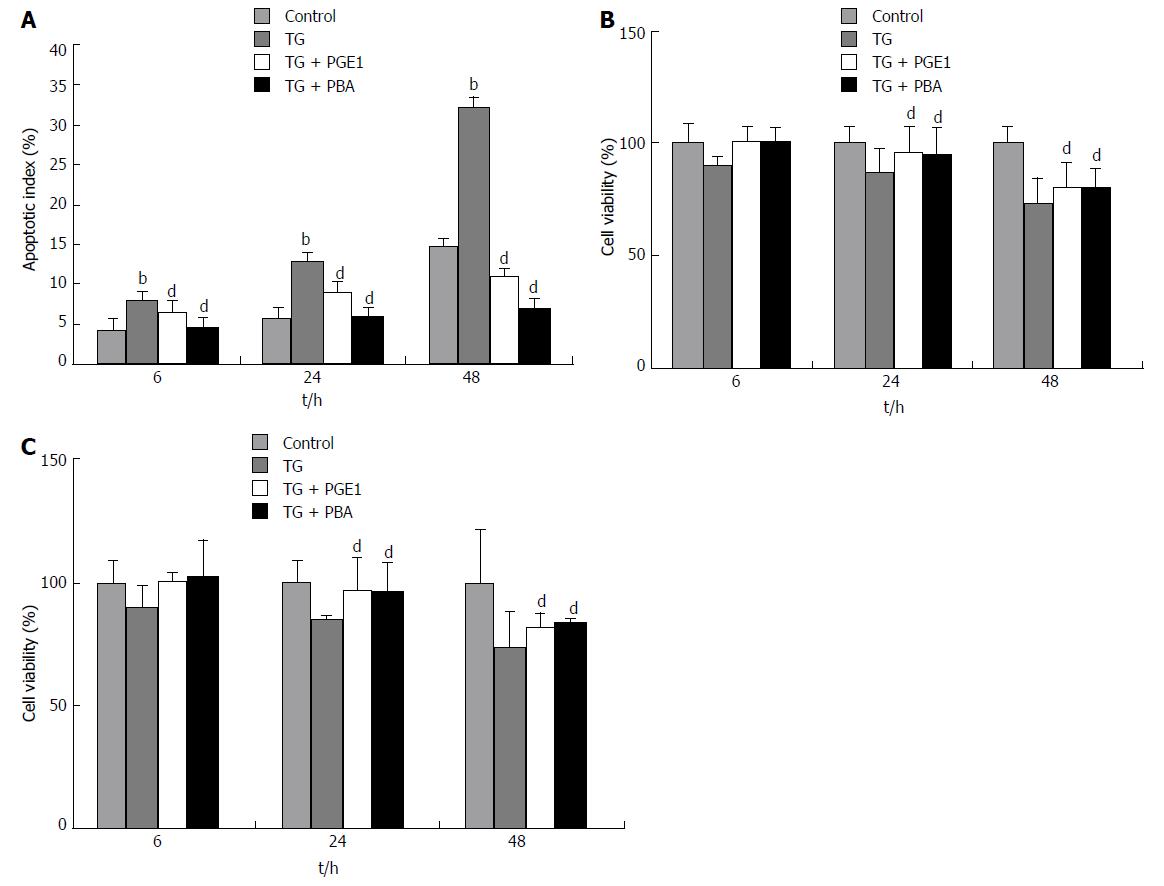
We also confirmed TG-induced apoptosis in both L02 and HepG2 cells by means of flow cytometry and MTS assay. As shown in Figure 2A, the apoptotic index of L02 cells after TG treatment was significantly higher than that of control from 6 h to 48 h (P < 0.01). At 48 h, the apoptotic index reached 32.66%. TG also showed a dose-dependent increase in apoptotic index with increase in the concentration of TG (1 μmol/L, 2 μmol/L and 3 μmol/L; data not shown). Cell viability of both L02 and HepG2 cells significantly decreased from 24 h to 48 h after TG treatment (Figure 2B and C).
We assessed the effect of PGE1 on ER stress-induced apoptosis in L02 cells (Figure 2A). From 6 h to 48 h, 1 μmol/L PGE1 pretreatment significantly decreased TG-induced apoptotic index. At 48 h, 0.5 μmol/L and 1 μmol/L PGE1 showed dose-dependent protection against TG-induced apoptosis (data not shown). PBA is a low molecular weight chemical chaperone and an ER stress inhibitor. PBA at 10 nmol/L significantly inhibited TG induced apoptosis (Figure 2A). MTS assay revealed that PGE1 significantly increased the viability of TG-treated L02 cells and HepG2 cells (Figure 2B and C). These results demonstrate the protective effect of PGE1 on ER stress-induced apoptosis in L02 cells and in HepG2 cells.
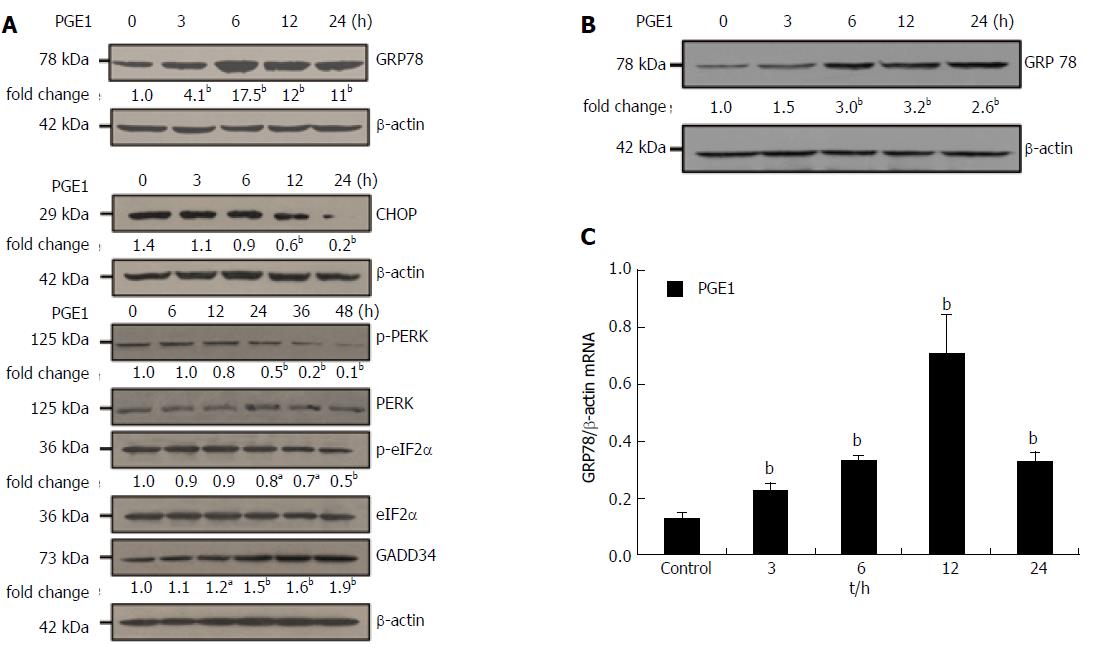
After demonstration of the cytoprotective effect of PGE1, we investigated the effects of PGE1 on UPR signals induced by TG. We selected three indicators from UPR, GRP78, CHOP and sXBP1, as GRP78 is a common downstream target of the three UPR signal pathways. CHOP is the major protein involved in apoptotic signals induced by ER stress, and sXBP1 is activated through the oligomerization of IRE1a[29]. As shown in Figure 3, 1 μmol/L PGE1 appeared to increase TG-induced GRP78, CHOP and sXBP1 expressions till 6 h post-treatment. However, from 24 h to 48 h, PGE1 suppressed TG-induced GRP78 and CHOP expressions. These results indicate that although PGE1 increased the early expression of UPR signals, it promoted the rapid recovery of ER stress.
The observation that PGE1 attenuated the ER stress-induced apoptosis and promoted the rapid recovery from ER stress prompted us to investigate the effect of PGE1 on GRP78, CHOP and GADD34 expressions. GRP78 is a critical chaperone which determines the outcome of ER stress. GADD34 is involved in both recovery and resumption of protein synthesis as well as in the ER stress-induced apoptosis[14,30]. We also investigated the effects of PGE1 on phosphorylation of eIF2α and PERK, since the activation of PERK and eIF2α is known to induce CHOP and GADD34. From 3 h to 24 h after treatment with 1 μmol/L PGE1, a significant increase in GRP78 expression was observed in both L02 cells and HepG2 cells (Figure 4A and B). PGE1 also decreased CHOP expression in L02 cells and induced the expression of GADD34; however, it suppressed the expression of p-PERK and p-eIF2α proteins (Figure 4A). Results of quantitative real-time RT-PCR also demonstrated that PGE1 induced the mRNA expression of GRP78 from 3 h to 24 h (Figure 4C).
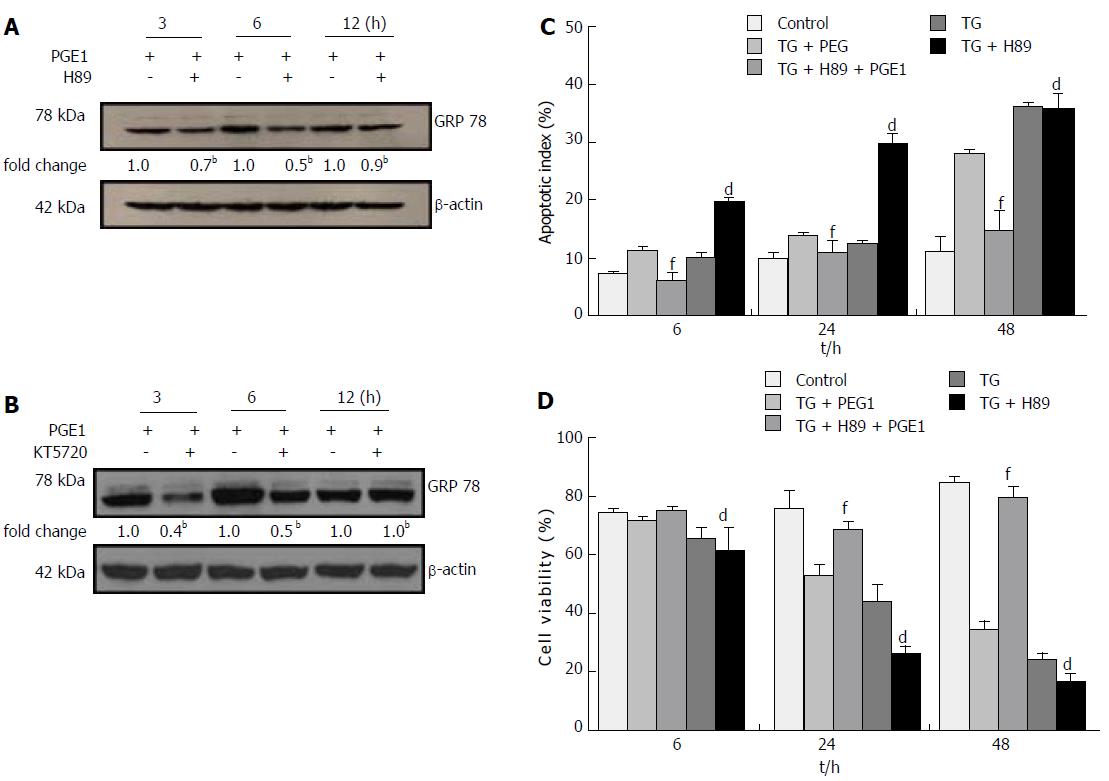
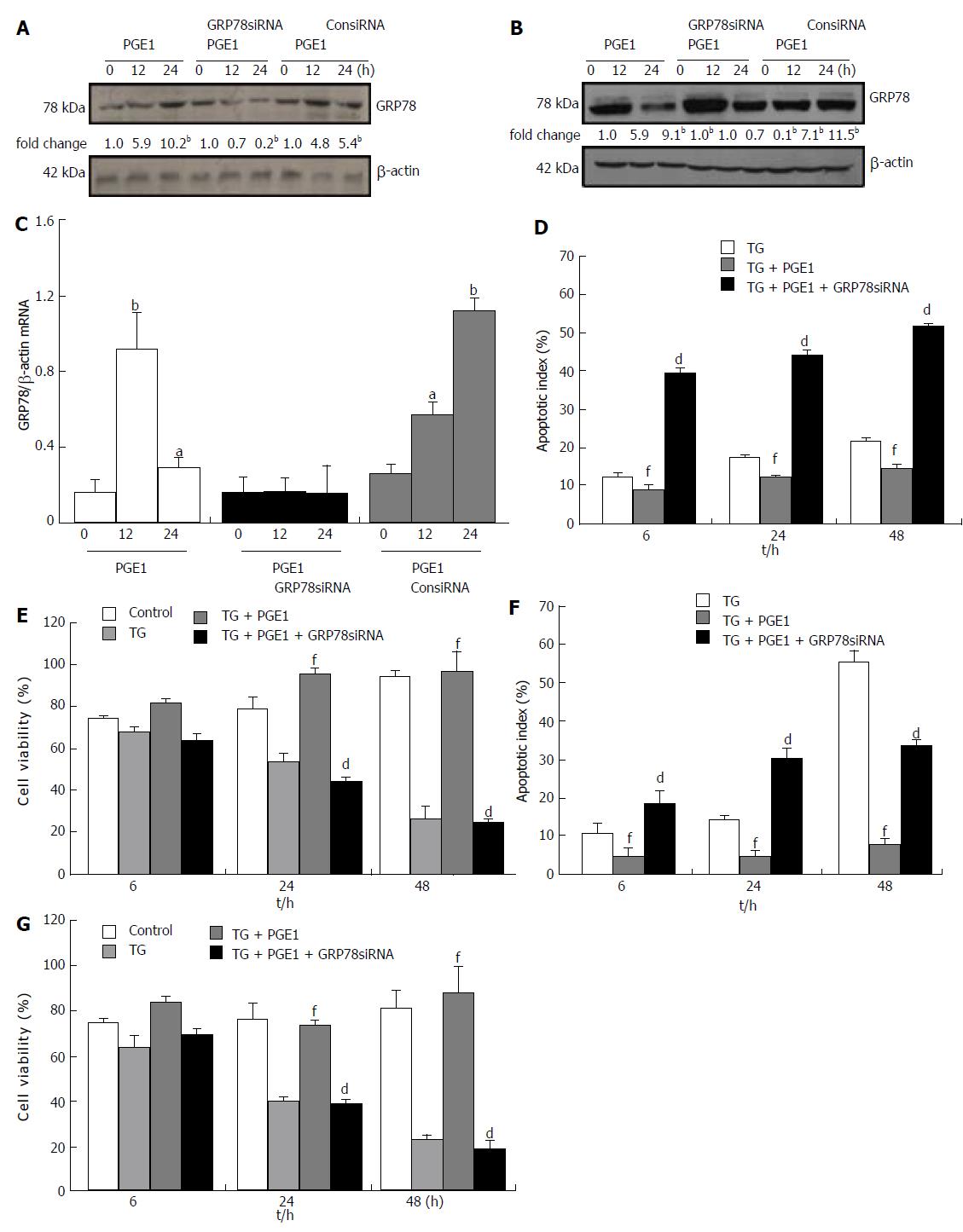
To explore the signal pathways that may mediate the effect of PGE1 on GRP78 expression, we inhibited the PKA pathway by H89 or KT5720. Treatment with 10 μmol/L of H89 or 1 μmol/L KT5720 appeared to counteract the effect of PGE1 on GRP78 expression (Figure 5A and B). These results indicate that PGE1 increased GRP78 expression via a PKA-dependent pathway.
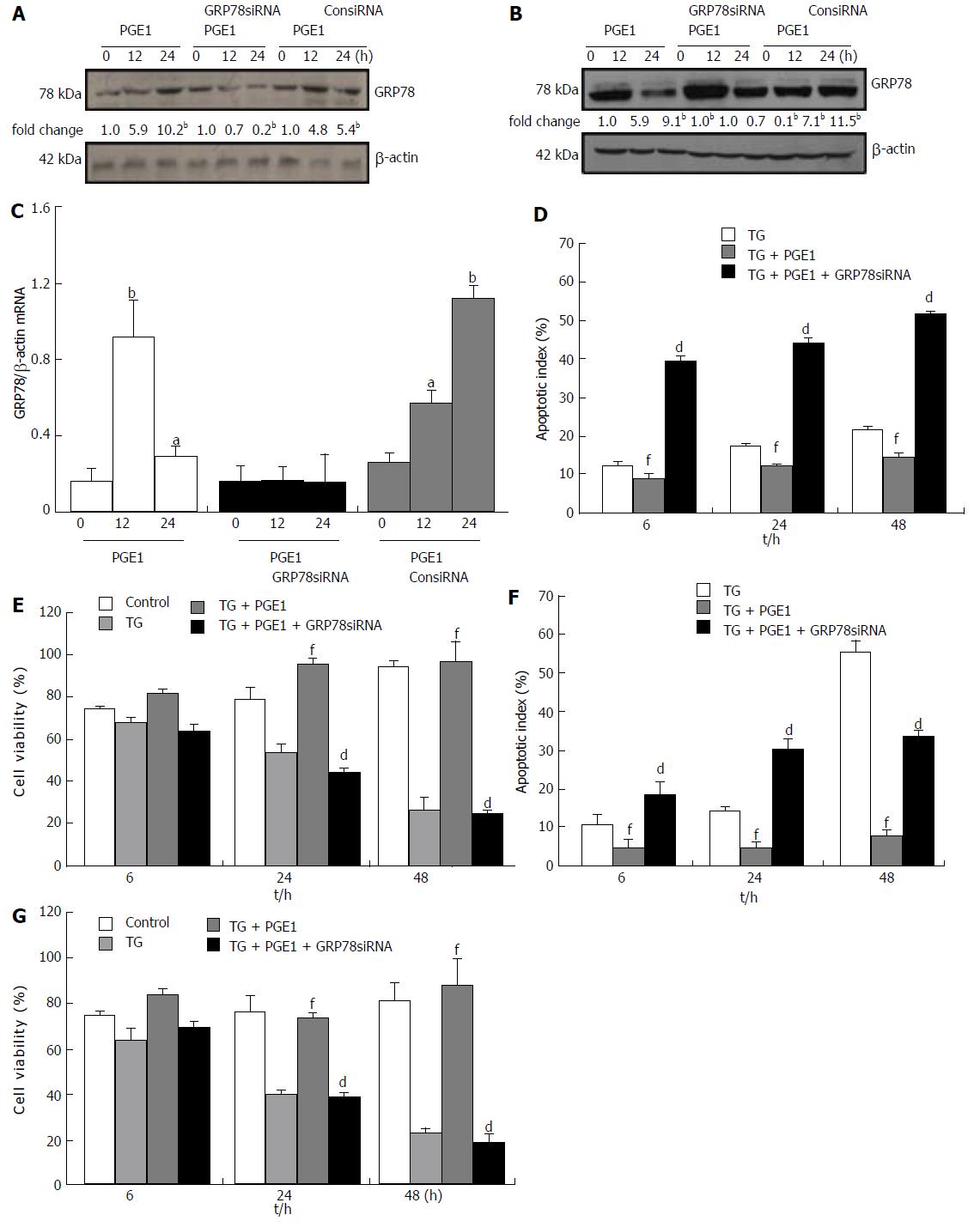
H89 at 10 μmol/L appeared to inhibit the protective effect of PGE1 on hepatocyte apoptosis, which suggests that the cytoprotective effect of PGE1 was mediated via the PKA signaling pathway (Figure 5C). To further test whether the protective effect of PGE1 was dependent on the induction of GRP78 expression, we inhibited GRP78 expression by transfection with siRNA specifically targeted against human GRP78. On blocking the PGE1-induced up-regulation of GRP78 expression by siRNA (Figure 6A-C), PGE1 appeared to lose its cytoprotective effect in both L02 cells and HepG2 cells (Figure 6D-G). These findings imply that PGE1 protected against hepatocyte apoptosis via PKA-dependent induction of GRP78 expression.
The key findings of this study are that PGE1 protected hepatocytes from ER stress-induced apoptosis, that PGE1 enhanced the expression of GRP78 via the PKA pathway, and that the cytoprotective effect of PGE1 on hepatocytes was mediated via PKA-dependent GRP78 induction.
TG is known to induce ER stress by blocking ER Ca2+ uptake, which leads to depletion of ER Ca2+ stores[31]. PGE1 can also increase intracellular Ca2+ level by promoting the influx of Ca2+ from the external medium as well as by mobilization of Ca2+ from intracellular stores[32]. As intracellular Ca2+ level is an important factor in hepatocyte apoptosis and necrosis, cotreatment with TG and PGE1 may have modulated apoptotic signals of L02 cells, which eventually led to cell death by necrosis. Therefore, we assessed cell apoptosis and viability on flow cytometry and by MTS assay. Our results strongly suggest that PGE1 protects hepatocytes from ER stress-induced apoptosis.
PGE1 is known to have a direct vasodilator as well as an anti-platelet effect[22,33]. Several in vivo studies have indicated that PGE1 protects against hepatocyte apoptosis and promotes hepatocyte proliferation via inducing down-regulation of proinflammatory cytokine levels[24,26], suppression of tumor necrosis factor-αreceptor and adhesion molecule expression[23,24], and by up-regulating cyclin C and cyclin D1 expressions[34]. PGE1 also inhibits oxidative stress and nitrosative stress-induced hepatocyte death by inhibiting production of superoxide anion, by enhancing nitric oxide synthase expression and by inhibiting nuclear factor-κB activation in vitro[19,35,36]. In this study, we demonstrated that PGE1 protected hepatocytes from ER stress-induced apoptosis. Our findings suggest that modulation of UPR may mediate the cytoprotective effect of PGE1 in various pathological conditions.
The mechanisms involved in ER stress-induced apoptosis are yet to be elucidated. The adaptive capacity of cells to ER stress is an important determinant of the outcomes of ER stress. Apoptosis results from sustained or strong ER stress when UPR fails to restore ER homeostasis[37,38]. In the present study, PGE1 treatment enhanced TG-induced CHOP, GRP78 and sXBP1 expressions at 6 h, and significantly attenuated TG-induced CHOP, GRP78 and sXBP1 expressions, as assessed at 48 h. These results suggest that PGE1 boosted the initial expression of UPR and promoted restoration of ER homeostasis during ER stress.
During ER stress, UPR may have been activated to restore ER homeostasis. UPR is precisely regulated by three ER stress transducers and their downstream signals. GRP78 is considered as a master regulator of response to ER stress[39,40]. GRP78 binds with all three major UPR sensors (PERK, IRE1a and ATF6) and keeps them inactivated while the homeostasis of ER is maintained. During ER stress, GRP78 dissociates from PERK, ATF6 and IRE1a and binds to unfolded or misfolded proteins, promotes their proper folding or directs them to degradation. The dissociation of GRP78 from PERK, ATF6 and IRE1a, triggers the UPR response and further enhances the expression of GRP78. Increased GRP78 augments the folding capacity of the ER, inactivates the three ER sensors and promotes restoration of ER homeostasis[29].
The protective role of GRP78 against apoptosis during ER stress has been demonstrated both in vivo and in vitro[41-44]. The insulin signaling pathway has been found to promote cell proliferation and improve cell survival via up-regulation of GRP78 expression[45]. In this study, PGE1 significantly inhibited TG-induced apoptosis. PGE1 induced protein and mRNA expressions of GRP78. Further, inhibition of GRP78 expression via either H89 or siRNA hindered the protective role of PGE1 on TG-induced apoptosis. These results demonstrate that the cytoprotective effect of PGE1 on hepatocytes was mediated via induction of GRP78 during ER stress. The protein expression of GRP78 induced by PGE1 peaked at 6 h; however, mRNA expression of GRP78 induced by PGE1 peaked at 12 h. It is difficult to explain the difference in the time frame for attainment of peak levels of mRNA and protein expressions of GRP78. One explanation is that PGE1 regulated the GRP78 expression not only at the transcriptional level but also at the translational or posttranslational level. Whether PGE1 regulates the expression of GRP78 at the translational or posttranslational level warrants further studies.
The other important signal pathways in ER stress-induced apoptosis are mediated via induction of CHOP and GADD34. During UPR, phosphorylation of eIF2αvia the PERK pathway results in inhibition of mRNA translation and general protein synthesis. However, mRNA for activating transcription factor (ATF) 4 is selectively up-regulated. Activation of ATF4 results in induction of CHOP and GADD34 expressions. GADD34 and protein phosphatase 1 were shown to promote dephosphorylation of eIF2α and allow protein synthesis[46]. However, recent studies have shown that induction of GADD34 exacerbates the disturbance of ER homeostasis and leads to cell apoptosis by increasing oxidative stress[47]. CHOP has been identified as one of the most important mediators of ER stress-induced apoptosis; it induces apoptosis through various signal pathways[48].
In the current study, we had anticipated inhibition of GADD34 by PGE1. However, we observed an enhanced expression of GADD34 and significant inhibition of p-PERK, CHOP and p-eIF2α expressions in L02 cells. Our results indicate that the cytoprotective effect of PGE1 against hepatocyte apoptosis does not depend on the inhibition of GADD34. Since PGE1 lost its protective effects after inhibition of GRP78 expression in this study, it is possible that the increased expression of GADD34 in our study was the result of induction of GRP78 by PGE1 and that this represented restoration of ER homeostasis. Further study is needed to clarify the role of GADD34 in ER stress-induced apoptosis.
The biological effects of PGE result from its binding to its receptors, EP1 to EP4, and previous studies have shown that L02 cells express EP1 receptors[49]. Binding of PGE1 with its receptors stimulates production of the second messenger cyclic 3, 5 adenosine monophosphate (cAMP). cAMP may act via distinct intracellular signaling effectors, such as PKA and the exchange proteins activated by cAMP[50]. A previous study has shown that cAMP has PKA-independent interaction with Ca2+ stored in lymphocytes[51]. To test whether the hepatoprotective effect of PGE1 is mediated via PKA-independent interaction with Ca2+ stores in L02 cells, we used H89 to block the PKA pathway. The results showed that the protective effects of PGE1 were largely dependent on the PKA pathway. However, H89 inhibited GRP78 expression most effectively at 6 h, and then at 12 h the inhibitory effect was alleviated; the increased apoptotic index lasted from 6 h to 48 h. It is difficult to explain this result. One explanation is that H89 may also have inhibited other kinases in addition to PKA. H89 has been shown to inhibit at least 8 kinases beside PKA[52]. The role of PKA and other kinases in ER stress-induced apoptosis remains to be studied in the future.
It is known that GRP78 expression is regulated at the transcriptional level by ER stress. Previous studies have shown that preconditioning to ER stress protects against cell death via induction of GRP78 or autophagy[53]. In this study, although PGE1 pretreatment significantly induced GRP78 expression and enhanced TG-induced early CHOP and sXBP1 expressions, PGE1 alone inhibited the expressions of p-PERK and p-eIF2α and CHOP. Therefore, whether PGE1 induced GRP78 expression via induction of ER stress is not known. In previous studies, leptin was shown to induce GRP78 expression in neuronal cells through the PI3K-mTOR pathway[54]; further, oncostatin M was also shown to induce GRP78 expression without triggering ER stress[55]. The mechanisms involved in the induction of GRP78 expression by PGE1 warrant further investigation.
In conclusion, this is the first study to demonstrate that the cytoprotective effect of PGE1 against ER stress-induced apoptosis is mediated via PKA-dependent induction of GRP78 expression in hepatocytes. Further studies are required for devising treatment strategies to protect hepatocytes against ER stress-induced apoptosis, which will be of much clinical relevance in the context of liver diseases.
Prostaglandin (PG) E1 has been shown to protect against hepatocyte apoptosis; however, the role of Prostaglandin E1 (PGE1) in endoplasmic reticulum (ER) stress-induced apoptosis of hepatocytes is largely unknown.
ER stress has been implicated in the pathogenesis of various liver diseases. Understanding the mechanisms underlying ER stress-induced apoptosis and devising a treatment strategy to protect hepatocytes from ER stress-induced apoptosis will benefit most patients with liver diseases.
Pretreatment with PGE1 protected hepatocytes against thapsigargin-induced apoptosis. PGE1 alone induced protein and mRNA expressions of glucose-regulated protein (GRP) 78. Treatment with protein kinase A (PKA)-inhibitor H89, KT5720 or small interfering (si)RNA specifically targeted against human GRP78 blocked PGE1-induced up-regulation of GRP78. The hepatoprotective effect of PGE1 was lost by blocking GRP78 expression by either H89 or siRNA. Our study demonstrates, for the first time, that PGE1 protects against ER stress-induced hepatocyte apoptosis via PKA pathway-dependent induction of GRP78 expression.
PKA and GRP78 may be new targets for pharmacological treatment in patients with liver diseases.
The strengths of this study lie in the experimental design, methodology and that the authors describe the caveats and potential pitfalls in great detail. Overall, the study was well-designed and the paper is interesting and sound.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cubero FJ, McMillin MA S- Editor: Wei LJ
L- Editor: Filipodia E- Editor: Ma YJ
| 1. | Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3:977-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 3. | Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 775] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 4. | Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460-3470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1537] [Article Influence: 128.1] [Reference Citation Analysis (1)] |
| 5. | Zha BS, Zhou H. ER Stress and Lipid Metabolism in Adipocytes. Biochem Res Int. 2012;2012:312943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Ben Mosbah I, Alfany-Fernández I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, Rodés J, Brenner C, Roselló-Catafau J, Peralta C. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis. 2010;1:e52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Asselah T, Bièche I, Mansouri A, Laurendeau I, Cazals-Hatem D, Feldmann G, Bedossa P, Paradis V, Martinot-Peignoux M, Lebrec D. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J Pathol. 2010;221:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23 Suppl 1:S16-S24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Yang X, Shao H, Liu W, Gu W, Shu X, Mo Y, Chen X, Zhang Q, Jiang M. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol Lett. 2015;234:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, Eckhoff DE, Contreras JL. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery. 2005;138:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Duvigneau JC, Kozlov AV, Zifko C, Postl A, Hartl RT, Miller I, Gille L, Staniek K, Moldzio R, Gregor W. Reperfusion does not induce oxidative stress but sustained endoplasmic reticulum stress in livers of rats subjected to traumatic-hemorrhagic shock. Shock. 2010;33:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda). 2007;22:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 340] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 13. | Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3842] [Cited by in RCA: 4533] [Article Influence: 348.7] [Reference Citation Analysis (0)] |
| 14. | Diehl JA, Fuchs SY, Koumenis C. The cell biology of the unfolded protein response. Gastroenterology. 2011;141:38-41, 41.e1-41.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2364] [Cited by in RCA: 3050] [Article Influence: 234.6] [Reference Citation Analysis (0)] |
| 16. | Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3:a004424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 293] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 17. | Siendones E, Jiménez-Gómez Y, Montero JL, Gómez-Díaz C, Villalba JM, Muntané J. PGE1 abolishes the mitochondrial-independent cell death pathway induced by D-galactosamine in primary culture of rat hepatocytes. J Gastroenterol Hepatol. 2005;20:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Togo S, Chen H, Takahashi T, Kubota T, Matsuo K, Morioka D, Watanabe K, Yamamoto H, Nagashima Y, Shimada H. Prostaglandin E1 improves survival rate after 95% hepatectomy in rats. J Surg Res. 2008;146:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Kishimoto S, Sakon M, Umeshita K, Miyoshi H, Taniguchi K, Meng W, Nagano H, Dono K, Ariyosi H, Nakamori S. The inhibitory effect of prostaglandin E1 on oxidative stress-induced hepatocyte injury evaluated by calpain-mu activation. Transplantation. 2000;69:2314-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Sterling RK, Luketic VA, Sanyal AJ, Shiffman ML. Treatment of fulminant hepatic failure with intravenous prostaglandin E1. Liver Transpl Surg. 1998;4:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Greig PD, Woolf GM, Sinclair SB, Abecassis M, Strasberg SM, Taylor BR, Blendis LM, Superina RA, Glynn MF, Langer B. Treatment of primary liver graft nonfunction with prostaglandin E1. Transplantation. 1989;48:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 185] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Weiner R, Kaley G. Influence of prostaglandin E1 on the terminal vascular bed. Am J Physiol. 1969;217:563-566. [PubMed] |
| 23. | Lozano JM, Collado JA, Medina T, Muntané J. Protection against liver injury by PGE1 or anti-TNF-alpha is associated with a reduction of TNF-R1 expression in hepatocytes. Scand J Gastroenterol. 2003;38:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Hafez T, Moussa M, Nesim I, Baligh N, Davidson B, Abdul-Hadi A. The effect of intraportal prostaglandin E1 on adhesion molecule expression, inflammatory modulator function, and histology in canine hepatic ischemia/reperfusion injury. J Surg Res. 2007;138:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Matsuo K, Togo S, Sekido H, Morita T, Kamiyama M, Morioka D, Kubota T, Miura Y, Tanaka K, Ishikawa T. Pharmacologic preconditioning effects: prostaglandin E1 induces heat-shock proteins immediately after ischemia/reperfusion of the mouse liver. J Gastrointest Surg. 2005;9:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Jia C, Dai C, Bu X, Peng S, Xu F, Xu Y, Zhao Y. Co-administration of prostaglandin E1 with somatostatin attenuates acute liver damage after massive hepatectomy in rats via inhibition of inflammatory responses, apoptosis and endoplasmic reticulum stress. Int J Mol Med. 2013;31:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Wang S, Jiang W, Chen X, Zhang C, Li H, Hou W, Liu Z, McNutt MA, Lu F, Li G. Alpha-fetoprotein acts as a novel signal molecule and mediates transcription of Fn14 in human hepatocellular carcinoma. J Hepatol. 2012;57:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9915] [Cited by in RCA: 11140] [Article Influence: 696.3] [Reference Citation Analysis (0)] |
| 29. | Kawaguchi S, Ng DT. Cell biology. Sensing ER stress. Science. 2011;333:1830-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Jäger R, Bertrand MJ, Gorman AM, Vandenabeele P, Samali A. The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol Cell. 2012;104:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Kiviluoto S, Vervliet T, Ivanova H, Decuypere JP, De Smedt H, Missiaen L, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate receptors during endoplasmic reticulum stress. Biochim Biophys Acta. 2013;1833:1612-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Adachi M, Ryo R, Yoshida A, Teshigawara K, Yamaguchi N, Hoshijima M, Takai Y, Sato T. Elevation of intracellular calcium ion by prostaglandin E1 and its inhibition by protein kinase C in a human megakaryocyte leukemia cell line. Cancer Res. 1989;49:3805-3808. [PubMed] |
| 33. | Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761-767. [PubMed] |
| 34. | Ishibe A, Togo S, Kumamoto T, Watanabe K, Takahashi T, Shimizu T, Makino H, Matsuo K, Kubota T, Nagashima Y. Prostaglandin E1 prevents liver failure after excessive hepatectomy in the rat by up-regulating Cyclin C, Cyclin D1, and Bclxl. Wound Repair Regen. 2009;17:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Ranchal I, González R, López-Sánchez LM, Barrera P, López-Cillero P, Serrano J, Bernardos A, De la Mata M, Rodríguez-Ariza A, Muntané J. The differential effect of PGE(1) on d-galactosamine-induced nitrosative stress and cell death in primary culture of human hepatocytes. Prostaglandins Other Lipid Mediat. 2006;79:245-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Siendones E, Fouad D, Díaz-Guerra MJ, de la Mata M, Boscá L, Muntané J. PGE1-induced NO reduces apoptosis by D-galactosamine through attenuation of NF-kappaB and NOS-2 expression in rat hepatocytes. Hepatology. 2004;40:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C. When ER stress reaches a dead end. Biochim Biophys Acta. 2013;1833:3507-3517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 38. | Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23:143-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 39. | Pluquet O, Pourtier A, Abbadie C. The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol. 2015;308:C415-C425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 40. | Hiramatsu N, Chiang WC, Kurt TD, Sigurdson CJ, Lin JH. Multiple Mechanisms of Unfolded Protein Response-Induced Cell Death. Am J Pathol. 2015;185:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Liu L, Chowdhury S, Fang X, Liu JL, Srikant CB. Attenuation of unfolded protein response and apoptosis by mReg2 induced GRP78 in mouse insulinoma cells. FEBS Lett. 2014;588:2016-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Li H, Zhu X, Fang F, Jiang D, Tang L. Down-regulation of GRP78 enhances apoptosis via CHOP pathway in retinal ischemia-reperfusion injury. Neurosci Lett. 2014;575:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Martin S, Lovat PE, Redfern CP. Cell-type variation in stress responses as a consequence of manipulating GRP78 expression in neuroectodermal cells. J Cell Biochem. 2015;116:438-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Inageda K. Insulin modulates induction of glucose-regulated protein 78 during endoplasmic reticulum stress via augmentation of ATF4 expression in human neuroblastoma cells. FEBS Lett. 2010;584:3649-3654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23:1292-1303. [PubMed] |
| 47. | Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1327] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 48. | Rao J, Zhang C, Wang P, Lu L, Qian X, Qin J, Pan X, Li G, Wang X, Zhang F. C/EBP homologous protein (CHOP) contributes to hepatocyte death via the promotion of ERO1α signalling in acute liver failure. Biochem J. 2015;466:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Jin J, Chang Y, Wei W, He YF, Hu SS, Wang D, Wu YJ. Prostanoid EP1 receptor as the target of (-)-epigallocatechin-3-gallate in suppressing hepatocellular carcinoma cells in vitro. Acta Pharmacol Sin. 2012;33:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Godinho RO, Duarte T, Pacini ES. New perspectives in signaling mediated by receptors coupled to stimulatory G protein: the emerging significance of cAMP efflux and extracellular cAMP-adenosine pathway. Front Pharmacol. 2015;6:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | de la Rosa LA, Vilariño N, Vieytes MR, Botana LM. Modulation of thapsigargin-induced calcium mobilisation by cyclic AMP-elevating agents in human lymphocytes is insensitive to the action of the protein kinase A inhibitor H-89. Cell Signal. 2001;13:441-449. [PubMed] |
| 52. | Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev. 2006;24:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 53. | Chandrika BB, Yang C, Ou Y, Feng X, Muhoza D, Holmes AF, Theus S, Deshmukh S, Haun RS, Kaushal GP. Endoplasmic Reticulum Stress-Induced Autophagy Provides Cytoprotection from Chemical Hypoxia and Oxidant Injury and Ameliorates Renal Ischemia-Reperfusion Injury. PLoS One. 2015;10:e0140025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Thon M, Hosoi T, Yoshii M, Ozawa K. Leptin induced GRP78 expression through the PI3K-mTOR pathway in neuronal cells. Sci Rep. 2014;4:7096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Vollmer S, Haan C, Behrmann I. Oncostatin M up-regulates the ER chaperone Grp78/BiP in liver cells. Biochem Pharmacol. 2010;80:2066-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |