Published online Oct 28, 2017. doi: 10.3748/wjg.v23.i40.7211
Peer-review started: June 8, 2017
First decision: August 10, 2017
Revised: August 4, 2017
Accepted: September 26, 2017
Article in press: September 26, 2017
Published online: October 28, 2017
Processing time: 143 Days and 16.5 Hours
To investigate whether glucagon-like peptide-2 (GLP-2) influences the neurally-induced responses in gastric strips from mice, since no data are available.
For functional experiments, gastric fundal strips were mounted in organ baths containing Krebs-Henseleit solution. Mechanical responses were recorded via force-displacement transducers, which were coupled to a polygraph for continuous recording of isometric tension. Electrical field stimulation (EFS) was applied via two platinum wire rings through which the preparation was threaded. The effects of GLP-2 (2 and 20 nmol/L) were evaluated on the neurally-induced contractile and relaxant responses elicited by EFS. Neuronal nitric oxide synthase (nNOS) enzyme was evaluated by immunohistochemistry.
In the functional experiments, electrical field stimulation (EFS, 4-16 Hz) induced tetrodotoxin (TTX)-sensitive contractile responses, which were reduced in amplitude by GLP-2 (P < 0.05). In the presence of the nitric oxide (NO) synthesis inhibitor L-NNA, GLP-2 no longer influenced the neurally-evoked contractile responses (P > 0.05). The direct smooth muscle response to methacholine was not influenced by GLP-2 (P > 0.05). In the presence of guanethidine and carbachol, the addition of GLP-2 to the bath medium evoked TTX-sensitive relaxant responses that were unaffected by L-NNA (P > 0.05). EFS induced a fast NO-mediated relaxation, whose amplitude was enhanced in the presence of the hormone (P < 0.05). Immunohistochemical experiments showed a significant increase (P < 0.05) in nNOS immunoreactivity in the nerve structures after GLP-2 exposure.
The results demonstrate that in gastric fundal strips, GLP-2 influences the amplitude of neurally-induced responses through the modulation of the nitrergic neurotransmission and increases nNOS expression.
Core tip:The results of the present study demonstrate for the first time that, in strips from the mouse gastric fundus, glucagon-like peptide-2 (GLP-2) depresses the amplitude of the neurally-induced contractile responses and enhances the amplitude of the relaxant ones through the modulation of the nitrergic neurotransmission. GLP-2 also increases neuronal nitric oxide synthase immunoreactivity in the nerve structures. All these inhibitory effects might contribute to gastric relaxation, thus increasing the organ capacity. Since gastric distension represents a peripheral satiety signal from a physiological point of view, it could be speculated that the relaxant effects of GLP-2 might concur to suppress feeding behavior in rodents.
- Citation: Garella R, Idrizaj E, Traini C, Squecco R, Vannucchi MG, Baccari MC. Glucagon-like peptide-2 modulates the nitrergic neurotransmission in strips from the mouse gastric fundus. World J Gastroenterol 2017; 23(40): 7211-7220
- URL: https://www.wjgnet.com/1007-9327/full/v23/i40/7211.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i40.7211
Glucagon-like peptide-2 (GLP-2), produced by enteroendocrine “L” cells[1,2], plays a role in the regulation of metabolism and energy homeostasis[3,4], and has been reported to influence many gastrointestinal functions[5,6]. GLP-2 acts on G protein-coupled receptors (GLP-2r)[7-9] largely distributed in the gastrointestinal tract[2,10]. In the gut, GLP-2r has been revealed in several cell types, including excitatory and inhibitory neurons[10-13]. In this view, GLP-2 has been demonstrated to exert a neuromodulatory action either by inhibiting intestinal transit in vivo[14] or by reducing colonic and duodenal cholinergic muscle contractions in vitro[10,11].
Concerning gastric motility, contrasting results have been reported in humans regarding the ability of GLP-2 to slow emptying[15-17]. In vivo experiments in pigs have shown that the hormone reduced antral motility through central nervous system mechanisms[18]. In addition to the central actions, experiments carried out in vitro have demonstrated that GLP-2 exerts peripheral effects on either gastric strips or isolated whole stomach from mice[19]. Particularly, the hormone has been shown to relax gastric smooth muscle acting indirectly through the stimulation of vasoactive intestinal polypeptide (VIP) release from myenteric neurons with site-related effects, exerting its action in the fundal region only[19]. However, to our knowledge, no data have been reported about the influence of GLP-2 on the neurally-induced gastric responses elicited by electrical stimulation in in vitro preparations. For the above reason, we presently investigated whether GLP-2 influences the neurally-induced contractile and relaxant responses in gastric fundal strips from mice. Along with VIP[20], nitric oxide (NO)[21] is considered one of the major non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmitters responsible for the proximal stomach relaxation; thus, we also evaluated the effects of GLP-2 on both the nitrergic neurotransmission and the neuronal nitric oxide (nNOS) expression.
Experiments were performed on 8- to 12-week-old female mice (CD1 Swiss strain; Envigo, Udine, Italy). The animals were kept under the following conditions: 12-h light/12-h dark photoperiod, constant temperature (21 ± 1 °C), and standard laboratory feed. The mice were killed by prompt cervical dislocation to minimize animal suffering.
As formerly reported[22-25], the stomach was promptly removed, and two full-thickness longitudinal strips (2 mm × 10 mm) were cut from each gastric fundal region. One end of each strip was tied to a platinum rod, while the other was connected to a force displacement transducer (Grass model FT03, Quincy, MA, United States) by a silk thread for continuous recording of isometric tension. The transducer was coupled to a polygraph (Grass model 7K, Quincy, MA, United States). Preparations were mounted in double-jacketed organ baths (5 mL) containing Krebs-Henseleit solution, gassed with a 95% O2-5% CO2 mixture, of the following composition (mmol
Electrical field stimulation (EFS) was applied by means of two platinum wire rings (2 mm diameter, 5 mm apart) through which the strip was threaded. Electrical pulses (rectangular waves, 80 V, 4-16 Hz, 0.5 ms, for 15 s) were delivered by a Grass model S8 stimulator. Preparations were allowed to equilibrate for 1 h under an initial load of 0.8 g: during this period, prolonged washes with Krebs-Henseleit solution were performed to avoid the accumulation of metabolites in the organ baths.
The influence of GLP-2 (2 nmol/L and 20 nmol/L) on either neurally-induced or direct smooth muscle contractile responses was firstly investigated. EFS (4-16Hz) was also performed in the presence of 1 μmol/L tetrodotoxin (TTX) or 200 μmol/L NG-nitro-
In a second series of experiments, the effects of GLP-2 (2 nmol/L and 20 nmol/L) on the EFS-induced NANC relaxant responses were investigated. For this purpose, functional experiments were carried out in the presence of 1 μmol/L guanethidine sulphate and 1 μmol/L carbachol (CCh) to rule out adrenergic and cholinergic influences, respectively. Elapsed time between two subsequent additions of CCh was at least 15 min, during which prolonged washes with Krebs-Henseleit solution were performed. When a stable plateau phase of the contraction evoked by CCh was reached, EFS or drugs were applied. The response to GLP-2 (2 nmol/L and 20 nmol/L) was also tested 20 min following the addition of TTX or the NO synthesis inhibitor
Two gastric fundus specimens per animal (6 mice) were taken, which were prepared as reported above and handled as follows. The specimens were stabilized in 5 mL organ baths containing Krebs-Henseleit solution; at the end of the steadying period, one half of the specimens was exposed to GLP-2 for 30 min, which was added directly to the bath reaching 20 nmol/L concentration. The second half was maintained in Krebs solution and used as a control. At the end of the exposure time, the specimens were transferred into a solution of paraformaldehyde (4%) dissolved in phosphate-buffered saline (0.1 mol/L, PBS, pH 7.4), maintained overnight (ON) at 4 °C. The day after, the specimens were dehydrated, cleared in xylene and embedded in paraffin. The rotary microtome (MR2, Boeckeler Instruments Inc. Tucson, Arizona, United States) allowed to cut 5 μm thick sections, which were collected on slides suitable to histological and immunofluorescent staining.
The sections were deparaffinized through consecutive passages in xylene and in decreasing ethanol concentration solutions up to the final step in distilled water. Some sections were H&E stained to visualize the integrity of the muscle wall. Some others underwent the immunofluorescent procedure. For antigen retrieval the sections were transferred for 20 min in a EDTA 1 mmol/L, pH 9.0 + tris buffer 10 mmol/L, maintained at the temperature of 90-92 °C; then they were allowed to cool in the same solution. After few washes in 0.1 mol/L PBS, the section were first incubated for 20 min with bovine serum albumin (1.5%, Sigma Aldrich, Milan, Italy) diluted in PBS and then ON at 4 °C in the presence of the nNOS antibody (rabbit polyclonal; 1:1500; Chemicon, Temecula, CA, United States). After several washes, the appropriate fluorochrome-conjugated secondary antibody (goat anti-rabbit, AlexaFluor 488; 1:333; Jackson ImmunoResearch, West Grove, PA, United States) diluted in PBS were applied for 2 h at RT. Finally, the sections were counterstained with Bisbenzimide Hoechst Trihydrochloride (BHT; Sigma Aldrich srl, Milan, Italy) to visualize the cell nuclei, and set in the aqueous medium (Immu-Mount, Thermo Scientific, Waltham, MA, United States). To verify the specificity of the primary antibody, the procedure has been performed omitting the antibody. An epifluorescence microscope (Zeiss Axioskop microscope, Oberkochen, Germany) allowed to visualize the fluorescent labelling. A digital camera (Leica DFC310 FX 1.4-megapixel camera, Leica Microsystems, Mannheim, Germany) coupled to an image acquisition software (LAS V3.8, Leica Microsystems, Germany) allowed to capture and archive the related images.
The quantitative analysis of the labelled structures was done acquiring digitized images for the whole length of the section (1 section/animal; 6 animals/group) using 20 x objective and the acquisition of overlapping images was avoided. The total number of images obtained was comparable between control and GLP-2 treated sections as confirmed by the similar length of the fundal specimens. ImageJ (NIH, Bethesda, MD, United States) was used to analyze the fluorescent labeling in the acquired images. The area of the labelled structures in the muscle wall was quantified and expressed as fraction of the total image area x 100. The labelling was converted to a binary image; the threshold value was set in control images and maintained in treated ones. Two researchers counted in blind the myenteric labelled neurons in each section (1 section/mouse; 6 mice/group) and the result was proposed as neuron number per mm.
The following drugs were used: guanethidine sulphate, CCh, GLP-2, methacholine, L-NNA, TTX. All of the above drugs were purchased from Sigma Chemical (St. Louis, MO, United States) with the exception of GLP-2 that was obtained from Tocris Bioscience (Bristol, United Kingdom). Solutions were freshly prepared, except for TTX, for which a stock solution was stored at -20 °C. Drug concentrations are referred as final bath concentrations and are in the range of those previously reported to be effective in in vitro gastric preparations[26-28]. Particularly, the chosen doses of GLP-2 employed are those reported able to cause gastric relaxation in mice[19].
Amplitude of contractions is given as percentage of the muscular contraction elicited by methacholine (2 μmol/L) taken as 100% or as absolute values (g). Amplitude of responses to methacholine was measured 30 s after a stable plateau phase was reached. Relaxant responses are calculated as percentage decrease relative to the muscular tension induced by CCh (1 μmol/L) just before obtaining relaxations. With respect to pre-stimulus level, amplitude values of EFS-induced fast relaxations refer to the maximal peak obtained during the stimulation period and amplitude values of EFS-induced sustained relaxations refer to the maximal peak, obtained following the stimulation period. The statistical significance was evaluated by paired or unpaired Student t-test to compare two experimental groups or one-way ANOVA followed by Newman-Keuls posttest when more than two groups were compared (Prism 3.0; GraphPad Software, San Diego, CA, United States). Differences were considered significant when P ≤ 0.05. Results are mean ± SE. The number of muscle strip preparations is indicated by n in the results.
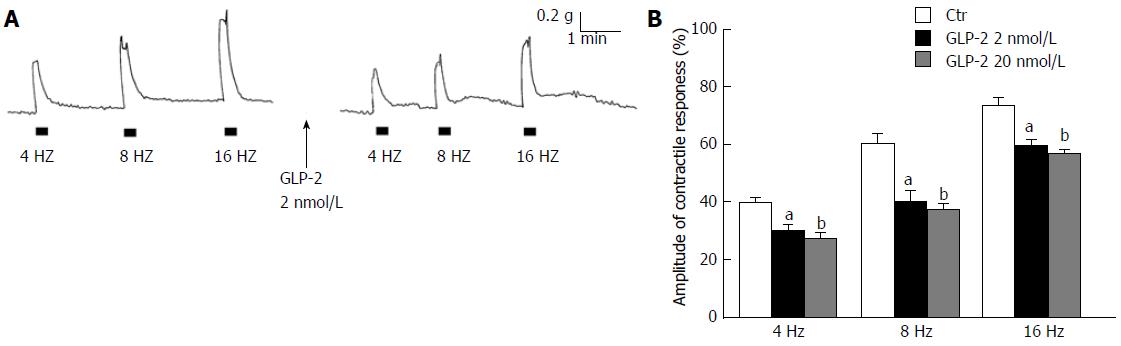
At basal tension (n = 28), EFS (4-16 Hz) induced contractile responses whose amplitude increased by increasing the stimulation frequency (Figure 1A and B). These responses were abolished by 1 μmol/L TTX (n = 4) indicating their nervous nature. The addition of GLP-2 (2 nmol/L or 20 nmol/L) to the bath medium (n = 16), other than causing a long-lasting decay of the basal tension (0.15 ± 0.02 and 0.20 ± 0.03 g at 2 nmol/L and 20 nmol/L, respectively), decreased the amplitude of the excitatory responses elicited by EFS (Figure 1A and B).
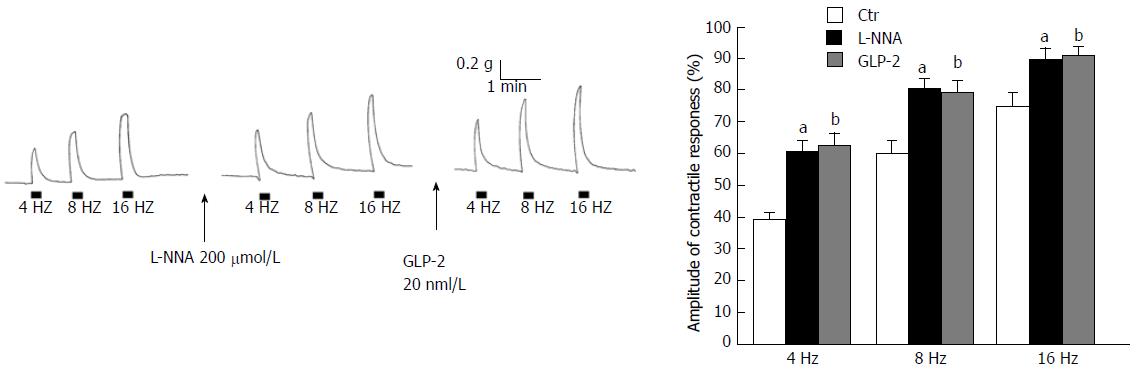
In the presence of the NO synthesis inhibitor L-NNA (200 μmol/L) the amplitude of the EFS-induced contractions was enhanced (Figure 2A and B). The effects of
Methacholine (2 μmol/L) caused a sustained contraction that was TTX-insensitive and reached a plateau phase (mean amplitude 0.98 ± 0.3 g) that persisted until washout. GLP-2 (2 nmol/L or 20 nmol/L) did not influence the response to methacholine (mean amplitude 1.02 ± 0.2 g, P > 0.05). No significant differences (P > 0.05) in amplitude of the contractile response to methacholine (2 μmol/L) were observed between that obtained at the end or at the beginning of the experiments, thus indicating that the viability of the preparations was not compromised.
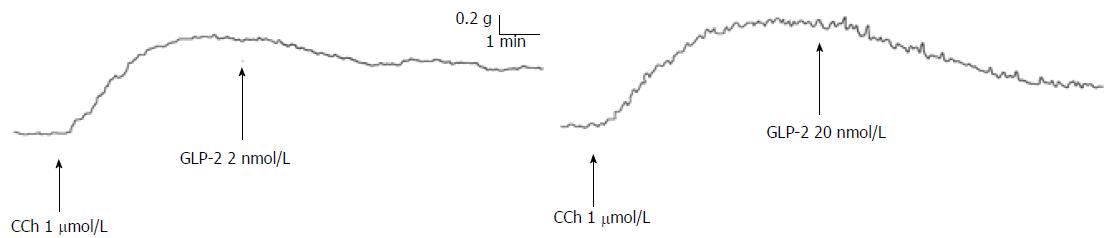
The effects of GLP-2 were also tested on the neurally-induced relaxant responses. In the presence of guanethidine, addition of CCh (1 μmol/L) to the bath medium (n = 28) caused a rapidly arising contraction (mean amplitude 1.2 ± 0.2 g), which persisted until washout. In CCh precontracted strips, GLP-2 (2 nmol/L or 20 nmol/L) elicited (n = 12) dose-dependent slow and long-lasting relaxations (Figure 3) that were abolished by 1 μmol/L TTX (n = 4; data not shown) and unaffected (P > 0.05) by L-NNA (200 μmol/L) (n = 4) (mean amplitude 27 ± 1.5% vs 25 ± 1.9% and 47 ± 1.7% vs 50 ± 1.6% at 2 nmol/L and 20 nmol/L, respectively).
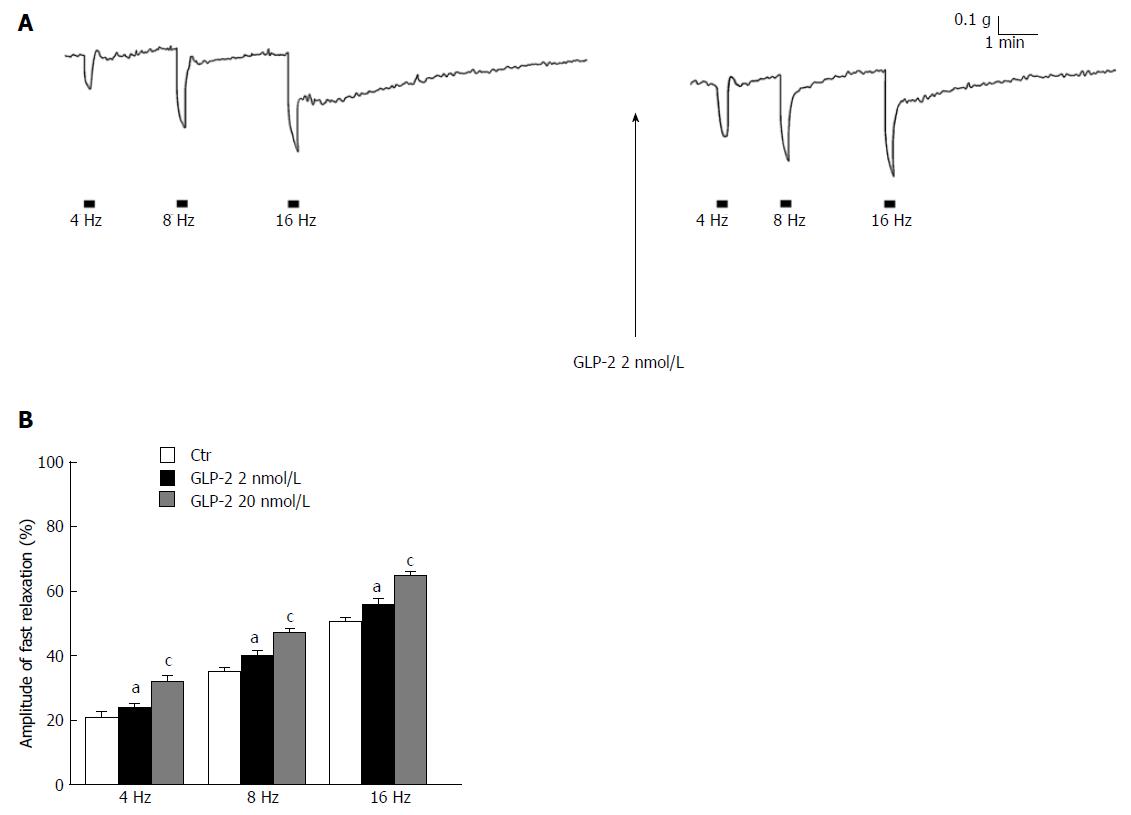
In CCh precontracted strips, EFS (4-16 Hz) elicited (n = 16) a fast relaxant response followed, at the higher stimulation frequencies, by a slow relaxation (Figure 4A). As previously observed in the mouse gastric fundus[23], the fast component of the response was abolished by 200 μmol/L L-NNA (n = 4), indicating its nitrergic nature. In the presence of GLP-2 (2 nmol/L or 20 nmol/L), the amplitude of the EFS-induced fast nitrergic relaxation was increased (P < 0.05; Figure 4A and B), whereas that of the slow relaxation was reduced (6.1 ± 0.6% vs 12.75 ± 2.3% and 19.68 ± 1.6% vs 30.46 ± 2.2%, at 8 and 16 Hz, respectively; P < 0.05) (Figure 4A) or even abolished at the highest dose employed.
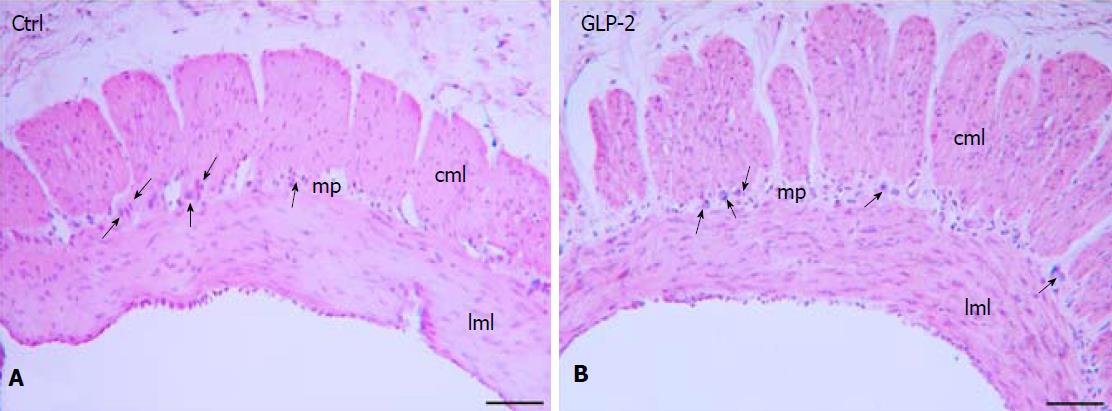
Anatomical and histological evaluation: At the end of the tissue sampling procedure, the control and GLP-2 treated specimens appeared anatomically intact and the H&E staining confirmed the integrity of the muscle wall (Figure 5).
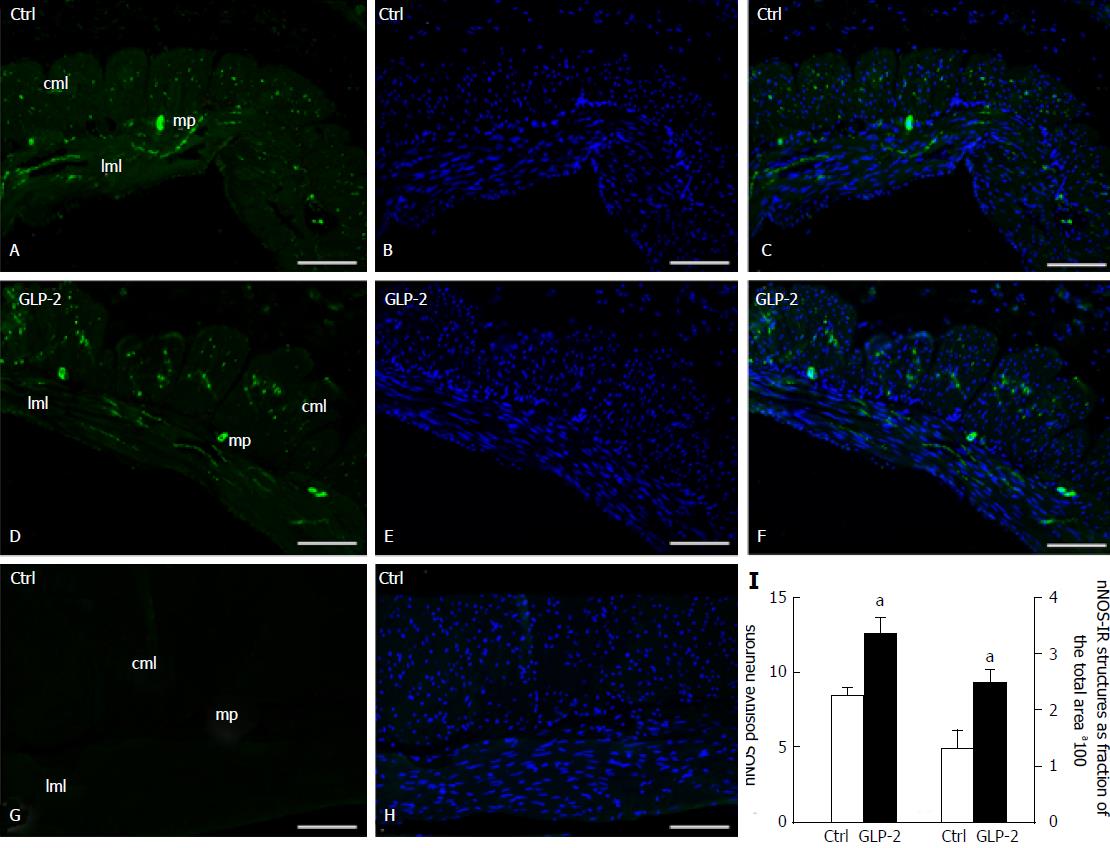
Immunofluorescence: In the gastric fundus of both control (Figure 6A-C) and GLP-2 (20 nmol/L) treated specimens (Figure 6D-F), the nNOS-immunoreactivity (IR) was observed in the soma of the myenteric neurons and in the fibers located in the longitudinal and circular muscle layers. No IR was detected when the primary antibody was omitted (Figure 6G-H). Quantitation of the IR showed a statistically significant increase in nNOS positive neuron number and nerve fibers (Figure 6I). The length of gastric fundal strips subjected to quantitative analysis was not different between control and GLP-2 treated specimens (4.17 ± 0.60 mm controls vs 4.07 ± 0.48 mm GLP-2 treated).
The present study demonstrates for the first time that GLP-2 influences the neurally-induced contractile and relaxant responses in mouse fundal strips through a modulatory action on nitrergic neurotransmission and increases nNOS expression. Particularly, the functional experiments show that GLP-2 is able to depress the amplitude of the EFS-induced contractile responses at each stimulation frequency applied.
It is known that gastric motor responses are a balance between nervous excitatory (mainly cholinergic) and inhibitory (NANC) influences exerted on the smooth muscle. Since during EFS both excitatory and inhibitory nervous fibers are simultaneously activated, the reduction in amplitude of the neurally-induced contractions caused by GLP-2 might be ascribable either to a minor activation of the excitatory component or to a major nervous inhibitory influence exerted on the smooth muscle. In this view, the increase in amplitude of the EFS-induced contractile responses by the NO synthesis inhibitor L-NNA indicates the removal of a nitrergic inhibitory nervous influence. Notably, in the presence of L-NNA, GLP-2 no longer depressed the amplitude of the neurally-induced excitatory responses. These data demonstrate that the hormone effects on the EFS-induced contractions occur through the modulation of the nitrergic neurotransmission and, in addition, exclude its inhibitory action on the cholinergic neurotransmission.
The lack of effects of GLP-2 on the direct smooth muscle responses elicited by methacholine further supports its neuromodulatory action. This observation also proves that the depressant actions of GLP-2 on the EFS-induced contractile responses are not ascribable to aspecific effects such as the decay of the basal tension. Moreover, the similar amplitude of the contraction to methacholine at the beginning and at the end of the experiments demonstrates that muscle responsiveness is not compromised.
In keeping with the above observations, the results of the experiments performed in the presence of guanethidine and CCh demonstrate, for the first time, that the hormone influences the amplitude of the neurally-induced relaxant responses in gastric fundal strips through a modulatory action on the nitrergic neurotransmission. As previously observed in strips from the mouse gastric fundus in the presence of guanethidine and CCh[23,26,28], EFS induced a fast NO-mediated relaxation, followed by a slow and sustained relaxant response at the highest stimulation frequencies. In the present experiments, the observation that GLP-2 increased the amplitude of the EFS-induced fast nitrergic relaxation in the whole range of stimulation frequencies in a dose-related manner further supports the involvement of the NO pathway in the hormone effects. To ascertain whether the effects of GLP-2 on both the neurally-induced contractile and relaxant responses were ascribable to an increased NO production, nNOS expression was evaluated in gastric preparations by immunohistochemistry. The results show that GLP-2 induces an increase in the nNOS labeling in the nerve structures of the gastric fundus muscle coat. Accordingly, an increased NO production in neurons induced by exogenous GLP-2 has been suggested to occur in the mouse duodenum in which the hormone inhibited the spontaneous mechanical activity[11]. In the present experiments, the increased nNOS expression in the nerve structures of the muscle wall fits well with the progressive decrease in amplitude of the EFS-induced contractile responses as well as with the increase of the fast relaxant responses caused by GLP-2. Thus, these hormone effects occur only during EFS, i.e., when the nitrergic pathway is engaged, also explaining the ineffectiveness of L-NNA to influence the relaxant response induced by the addition of GLP-2 to bath medium. Indeed, gastric relaxation to GLP-2 in mice has been reported to occur through the release of VIP from myenteric neurons[19]. In this view, immunohistochemical studies in mouse, human and porcine intestine reported the presence of the GLP-2 receptor in the enteric neurons[10-12,29] and showed that some of them co-localized with VIP[11,12]. We previously observed[26] that, in strips from the mouse gastric fundus, the EFS-induced slow relaxation was abolished following VIP receptors desensitization, suggesting the involvement of the peptide in this kind of response. On this basis, it could be speculated that the reduction/abolition of the neurally-induced slow relaxation by GLP-2 observed in the present experiments occurred because VIP, released earlier from the nerve terminal by the hormone, has already occupied the muscular receptors engaged in the EFS-induced vipergic relaxant response. However, even if the validation of this hypothesis was beyond the aim of the present work, the observation that GLP-2 influences in an opposite manner the two components of the neurally-induced relaxant responses excludes an aspecific effect.
The present results demonstrate that the hormone depresses the amplitude of the neurally-induced contractile responses, enhances the amplitude of the NO-mediated fast relaxant responses and increases the nNOS expression in the nerve fibers. All of these effects of GLP-2 might contribute to gastric relaxation, thus increasing the organ capacity. Since gastric distension represents a peripheral satiety signal[30] from a physiological point of view, it could be hypothesized that the relaxant effects of GLP-2 might contribute to suppress feeding behavior in rodents[31,32].
In conclusion, this study shows for the first time that GLP-2 influences the neurally-induced responses in gastric strips from mice through a modulatory action on the nitrergic neurotransmission and increases nNOS expression.
Glucagon-like peptide-2 (GLP-2) has been shown to relax gastric smooth muscle acting on myenteric neurons. However, to our knowledge, no data are present in the literature on the effects of GLP-2 on the neurally-induced gastric responses elicited by electrical stimulation in in vitro preparations. For the above reason, they presently investigated whether GLP-2 influences the neurally-induced contractile and relaxant responses in strips from the mouse gastric fundus. Moreover, since nitric oxide (NO) is considered one of the major NANC inhibitory neurotransmitters responsible for the proximal stomach relaxation, they also evaluated the effects of GLP-2 on both the nitrergic neurotransmission and the neuronal nitric oxide (nNOS) expression.
Previous studies have indicated that GLP-2 might contribute to suppress feeding behavior in rodents. Thus, the relaxant effects of GLP-2 on gastric preparations, leading to organ distension, might represent peripheral satiety signals.
This is the first study showing that GLP-2 is able to influence the neurally-induced responses in gastric strips from mice through a modulatory action on nitrergic neurotransmission and to increase nNOS expression.
The evidence that GLP-2 is able to induce gastric relaxation, through the modulation of the nitrergic neurotransmission, contributes to the knowledge of its peripheral effects. The results highlight an additional site of action that might be implicated in the hormone control of feeding behavior.
Gastric motor responses are the result of a balance between nervous excitatory, mainly cholinergic, and NANC inhibitory influences exerted on smooth muscle. During EFS, both excitatory and NANC inhibitory nervous fibers are simultaneously activated. The release of neurotransmitters from enteric neurons may be modulated by a variety of substances, including hormones.
This is a very interesting study investigating the influence of GLP2 on neurally-induced responses in gastric fundal strips.
We thank Mr. Adrio Vannucchi for preparation of Figures.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Wei DY, Zhu YL S- Editor: Ma YJ L- Editor: A
E- Editor: Ma YJ
| 1. | Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Marathe CS, Rayner CK, Jones KL, Horowitz M. Glucagon-like peptides 1 and 2 in health and disease: a review. Peptides. 2013;44:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Baldassano S, Amato A, Caldara GF, Mulè F. Glucagon-like peptide-2 treatment improves glucose dysmetabolism in mice fed a high-fat diet. Endocrine. 2016;54:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Baldassano S, Amato A, Mulè F. Influence of glucagon-like peptide 2 on energy homeostasis. Peptides. 2016;86:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Dubé PE, Brubaker PL. Frontiers in glucagon-like peptide-2: multiple actions, multiple mediators. Am J Physiol Endocrinol Metab. 2007;293:E460-E465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Janssen P, Rotondo A, Mulé F, Tack J. Review article: a comparison of glucagon-like peptides 1 and 2. Aliment Pharmacol Ther. 2013;37:18-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA. 1999;96:1569-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 261] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Rowland KJ, Brubaker PL. The “cryptic” mechanism of action of glucagon-like peptide-2. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1-G8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119:744-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 263] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Amato A, Rotondo A, Cinci L, Baldassano S, Vannucchi MG, Mulè F. Role of cholinergic neurons in the motor effects of glucagon-like peptide-2 in mouse colon. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1038-G1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Cinci L, Faussone-Pellegrini MS, Rotondo A, Mulè F, Vannucchi MG. GLP-2 receptor expression in excitatory and inhibitory enteric neurons and its role in mouse duodenum contractility. Neurogastroenterol Motil. 2011;23:e383-e392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Ørskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept. 2005;124:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | McDonagh SC, Lee J, Izzo A, Brubaker PL. Role of glial cell-line derived neurotropic factor family receptor alpha2 in the actions of the glucagon-like peptides on the murine intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G461-G468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Meier JJ, Nauck MA, Pott A, Heinze K, Goetze O, Bulut K, Schmidt WE, Gallwitz B, Holst JJ. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Nagell CF, Wettergren A, Pedersen JF, Mortensen D, Holst JJ. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. Scand J Gastroenterol. 2004;39:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Schmidt PT, Näslund E, Grybäck P, Jacobsson H, Hartmann B, Holst JJ, Hellström PM. Peripheral administration of GLP-2 to humans has no effect on gastric emptying or satiety. Regul Pept. 2003;116:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Wøjdemann M, Wettergren A, Hartmann B, Holst JJ. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol. 1998;33:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Amato A, Baldassano S, Serio R, Mulè F. Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. Am J Physiol Gastrointest Liver Physiol. 2009;296:G678-G684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Lefebvre RA. Non-adrenergic non-cholinergic neurotransmission in the proximal stomach. Gen Pharmacol. 1993;24:257-266. [PubMed] |
| 21. | Rand MJ. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992;19:147-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Baccari MC, Bani D, Calamai F. Evidence for a modulatory role of orexin A on the nitrergic neurotransmission in the mouse gastric fundus. Regul Pept. 2009;154:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Baccari MC, Calamai F. Modulation of nitrergic relaxant responses by peptides in the mouse gastric fundus. Regul Pept. 2001;98:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Garella R, Baccari MC. Contribution of endogenous nitrergic and peptidergic influences to the altered neurally-induced gastric contractile responses in strips from dystrophic (mdx) mice. Regul Pept. 2010;160:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Pini A, Garella R, Idrizaj E, Calosi L, Baccari MC, Vannucchi MG. Glucagon-like peptide 2 counteracts the mucosal damage and the neuropathy induced by chronic treatment with cisplatin in the mouse gastric fundus. Neurogastroenterol Motil. 2016;28:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Garella R, Baccari MC. Endocannabinoids modulate non-adrenergic, non-cholinergic inhibitory neurotransmission in strips from the mouse gastric fundus. Acta Physiol (Oxf). 2012;206:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Squecco R, Garella R, Francini F, Baccari MC. Influence of obestatin on the gastric longitudinal smooth muscle from mice: mechanical and electrophysiological studies. Am J Physiol Gastrointest Liver Physiol. 2013;305:G628-G637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Vannucchi MG, Garella R, Cipriani G, Baccari MC. Relaxin counteracts the altered gastric motility of dystrophic (mdx) mice: functional and immunohistochemical evidence for the involvement of nitric oxide. Am J Physiol Endocrinol Metab. 2011;300:E380-E391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Baldassano S, Amato A. GLP-2: what do we know? What are we going to discover? Regul Pept. 2014;194-195:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Reinehr T, Roth CL. The gut sensor as regulator of body weight. Endocrine. 2015;49:35-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Baldassano S, Bellanca AL, Serio R, Mulè F. Food intake in lean and obese mice after peripheral administration of glucagon-like peptide 2. J Endocrinol. 2012;213:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Guan X, Shi X, Li X, Chang B, Wang Y, Li D, Chan L. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. Am J Physiol Endocrinol Metab. 2012;303:E853-E864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |