Published online Jan 28, 2017. doi: 10.3748/wjg.v23.i4.676
Peer-review started: October 8, 2016
First decision: October 28, 2016
Revised: November 14, 2016
Accepted: January 2, 2017
Article in press: January 3, 2017
Published online: January 28, 2017
Processing time: 107 Days and 9.5 Hours
To investigate the association between postoperative pain control and oncologic outcomes in resected pancreatic ductal adenocarcinoma (PDAC).
From January 2009 to December 2014, 221 patients were diagnosed with PDAC and underwent resection with curative intent. Retrospective review of the patients was performed based on electronic medical records system. One patient without records of numerical rating scale (NRS) pain intensity scores was excluded and eight patients who underwent total pancreatectomy were also excluded. NRS scores during 7 postoperative days following resection of PDAC were reviewed along with clinicopathologic characteristics. Patients were stratified into a good pain control group and a poor pain control group according to the difference in average pain intensity between the early (POD 1, 2, 3) and late (POD 5, 7) postoperative periods. Cox-proportional hazards multivariate analysis was performed to determine association between postoperative pain control and oncologic outcomes.
A total of 212 patients were dichotomized into good pain control group (n = 162) and poor pain control group (n = 66). Median follow-up period was 17 mo. A negative impact of poor postoperative pain control on overall survival (OS) was observed in the group of patients receiving distal pancreatectomy (DP group; 42.0 mo vs 5.0 mo, P = 0.001). Poor postoperative pain control was also associated with poor disease-free survival (DFS) in the DP group (18.0 mo vs 8.0 mo, P = 0.001). Patients undergoing pancreaticoduodenectomy or pylorus-preserving pancreaticoduodenectomy (PD group) did not show associations between postoperative pain control and oncologic outcomes. Poor patients’ perceived pain control was revealed as an independent risk factor of both DFS (HR = 4.157; 95%CI: 1.938-8.915; P < 0.001) and OS (HR = 4.741; 95%CI: 2.214-10.153; P < 0.001) in resected left-sided pancreatic cancer.
Adequate postoperative pain relief during the early postoperative period has important clinical implications for oncologic outcomes after resection of left-sided pancreatic cancer.
Core tip: This is a retrospective review to evaluate the association between postoperative pain control and oncologic outcomes in resected pancreatic ductal adenocarcinoma. In multivariate analysis, poor patients’ perceived pain control was an independent risk factor for both disease-free survival (HR = 4.157; 95%CI: 1.938-8.915; P < 0.001) and overall survival (HR = 4.741; 95%CI: 2.214-10.153; P < 0.001) in resected left-sided pancreatic cancer. Adequate postoperative pain control to reduce patients’ perceived pain during immediate postoperative period may be as important as adjuvant therapy in resected left-sided pancreatic cancer.
- Citation: Min EK, Chong JU, Hwang HK, Pae SJ, Kang CM, Lee WJ. Negative oncologic impact of poor postoperative pain control in left-sided pancreatic cancer. World J Gastroenterol 2017; 23(4): 676-686
- URL: https://www.wjgnet.com/1007-9327/full/v23/i4/676.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i4.676
Pancreas cancer is one of the most fatal malignancies in the world and is currently the fourth leading cause of cancer death in the United States[1]. Surgical excision remains the only curative therapy for pancreatic cancer. However, the resection rate is less than 20% at the time of initial diagnosis, and the rate of recurrence is extremely high even after surgery, occurring in up to 65% to 95% of patients[2-4]. To overcome the high incidence of micrometastatic disease, margin-negative resection[5] and the use of adjuvant treatment[2,3,6] have been considered as important prognostic factors of long-term survival. Nonetheless, 5-year overall survival remains less than 25% even after receiving adjuvant chemotherapy following resection[2,3].
Recently, the importance of the perioperative period on oncologic outcome after cancer surgery has been emphasized in several review studies[7-10]. These studies underlined that the paracrine and neuroendocrine responses caused by surgical stress could promote tumor metastasis through direct action on residual malignant cells and by suppressing natural killer (NK) cell activity, thus compromising antimetastatic cell-mediated immunity (CMI)[8,11,12]. Downregulation of immunity after surgery is known to peak at postoperative day (POD) 3[13], and the decline in NK cell cytotoxicity has been documented to last until POD 7 to 9, depending on the surgical procedure[14-16]. A decrease in NK cell cytotoxicity following pancreaticoduodenectomy (PD) at POD 7 was also recently reported[17]. These results indicate that the early postoperative period harbors potential for the initiation of cancer metastasis, either de novo or from pre-existing micrometastasis.
Even when surgeons achieve R0 resection, various factors of this disproportionally pivotal perioperative period can facilitate growth of potential residual cancer beyond a critical immunological threshold, leading to cancer recurrence. Suggested perioperative risk factors that modulate surgery-induced immunosuppression include anesthetic technique, analgesic agents, blood transfusion, hypothermia, and pain[7-10].
Among these factors, acute pain is known to suppress NK cell activity[18], and its immunosuppressive properties have been shown to promote tumor growth in animal models[19-22]. Postsurgical pain activates the sympathetic nervous system (SNS), leading to catecholamine secretion[23], which directly inhibits NK cells. Furthermore, postoperative pain is not only a result of surgical tissue damage and nociception, but also reflects psychological stress, which has been reported as a risk factor of metastatic progression in some clinical trials[24,25].
In spite of its potential role as an immunomodulator promoting tumor growth and metastasis, there has been no study to evaluate the oncologic significance of postoperative pain following resection of pancreas cancer. In this study, we investigated the association between postoperative pain control and oncologic outcomes in resected pancreatic ductal adenocarcinoma (PDAC).
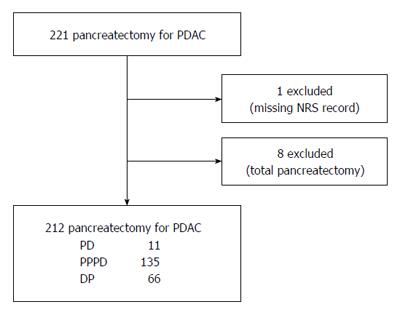
From January 2009 to December 2014, 221 patients with PDAC underwent pancreatectomy with curative intent in our center. We retrospectively reviewed clinicopathologic characteristics and numerical rating scale (NRS) pain intensity score recorded in the nursing records system. One patient was excluded because of missing NRS data for an unknown reason and eight patients who required total pancreatectomy were also excluded (Figure 1). The study was reviewed and approved by the Institutional Review Board of Yonsei University College of Medicine.
NRS score from the nursing records system was available from 2009. Nurses administered the 11-point NRS, with the score ranging from 0 to 10, to evaluate pain intensity whenever the patients reported pain. Patients were instructed to rate 0 as “no pain at all”and 10 as “the worst possible pain”. NRS scores during 7 postoperative days following resection of PDAC were reviewed.
We defined early pain score as the average of all pain scores reported on POD 1, 2, and 3 and late pain score as the average of scores reported on POD 5 and 7. In consideration of the subjective nature of pain and the importance of “perceived control”, we applied the concept of pain control expressed as difference in pain intensity between the two periods rather than objective pain intensity value. We defined the “good pain control group” as the group of patients whose late pain intensity was lower than that of early pain intensity and the “poor pain control group” as the group of patients whose late pain intensity was the same or higher than the early pain intensity.
Postoperative complications were defined using the Clavien-Dindo classification of surgical complications[26]. Major complications were defined as complications with a Clavien-Dindo score of III or higher, which require additional interventional and/or medical treatment associated with prolonged hospital stay. TNM stages were classified according to the American Joint Committee on Cancer (AJCC; 7th edition) staging system[27]. Multivisceral resection was defined as resection of any organ or a part of an organ other than the pancreas and spleen. Combined resection was defined as any multivisceral resection or vascular resection.
Statistical analyses were performed using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL, United States) and MedCalc 16.8.4 for Windows (MedCalc Inc., Mariakerke, Belgium). For continuous variables, t-test was performed and reported as mean and standard deviation. For matched data analysis, paired t-test was performed. Categorical variables were compared using the Chi-Square test or Fisher’s exact test and reported as number (n) and percentage (%). Overall survival (OS) rates and disease-free survival (DFS) rates were estimated using the Kaplan-Meier method. Log-rank test was performed to compare the categorical groups in univariate analysis. A multivariable Cox proportional hazards regression model was used to determine independent risk factors associated with OS and DFS. This model included all of the categorized patient, resection, and tumor characteristics with log-rank P values ≤ 0.150. Exponential (β) measures were reported with 95%CI to evaluate the risks associated with each factor. Statistical significance was achieved at P < 0.05.
A total of 212 patients who underwent pancreatectomy for PDAC were retrospectively reviewed. The clinicopathological characteristics are summarized in Table 1. Median follow-up period was 17 mo. Sixty-six patients (31.3%) received neoadjuvant concurrent chemoradiotherapy (CCRT) before pancreas resection, and 154 patients (72.6%) received adjuvant treatment of chemotherapy, radiotherapy, or CCRT according to their general condition. R0 resection was achieved in 187 patients (88.2%). In terms of resection methods, 146 patients (68.9%) underwent pancreaticoduodenectomy (PD) or pylorus-preserving pancreatoduodenectomy (PPPD), and 66 patients (31.1%) underwent distal pancreatectomy (DP) with or without spleen preservation.
| Characteristic (n = 212) | Frequency, mean ± SD |
| Age (yr) | 62.8 ± 9.5 |
| Male gender | 125 (59.0) |
| BMI (kg/m2) | 22.8 ± 2.9 |
| Diabetes | 73 (34.4) |
| ASA | |
| 1 | 66 (31.1) |
| 2 | 92 (43.4) |
| 3 | 49 (23.1) |
| 4 | 2 (0.9) |
| Operative time (min) | 402.2 ± 129.3 |
| Intraoperative bleeding (mL) | 626.0 ± 482.8 |
| Intraoperative transfusion | 47 (22.2) |
| pT stage | |
| T0 | 9 (4.2) |
| T1 | 16 (7.5) |
| T2 | 3 (1.4) |
| T3 | 182 (85.8) |
| T4 | 2 (0.9) |
| pN stage | |
| N0 | 106 (50.0) |
| N1 | 106 (50.0) |
| pTNM staging | |
| I | 18 (8.5) |
| II | 182 (85.8) |
| III | 2 (0.9) |
| IV | 1 (0.5) |
| R status | |
| R0 | 187 (88.2) |
| R1 | 23 (10.8) |
| R2 | 2 (0.9) |
| Cell differentiation | |
| Well | 24 (11.3) |
| Moderate | 152 (71.7) |
| Poor | 17 (8.0) |
| Undifferentiated | 1 (0.5) |
| Retrieved lymph nodes | 17.3 ± 9.9 |
| Vascular resection | 55 (25.9) |
| Mutivisceral resection | 36 (17.5) |
| Combined resection | 71 (33.5) |
| Lymphovascular invasion | 71 (33.5) |
| Perineural invasion | 146 (68.9) |
| Neoadjuvant CCRT | 66 (31.3) |
| Adjuvant treatment | 154 (72.6) |
| Complications | |
| Minor | 112 (52.8) |
| Major(≥ G3) | 20 (9.4) |
| Length of hospital stay (d) | 21.1 ± 15.0 |
| Recurrence | 137 (64.6) |
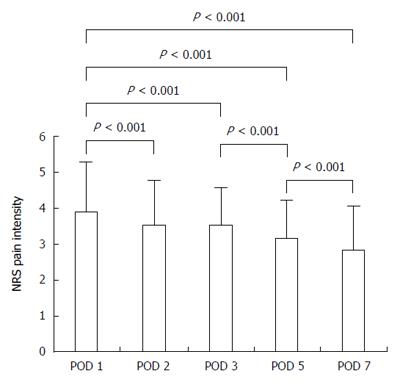
For the overall patient population, postoperative pain intensity decreased significantly at POD 2, 3, 5, and 7 compared with POD 1 (P < 0.001 for each; Figure 2). There was a significant decrease in pain intensity between each two successive days (P < 0.001), except between POD 2 and 3 (P = 0.916).
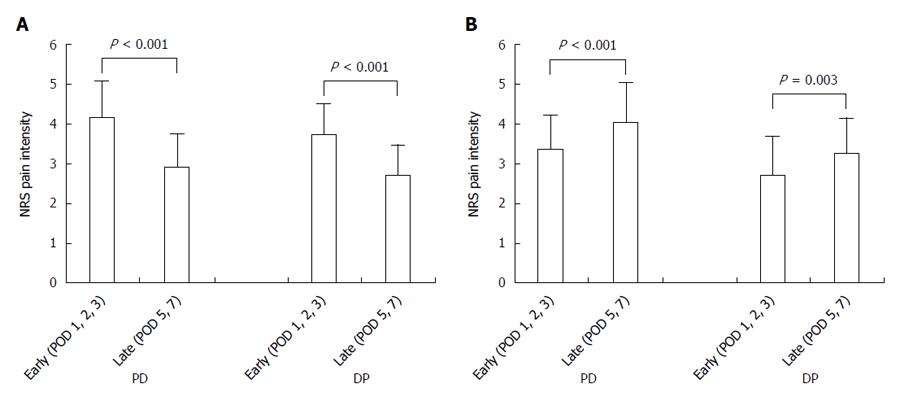
Patients were divided into a good pain control group (n = 162) and poor pain control group (n = 50). The good pain control group showed a reduction of pain intensity from 4.13 ± 0.93 to 2.87 ± 0.86 for the PD group (n = 109, P < 0.001) and from 3.71 ± 0.77 to 2.69 ± 0.78 for the DP group (n = 53, P < 0.001, Figure 3A). The poor pain control group showed an increase of pain intensity from 3.35 ± 0.87 to 4.03 ± 1.02 for the PD group (n = 37, P < 0.001) and from 2.71 ± 0.99 to 3.26 ± 0.88 for the DP group (n = 13, P = 0.003, Figure 3B).
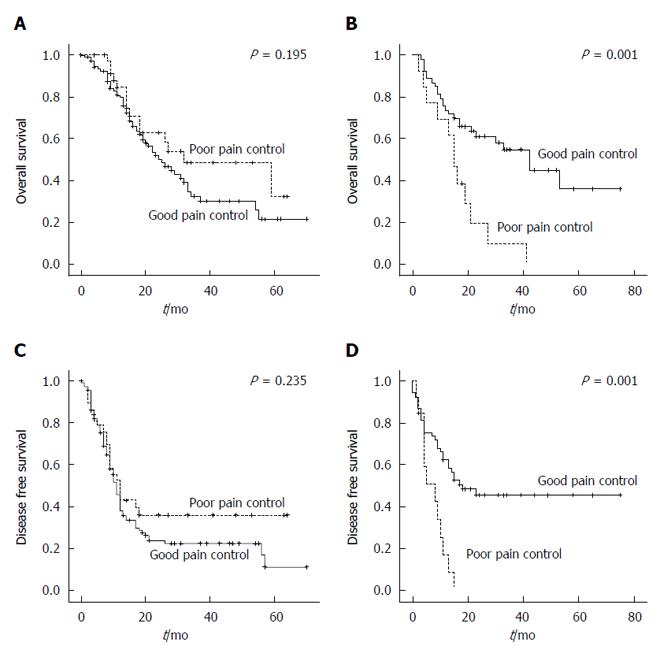
A negative impact of poor postoperative pain control on OS was observed in the DP group [good pain control vs poor pain control, median survival 42.0 mo (95%CI: 26.2-57.8) vs 15.0 mo (95%CI: 11.5-18.5), P = 0.001, Figure 4B]. Also, poor pain control exerted a negative effect on DFS in the DP group (good pain control vs poor pain control, median 18.0 mo vs 8.0 mo, P = 0.001, Figure 4D). Patients undergoing pancreaticoduodenectomy or pylorus-preserving pancreaticoduodenectomy (PD group) did not show associations between postoperative pain control and oncologic outcomes.
There were no significant differences in preoperative, intraoperative, or postoperative outcomes between the good pain control group and the poor pain control group (P > 0.05, Table 2). Other interesting finding was that surgical approach, such as open or minimally invasive, did not influence categorization of pain response (P = 0.523). Whether the patients received neoadjuvant CCRT or not also did not affect grouping of pain control (P = 0.719). There were also no differences in method of postoperative pain management techniques between the group (P = 0.445).
| Pain control group | P value | ||
| Good(n = 53) | Poor(n = 13) | ||
| Age (yr) | 65.6 ± 8.2 | 63.2 ± 11.3 | 0.378 |
| Male gender | 30 (56.6) | 7 (53.8) | 0.858 |
| BMI (kg/m2) | 23.1 ± 2.7 | 23.1 ± 2.4 | 0.993 |
| Diabetes | 17 (32.1) | 3 (23.1) | 0.739 |
| ASA | |||
| 1 | 16 (30.2) | 5 (41.7) | 0.342 |
| 2 | 22 (41.5) | 6 (50.0) | |
| 3 | 15 (28.3) | 1 (8.3) | |
| Surgical approach | |||
| Open | 35 (66.0) | 7 (53.8) | 0.523 |
| MIS | 18 (34.0%) | 6 (46.2) | |
| Spleen preservation | 7 (13.2) | 2 (15.4) | > 0.999 |
| Operation time (min) | 254.1 ± 95.3 | 304.6 ± 89.0 | 0.088 |
| Intraoperative bleeding (mL) | 364.2 ± 296.0 | 470.38 ± 624.8 | 0.373 |
| Intraoperative transfusion | 5 (9.4) | 3 (23.1) | 0.185 |
| Tumor size (cm) | 0.407 | ||
| < 3 | 30 (56.6) | 9 (69.2) | |
| ≥ 3 | 23 (43.4) | 4 (30.8) | |
| pT stage | 0.148 | ||
| T0 | 3 (5.7) | 0 (0) | |
| T1 | 3 (5.7) | 2 (15.4) | |
| T2 | 2 (3.8) | 1 (7.7) | |
| T3 | 45 (84.9) | 9 (69.2) | |
| T4 | 0 (0.0) | 1 (7.7) | |
| pN stage | 0.851 | ||
| N0 | 27 (50.9) | 7 (53.8) | |
| N1 | 26 (49.1) | 6 (46.2) | |
| pTNM staging | 0.429 | ||
| I | 5 (9.5) | 2 (15.4) | |
| II | 44 (83.0) | 10 (77.0) | |
| III | 0 (0.0) | 1 (7.7) | |
| IV | 1 (1.9) | 0 (0.0) | |
| R status | 0.121 | ||
| R0 | 47 (88.7) | 11 (84.6) | |
| R1 | 6 (11.3 ) | 1 (7.7) | |
| R2 | 0 (0.0 ) | 1 (7.7) | |
| Retrieved lymph nodes | 14.8 ± 11.0 | 14.2 ± 6.6 | 0.735 |
| Multivisceral resection | 12 (22.6) | 2 (15.4) | 0.718 |
| Combined resection | 14 (26.4) | 2 (15.4) | 0.496 |
| Lymphovascular invasion | 14 (27.5) | 4 (33.3) | 0.729 |
| Perineural invasion | 34 (66.7) | 5 (41.7) | 0.185 |
| Grade | 0.499 | ||
| Well | 3 (6.4) | 2 (16.7) | |
| Moderate | 38 (80.9) | 9 (75.0) | |
| Poor | 6 (12.8) | 1 (8.3) | |
| Neoadjuvant CCRT | 12 (22.6) | 4 (30.8) | 0.719 |
| Preoperative CA19-9 | 0.154 | ||
| < 300 | 34 (69.4) | 12 (92.3) | |
| ≥ 300 | 15 (30.6) | 1 (7.7) | |
| Adjuvant treatment | 39 (73.6) | 9 (69.2) | 0.739 |
| Time to adjuvant treatment (d) | 53.7 ± 36.8 | 63.0 ± 47.0 | 0.520 |
| Complications | |||
| Minor | 33 (62.3) | 7 (53.8) | 0.578 |
| Major(≥ G3) | 3 (7.3) | 2 (28.6) | 0.148 |
| Use of PCA | 0.445 | ||
| IV PCA | 34 (64.2) | 10 (76.9) | |
| Epidural PCA | 17 (32.1) | 2 (15.4) | |
| None | 2 (3.8) | 1 (7.7) | |
| Length of hospital stay (d) | 17.1 ± 11.0 | 27.2 ± 45.9 | 0.446 |
In univariate analysis, intraoperative transfusion, positive lymph node status, greater tumor diameter (≥ 3 cm), and poor pain control were identified as prognostic factors for predicting DFS in resected left-sided pancreatic cancer (P = 0.005, P = 0.011, P = 0.028, P = 0.001, respectively; Table 3). For OS, longer operation time (≥ 300 min), positive lymph node status, greater tumor diameter (≥ 3 cm), multivisceral resection, not receiving adjuvant treatment, and poor pain control were significant prognostic factors in univariate analysis (P = 0.035, P = 0.020, P = 0.023, P = 0.043, P = 0.017, P = 0.001, respectively; Table 3). Subsequent multivariate analysis revealed positive lymph node status, greater tumor diameter (≥ 3 cm), not receiving adjuvant treatment, and poor pain control as independent risk factors for both DFS and OS in resected left-sided pancreatic cancer (Table 4).
| n | DFS | OS | |||
| Median survival (mo) | P value1 | Median survival (mo) | P value1 | ||
| Age | |||||
| < 65 | 30 | 15 | 0.216 | 42 | 0.150 |
| ≥ 65 | 36 | 11 | 23 | ||
| Sex | |||||
| Female | 29 | 11 | 0.136 | 27 | 0.829 |
| Male | 37 | 18 | 33 | ||
| BMI (kg/m2) | |||||
| < 25 | 52 | 11 | 0.209 | 30 | 0.384 |
| ≥ 25 | 14 | 27 | |||
| Diabetes | |||||
| No | 46 | 13 | 0.674 | 27 | 0.654 |
| Yes | 20 | 15 | |||
| ASA | |||||
| 1/2 | 49 | 13 | 0.569 | 30 | 0.903 |
| 3/4 | 16 | 15 | |||
| Surgical approach | |||||
| Open | 42 | 14 | 0.971 | 30 | 0.645 |
| MIS | 24 | 11 | 41 | ||
| Spleen preservation | |||||
| Yes | 9 | 15 | 0.931 | 27 | 0.619 |
| No | 57 | 13 | 33 | ||
| Operation time (min) | |||||
| < 300 | 39 | 17 | 0.254 | 41 | 0.035 |
| ≥ 300 | 27 | 13 | 15 | ||
| Bleeding (mL) | |||||
| < 500 | 42 | 11 | 0.632 | 30 | 0.881 |
| ≥ 500 | 23 | 15 | 33 | ||
| Intraoperative transfusion | |||||
| No | 58 | 15 | 0.005 | 33 | 0.056 |
| Yes | 8 | 4 | 13 | ||
| Resection status | |||||
| R0 | 58 | 13 | 0.689 | 30 | 0.382 |
| R1/R2 | 8 | 13 | 21 | ||
| Lymph node status | |||||
| N0 | 34 | 0.011 | 41 | 0.020 | |
| N1 | 32 | 9 | 17 | ||
| Tumor size (cm) | |||||
| < 3 | 39 | 18 | 0.028 | 42 | 0.023 |
| ≥ 3 | 27 | 8 | 15 | ||
| pT stage | |||||
| ≤ 2 | 11 | 0.293 | 21 | 0.949 | |
| ≥ 3 | 55 | 13 | 33 | ||
| Multivisceral resection | |||||
| No | 52 | 15 | 0.111 | 33 | 0.043 |
| Yes | 14 | 10 | 13 | ||
| Combined resection | |||||
| No | 50 | 15 | 0.474 | 33 | 0.303 |
| Yes | 16 | 10 | 15 | ||
| Lymphovascular invasion | |||||
| No | 45 | 11 | 0.259 | 23 | 0.762 |
| Yes | 18 | 18 | 30 | ||
| Perineural invasion | |||||
| No | 24 | 11 | 0.947 | 21 | 0.621 |
| Yes | 39 | 15 | 27 | ||
| Neoadjuvant CCRT | |||||
| Yes | 16 | 15 | 0.351 | 33 | 0.433 |
| No | 50 | 11 | 21 | ||
| Preop CA19-9 (U/mL) | |||||
| < 300 | 46 | 15 | 0.782 | 27 | 0.540 |
| ≥ 300 | 16 | 13 | 42 | ||
| Adjuvant treatment | |||||
| Yes | 48 | 15 | 0.094 | 41 | 0.017 |
| No | 18 | 4 | 8 | ||
| Major complications (≥ G3) | |||||
| No | 43 | 15 | 0.813 | 42 | 0.916 |
| Yes | 5 | 4 | 21 | ||
| Use of PCA | |||||
| Epidural | 19 | 13 | 0.757 | 27 | 0.943 |
| IV | 44 | 15 | 33 | ||
| Pain control | |||||
| Good | 53 | 18 | 0.001 | 42 | 0.001 |
| Poor | 13 | 8 | 15 | ||
| Variables | Disease-free survival | Overall survival | ||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| Exp (β) | 95%CI | P value | Exp (β) | 95%CI | P value | Exp (β) | 95%CI | P value | Exp (β) | 95%CI | P value | |
| Positive lymph node status | 2.183 | 1.162-4.101 | 0.011 | 2.259 | 1.150-4.437 | 0.018 | 2.105 | 1.094-4.053 | 0.02 | 2.501 | 1.218-5.134 | 0.012 |
| Tumor size (≥ 3 cm) | 1.943 | 0.999-3.781 | 0.028 | 2.215 | 1.130-4.341 | 0.021 | 2.030 | 1.016-4.055 | 0.023 | 2.662 | 1.282-5.529 | 0.009 |
| No adjuvant treatment | 1.742 | 0.800-3.794 | 0.094 | 2.468 | 1.196-5.093 | 0.015 | 2.205 | 0.981-4.955 | 0.017 | 4.649 | 2.124-10.172 | < 0.001 |
| Poor pain control | 2.934 | 1.158-7.430 | 0.001 | 4.157 | 1.938-8.915 | < 0.001 | 2.915 | 1.156-7.350 | 0.001 | 4.741 | 2.214-10.153 | < 0.001 |
| Age (≥ 65 yr) | ND | ND | 1.608 | 0.844-3.064 | 0.150 | 1.706 | 0.799-3.640 | 0.167 | ||||
| Sex (Male) | 0.632 | 0.338-1.181 | 0.136 | 0.614 | 0.318-1.186 | 0.146 | ND | ND | ||||
| Operation time (≥ 300 min) | ND | ND | 1.949 | 0.981-3.873 | 0.035 | 1.890 | 0.923-3.868 | 0.082 | ||||
| Intraoperative transfusion | 2.788 | 0.903-8.612 | 0.005 | 1.745 | 0.688-4.425 | 0.241 | 2.159 | 0.729-6.396 | 0.056 | 1.986 | 0.750-5.257 | 0.167 |
| Multivisceral resection | 1.720 | 0.769-3.849 | 0.111 | 1.166 | 0.532-2.557 | 0.701 | 2.046 | 0.837-5.006 | 0.043 | 1.273 | 0.563-2.876 | 0.562 |
Evidence showing the association of pain relief and reduced surgery-induced tumor growth was first documented by Yeager et al[28] in colon carcinoma. Since then, the protective effect of various analgesics on surgery-induced metastasis has been reported by many studies[19,20,29-31]. However, there has been no study to evaluate the oncologic significance of postoperative pain control following resection of pancreatic cancer. To the best of our knowledge, the current retrospective study is the first to suggest that early postoperative pain control can influence patient survival after DP for PDAC, regardless of the biology of the tumor, surgical approach, or treatment modality.
A possible mechanism explaining the association of poor pain control and negative oncologic outcome could include interaction of inflammation, pain, and suppressed NK cell activity in the early postsurgical period, resulting in immunosuppression, which is known to peak on POD 3. The immunosuppressive property of pain is attributed to the direct inhibition of NK cell cytotoxicity by catecholamine secreted upon SNS activation[11,12]. Also, postoperative pain is associated with increased secretion of proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α. Sommer et al[32] indicated that pain and proinflammatory cytokines interact reciprocally. Pain affects the production and secretion of cytokines, and those cytokines reduce the activation threshold of peripheral nociceptors, resulting in pain augmentation. Several studies[33-35] have shown the association of pain relief in the immediate postoperative period and attenuated production of proinflammatory cytokines. The correlation between changes in proinflammatory cytokine levels and decreased NK cell response was also reported by Baxevanis et al[15]. Considering these findings, unrelieved or elevated pain intensity at the late postsurgical period (POD 5, 7) might reflect prolonged inflammation and a state of immunosuppression and thus a higher chance of tumor metastasis and recurrence, explaining the negative oncologic outcome.
Poor pain control is basically attributed to a failure of appropriate and adequate postoperative analgesic care. However, it has also been suggested that poor pain response can be a result of the patient-specific immune state before surgery[36], a sign of ongoing or forthcoming complications[37,38], or a contributory effect of perioperative psychological factors[39,40]. Since there were no significant differences in any clinicopathologic factors between the good pain control group and poor pain control group undergoing DP (Table 2), we can only speculate that inadequate postoperative pain control was the major reason for poor postoperative pain control in left-sided pancreatic cancer. Further study should include establishment of appropriate pain control protocol to minimize influence of inadequate pain control and evaluate whether there are other possible reasons for poor postoperative pain control in left-sided pancreatic cancer.
Currently, it is routine for patients undergoing pancreas cancer surgery to receive adjuvant treatment irrespective of whether R0 resection is achieved. Therefore, the postsurgical period has been viewed as a time for managing complications and improving the general condition of the patient in order to meet the physiological requirements for receiving adjuvant treatment. During this approximately two-months period, patients do not receive anticancer treatment or intervention. However, this period - especially the immediate early period - harbors a therapeutic window of anticancer treatment, as surgery-induced immunosuppression is still in its recovery phase, and the tumor burden could start to increase again. Although, present study is based on a small sample size and retrospective observation, our data suggest that pain management after DP could be more than a matter of patient recovery to receive adjuvant treatment at the appropriate time. Rather, adequate and appropriate pain control during the early postoperative period might exert a direct curative effect on left-sided pancreatic cancer.
Interestingly, the negative oncologic impact of postoperative pain control was not observed following PD. This may have been influenced by wider surgical extent involved with PD and the impact of postoperative pain control during the postoperative 7 days may be rendered ineffective. The operation time, intraoperative blood loss, and rate of intraoperative transfusion were all higher after PD compared to DP [448.3 min vs 264.1 min, P < 0.001; 717.9 cc vs 385.5 cc, P < 0.001; 39 (26.9%) vs 8 (12.1%), P = 0.017, respectively, data not shown], reflecting greater surgical stress. Increased surgical extent has been shown to be associated with higher rates of tumor metastasis[41] and delayed recovery of NK cell cytotoxicity[15]. Also, intraoperative transfusion has been repeatedly reported to modulate the postoperative immune response[42,43]. These factors may overcome the potential immune modulation and oncologic effect of pain control during the period of assessment.
Our study has several limitations. It is a retrospective study, and the number of patients in the poor pain control group after DP was small, making it difficult to reach sound conclusions. We grouped patients into good and poor pain control groups according to differences in NRS pain intensity between early (POD 1, 2, 3) and late (POD 5, 7) periods, because inflammation and immunosuppression peak on around POD 3. However, this time frame might not fit all cases and may vary according to surgical extent or approach. Future studies are needed to test various analytic approaches targeting the critical time point when postoperative pain most significantly mediates immunomodulation.
In addition, our definition of pain control groups may not fully represent pain control state. There have been reports of using satisfaction score along with pain score in fully assessing adequate pain control[44,45]. In determining pain control state, we were limited to the use of NRS pain intensity. Further studies with assessment of satisfaction score and refined definitions for pain control groups should be undertaken.
Lastly, for pain control, patients received intravenous patient-controlled analgesia (PCA) or epidural PCA (both based on fentanyl) or opiates on demand. However, disconnect timing of PCA, as well as the type and amount of analgesics used after clamping, were not investigated in this study. Opioids are believed to exert an immunosuppressive effect when they are used in the absence of pain[22]. Also, certain types of opioids, such as tramadol (but not all opioids) can overcome the immunosuppressive effects of pain, reversing the capacity of surgical stress to suppress NK cell cytotoxicity and promote tumor metastasis in animal models[20,46]. The complex interaction of pain, opioids, non-opioid analgesics, and their net effect on immunosuppression, which might have impacted oncologic outcome, was not assessed in this study. However, relationship between pain control method and postoperative pain control should be investigated further with a well-designed pain control protocol.
This study suggests that a change in patients’ perceived pain intensity in the postoperative period could influence survival outcome in resected left-sided pancreatic cancer. Unlike other prognostic factors, such as tumor size, lymph node metastasis, differentiation, lymphovascular invasion, and perineural invasion, postoperative pain is a controllable factor. Surgeons play a leading role in controlling pain during the postoperative period. In spite of compelling evidence supporting the immunologic and oncologic importance of the perioperative period, its application to the clinical field is still in its infancy. More research on underutilized modulators of this period - not only postoperative pain, but also intraoperative hypothermia, transfusion, nutritional support, and psychological intervention - is needed for the development of patient-oriented perioperative therapy against pancreatic cancer.
In conclusion, adequate postoperative pain relief during the early postoperative period has important clinical implications for oncologic outcomes after resection of left-sided pancreatic cancer.
Acute pain is known to suppress natural killer (NK) cell cytotoxicity and promote tumor growth and metastasis in preclinical models. Therefore, postoperative pain has been suggested as one of the risk factors for cancer metastasis and recurrence after oncologic surgery. Surgery-induced immunosuppression and inflammation are known to peak around postoperative day (POD) 3, and suppression of NK cell cytotoxicity lasts from approximately POD 7 to 9. The clinical significance of postoperative pain control during this critical period in patients undergoing pancreatectomy for pancreatic ductal adenocarcinoma (PDAC) has yet to be investigated.
This study contributes in discovering associations between postoperative pain control and oncologic outcomes in resected pancreatic cancer.
In this study, patients undergoing distal pancreatectomy for left-sided pancreatic cancer were found to have poor oncologic outcomes under poor postoperative pain control. Adequate postoperative pain relief during the early postoperative period has important clinical implications for oncologic outcomes after resection of left-sided pancreatic cancer.
Surgeons play a leading role in controlling pain during the postoperative period. Patients’ perceived postoperative pain should be actively relieved after distal pancreatectomy for pancreatic cancer to improve oncologic outcomes.
PD group: Patients underwent pancreaticoduodenectomy or pylorus-preserving pancreaticoduodenectomy. DP group: Patients underwent distal pancreatectomy. Good pain control group: Patients whose late pain intensity was lower than that of early pain intensity. Poor pain control group: Patients whose late pain intensity was the same or higher than the early pain intensity. Early pain intensity: Mean of all pain scores reported on POD 1, 2, and 3. Late pain intensity: Mean of pain scores reported on POD 5 and 7. Pain scores: Measurement of numerical rating scale (NRS) pain intensity score. 11-point NRS with the score ranging from 0 to 10 was used to evaluate pain intensity whenever the patients reported pain. Patients were instructed to rate 0 as “no pain at all” and 10 as “the worst possible pain”.
The study evaluated the association between postoperative pain control and oncologic outcomes in resected PDAC. The results showed that poor pain control was an independent risk factor for both DFS and OS in resected left-sided pancreatic cancer, but not in patients received PD. This is very interesting.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arai YC, Fu DL, Yang F S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 2. | Ueno H, Kosuge T, Matsuyama Y, Yamamoto J, Nakao A, Egawa S, Doi R, Monden M, Hatori T, Tanaka M. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 3. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1361] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 4. | Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, Hwang R, Vauthey JN, Abdalla EK, Lee JE. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 446] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 716] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 6. | Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 7. | Neeman E, Zmora O, Ben-Eliyahu S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res. 2012;18:4895-4902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30 Suppl:S32-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 9. | Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 366] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 10. | Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110:1636-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251-3258. [PubMed] |
| 13. | Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Pollock RE, Lotzová E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991;126:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Baxevanis CN, Papilas K, Dedoussis GV, Pavlis T, Papamichail M. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin Immunol Immunopathol. 1994;71:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Quintiliani L, Pescini A, Di Girolamo M, Iudicone P, Martini F, Guglielmetti M, Buzzonetti A, Fascioli S. Relationship of blood transfusion, post-operative infections and immunoreactivity in patients undergoing surgery for gastrointestinal cancer. Haematologica. 1997;82:318-323. [PubMed] |
| 17. | Iannone F, Porzia A, Peruzzi G, Birarelli P, Milana B, Sacco L, Dinatale G, Peparini N, Prezioso G, Battella S. Effect of surgery on pancreatic tumor-dependent lymphocyte asset: modulation of natural killer cell frequency and cytotoxic function. Pancreas. 2015;44:386-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Shavit Y, Martin FC, Yirmiya R, Ben-Eliyahu S, Terman GW, Weiner H, Gale RP, Liebeskind JC. Effects of a single administration of morphine or footshock stress on natural killer cell cytotoxicity. Brain Behav Immun. 1987;1:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Page GG, Blakely WP, Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 194] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Gaspani L, Bianchi M, Limiroli E, Panerai AE, Sacerdote P. The analgesic drug tramadol prevents the effect of surgery on natural killer cell activity and metastatic colonization in rats. J Neuroimmunol. 2002;129:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Page GG. The immune-suppressive effects of pain In. Madame Curie Bioscience Database. Auxtin, TX: Landes Bioscience 2000; . |
| 22. | Page GG. Immunologic effects of opioids in the presence or absence of pain. J Pain Symptom Manage. 2005;29:S25-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Traynor C, Hall GM. Endocrine and metabolic changes during surgery: anaesthetic implications. Br J Anaesth. 1981;53:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Andersen BL, Yang HC, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, Young DC, Carson WE. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 283] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 25. | Küchler T, Bestmann B, Rappat S, Henne-Bruns D, Wood-Dauphinee S. Impact of psychotherapeutic support for patients with gastrointestinal cancer undergoing surgery: 10-year survival results of a randomized trial. J Clin Oncol. 2007;25:2702-2708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8636] [Article Influence: 539.8] [Reference Citation Analysis (0)] |
| 27. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6465] [Article Influence: 431.0] [Reference Citation Analysis (0)] |
| 28. | Yeager MP, Colacchio TA. Effect of morphine on growth of metastatic colon cancer in vivo. Arch Surg. 1991;126:454-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Sacerdote P, Bianchi M, Gaspani L, Manfredi B, Maucione A, Terno G, Ammatuna M, Panerai AE. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. 2000;90:1411-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 30. | Page GG, McDonald JS, Ben-Eliyahu S. Pre-operative versus postoperative administration of morphine: impact on the neuroendocrine, behavioural, and metastatic-enhancing effects of surgery. Br J Anaesth. 1998;81:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Page GG, Ben-Eliyahu S, Yirmiya R, Liebeskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 639] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 33. | Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessler H. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesth Analg. 2009;109:1464-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Beilin B, Shavit Y, Trabekin E, Mordashev B, Mayburd E, Zeidel A, Bessler H. The effects of postoperative pain management on immune response to surgery. Anesth Analg. 2003;97:822-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Beilin B, Bessler H, Mayburd E, Smirnov G, Dekel A, Yardeni I, Shavit Y. Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology. 2003;98:151-155. [PubMed] |
| 36. | Fragiadakis GK, Gaudillière B, Ganio EA, Aghaeepour N, Tingle M, Nolan GP, Angst MS. Patient-specific Immune States before Surgery Are Strong Correlates of Surgical Recovery. Anesthesiology. 2015;123:1241-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Kalff JC, Türler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, Billiar TR, Simmons RL, Bauer AJ. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. 2003;237:301-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Lu CH, Chao PC, Borel CO, Yang CP, Yeh CC, Wong CS, Wu CT. Preincisional intravenous pentoxifylline attenuating perioperative cytokine response, reducing morphine consumption, and improving recovery of bowel function in patients undergoing colorectal cancer surgery. Anesth Analg. 2004;99:1465-171; table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Kiecolt-Glaser JK, Page GG, Marucha PT, MacCallum RC, Glaser R. Psychological influences on surgical recovery. Perspectives from psychoneuroimmunology. Am Psychol. 1998;53:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 333] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 40. | Potter MQ, Sun GS, Fraser JA, Beckmann JT, Swenson JD, Maak TG, Aoki SK. Psychological distress in hip arthroscopy patients affects postoperative pain control. Arthroscopy. 2014;30:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Tsuchiya Y, Sawada S, Yoshioka I, Ohashi Y, Matsuo M, Harimaya Y, Tsukada K, Saiki I. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | Blumberg N, Heal JM. Effects of transfusion on immune function. Cancer recurrence and infection. Arch Pathol Lab Med. 1994;118:371-379. [PubMed] |
| 43. | Kao KJ. Mechanisms and new approaches for the allogeneic blood transfusion-induced immunomodulatory effects. Transfus Med Rev. 2000;14:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Farooq F, Khan R, Ahmed A. Assessment of patient satisfaction with acute pain management service: Monitoring quality of care in clinical setting. Indian J Anaesth. 2016;60:248-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013;26:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 274] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 46. | Afsharimani B, Doornebal CW, Cabot PJ, Hollmann MW, Parat MO. Comparison and analysis of the animal models used to study the effect of morphine on tumour growth and metastasis. Br J Pharmacol. 2015;172:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |