Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7119
Peer-review started: July 27, 2017
First decision: August 10, 2017
Revised: August 23, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: October 21, 2017
Processing time: 86 Days and 14.7 Hours
To assess the diagnostic value of a laparoscopic finding of a hepatic subcapsular spider-like telangiectasis (HSST) sign in biliary atresia.
A retrospective study was conducted first and then a validation set was used to investigate the value of an HSST sign in predicting biliary atresia (BA). In the retrospective study, laparoscopic images of the liver surface were reviewed in 126 patients with infantile cholestasis (72 BA patients and 54 non-BA cholestasis patients) and a control group of 38 patients with non-hepatic conditions. Analysis was first made by two observers separately and finally, a consensus conclusion was achieved. Then, the diagnostic value of the HSST sign was validated in an independent cohort including 45 BA and 45 non-BA patients.
In the retrospective investigation, an amplified HSST sign was found in all BA patients, while we were unable to detect the HSST sign in 98.1% of the 54 non-BA patients. There was no HSST sign in any of the control subjects. In the first review, the sensitivity and specificity from one reviewer were 100% and 98.1%, respectively, and the results from the other reviewer were both 100%. The consensus sensitivity and specificity were 100% and 98.1%, respectively. The HSST sign was defined as being composed of several enlarged tortuous spider-like vascular plexuses with two to eight branches distributed on all over the liver surface, which presented as either a concentrated type or a dispersed type. In the independent validation group, the sensitivity, specificity, positive predictive value and negative predictive value of the HSST sign were 100%, 97.8%, 97.8% and 100%, respectively.
The HSST sign is characteristic in BA, and laparoscopic exploration for the HSST sign is valuable in the diagnosis of BA.
Core tip: Color Doppler ultrasound finding of hepatic subcapsular flow has shown much potential for discriminating biliary atresia (BA). While in clinical practice, we have noticed a similar but more intuitive phenomenon - a laparoscopic finding of hepatic subcapsular spider-like telangiectasis (HSST) sign, which may be a specific marker for BA. Based on the current data, we found that the sensitivity and specificity of the HSST sign were each generally close to 100%.
- Citation: Zhou Y, Jiang M, Tang ST, Yang L, Zhang X, Yang DH, Xiong M, Li S, Cao GQ, Wang Y. Laparoscopic finding of a hepatic subcapsular spider-like telangiectasis sign in biliary atresia. World J Gastroenterol 2017; 23(39): 7119-7128
- URL: https://www.wjgnet.com/1007-9327/full/v23/i39/7119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i39.7119
Biliary atresia (BA), as a common neonatal cholangiopathy that leads to cirrhosis[1], is the most common indication for liver transplantation in infants[2,3]. The incidence of BA ranges from 1 in 5000 (Asian countries) to 1 in 19000 (European countries) live births, and it presents with typical symptoms and signs such as persistent jaundice, acholic stools and hepatomegaly within the first months of life[2]. This disease is characterized by a progressive fibro-obliterative obstruction of extrahepatic bile ducts. A younger age (in the first 2 mo of life) at the time of Kasai portoenterostomy has been found to be life-saving in restoring bile flow and preventing the worsening of liver function[4,5]. Thus, early discrimination is vital for the prognosis. Nevertheless, it is still challenging to differentiate BA from other cholestatic diseases as there are no characteristic manifestations found in the early stage of BA.
Clinic features, such as prolonged jaundice, pale stools and high conjugated hyperbilirubinemia, are remarkable, but they are not specific[6]. The ultrasonographic findings of an abnormal gall bladder and triangular cord sign have been widely used in early discrimination of BA[6,7], but the accuracy of these measurements varies[8-15]. A recent study showed that the absence of a gallbladder and the triangular cord sign could both have the highest specificity, up to 99%, but a sensitivity of 28% and 80%, respectively[14]. Other examinations are also compelling, but the value is limited. A duodenal fluid test is difficult to perform. Definite biliary excretion shown by scintigraphy can exclude BA, however, the absence of excretion is not specific in BA because of it can be attributed to any form of severe cholestasis[2,16]. Due to the small diameter of and inadequate fluid in biliary ducts, magnetic resonance cholangiopancreatography (MRCP) plays a limited role in showing the biliary tree in normal infants younger than 3 mo[17]. Therefore, the high false positive rate in predicting BA is inevitable. Moreover, the pathological features of BA are bile ductular proliferation, canalicular and cellular bile stasis, bile plugs, and portal or perilobular fibrosis with preservation of the basic hepatic lobular architecture[5,18,19], which overlap with α-1-antitrypsin deficiency, and occasionally Alagille syndrome, cystic fibrosis, and total parenteral nutrition (TPN)-related cholestasis[5].
Cholangiography is the gold standard, and a definitive diagnosis of BA can be made when cholangiography fails to show the biliary tree[1]. Laparotomy cholangiography was previously used and was refused by some parents because of the abdominal trauma and incision complications, which finally led to the delay of diagnosis. Recently, with the application of laparoscopy in pediatric surgery, laparoscopy-assisted cholangiography (LAC) has become possible. In our center, we performed laparoscopy-assisted cholangiography on infants who were highly suspected of having BA before the Kasai portoenterostomy was developed[20]. During the procedure, we found that a prominent pathological change on the liver surface - a hepatic subcapsular spider-like telangiectasis (HSST) sign - had a strong correlation with BA.
The current report includes a retrospective study and a prospective study, which aimed to investigate the significance of the laparoscopic finding of an HSST sign in differentiating BA from other causes of neonatal cholestasis.
The study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology, and all parents of the patients signed the inform consent. Before the parents signed the consent form for surgery, they were informed that the procedure was minimally invasive and that the procedure would be carried out via a small incision at the umbilicus ring and one stab incision in the left abdominal wall. Parents were told that there was a chance of needing additional trocars or conversion to an open technique. Advantages and disadvantages were reviewed to ensure that the parents were fully educated about the procedure.
An HSST sign in laparoscopic images was evaluated in cholestasis cases and a control group.
Patients: Laparoscopic images of the liver surface were reviewed in 126 patients with infantile cholestasis who underwent laparoscopic cholecystocholangiography or laparoscopic exploration at our center between February 2011 and February 2015. Among the 126 patients, 72 (31 boys, 41 girls; mean age, 58 d ± 29 d) were diagnosed with BA on the basis of laparoscopic cholangiography or exploration (when cholangiography failed due to the presence of atrophic gallbladder), and the other 54 (24 boys, 30 girls; mean age, 59 d ± 21 d) were diagnosed with non-BA conditions, which included infantile hepatitis (n = 46), biliary hypoplasia (n = 5) and total parenteral nutrition (TPN) induced cholestasis (n = 3). Their final diagnoses were confirmed by LAC, pathological examination or clinical follow-up. Another 38 patients (19 boys, 19 girls; mean age, 63 d ± 22 d) without jaundice or liver disease who underwent laparoscopic surgery served as controls. Their clinical diagnoses were Hirschsprung’s disease (n = 34) and intussusception (n = 4).
Laparoscopic findings: Laparoscopic images (5 mm or 10 mm optical laparoscopy, pediatric HOPKINS II26003BA, STORZ, Germany) of the liver surface were evaluated by two observers separately and blindly (Zhou Y and Jiang M) to screen for the presence or absence of the HSST sign. The HSST sign was preliminarily defined as enlarged tortuous spider-like vascular plexuses distributed on the liver surface.
Statistical analysis: The results are expressed as the mean ± SD or number (percentage). For quantitative data, statistical significance between groups was tested using a two-sample t test. For qualitative data (sex), significance between groups was tested by a χ2 test. Sensitivity and specificity were calculated for the HSST sign with the method of Newcombe[21]. Individual readings were calculated first; consensus readings were calculated thereafter (by professor Tang ST). Meanwhile, we applied Cohen’s κ coefficient to assess the agreement between the analyses conducted by the two observers. Logistic regression analysis was applied to determine whether the presence of the HSST sign was useful in discriminating BA. A P-value of < 0.05 was considered statistically significant. SPSS 13.0 software (SPSS, IL, United States) was used for data analyses.
The diagnostic value of the HSST sign was validated in an independent cohort composed of 45 BA and 45 non-BA patients.
Patients: Between February 2015 and April 2017, we enrolled a consecutive series of patients who were highly suspected of having BA. The enrollment criteria in our series included the following: (1) icterus was gradually aggravated after the first 3-4 wk; (2) consistent depigmented stool; and (3) traditional diagnostic examinations (i.e., physical examination, blood biochemical examination, ultrasonography, hepatobiliary scintigraphy, MRCP, etc.) failed to achieve a definitive diagnosis[20]. The children’s general information, including gender, age and biochemical parameters [the level of total and direct serum bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gammaglutamyl transpeptidase (GGT)] were carefully documented.
Validation protocol: All enrolled patients underwent laparoscopic exploration for the HSST sign before LAC. The patient was considered to be BA when the HSST sign was detected. The final diagnosis was confirmed by LAC.
Pathologic evaluation: After surgery, patients underwent pathologic examination. The hepatic subcapsular spider-like vessels were confirmed by gross inspection, well preserved on the prepared tissue, and evaluated with a microscope.
Statistical analysis: The results are expressed as the mean ± SD or number (percentage). A two-sample t test was used to compare quantitative parameters between patients with BA and non-BA patients. A χ2 test was used to compare the male-to-female ratio between patients with BA and non-BA patients. The cut-off values for optimal clinical performance (best sensitivity and best specificity simultaneously) of individual parameters were determined from the receiver-operating characteristic (ROC) curve. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for the HSST sign using the method of Newcombe[21]. A P-value of < 0.05 was considered statistically significant. SPSS 13.0 software (SPSS, IL, United States) was used for data analysis.
The two groups were age- and sex- matched (P > 0.05 for both). The mean levels of total bilirubin, direct bilirubin, AST and ALT in the BA group showed no significant difference compared to the non-BA group. The mean level of GGT was significantly higher in the BA group than in the non-BA group (P = 0.02). Total bilirubin, direct bilirubin, ALT, AST and GGT levels were normal in the controls (Table 1).
| Characteristic | BA (n = 72) | Non-BA (n = 54) | P value1 | Control (n = 38) | P value2 | P value3 |
| Age (d) | 58 ± 29 (20-180) | 59 ± 21 (35-125) | 0.89 | 63 ± 22 (36-109) | 0.35 | 0.34 |
| Male-to-female ratio | 31:41 | 24:30 | 0.88 | 19:19 | 0.49 | 0.60 |
| Total bilirubin (μmol/L) | 197.6 ± 72.8 | 163.6 ± 62.1 | 0.062 | 19.3 ± 18.3 | <0.0001 | <0.0001 |
| Direct bilirubin (μmol/L) | 132.8 ± 143.8 | 99.3 ± 50.8 | 0.29 | 5.7 ± 5.6 | 0.003 | <0.0001 |
| ALT (U/L) | 162.9 ± 122.6 | 170.2 ± 362.6 | 0.91 | 21.3 ± 7.4 | <0.0001 | 0.15 |
| AST (U/L) | 239.7 ± 156.7 | 183.5 ± 253.1 | 0.27 | 30.0 ± 11.1 | <0.0001 | 0.037 |
| GGT(U/L) | 684.7 ± 450.6 | 454.2 ± 450.9 | 0.052 | 46.5 ± 22.4 | <0.0001 | 0.003 |
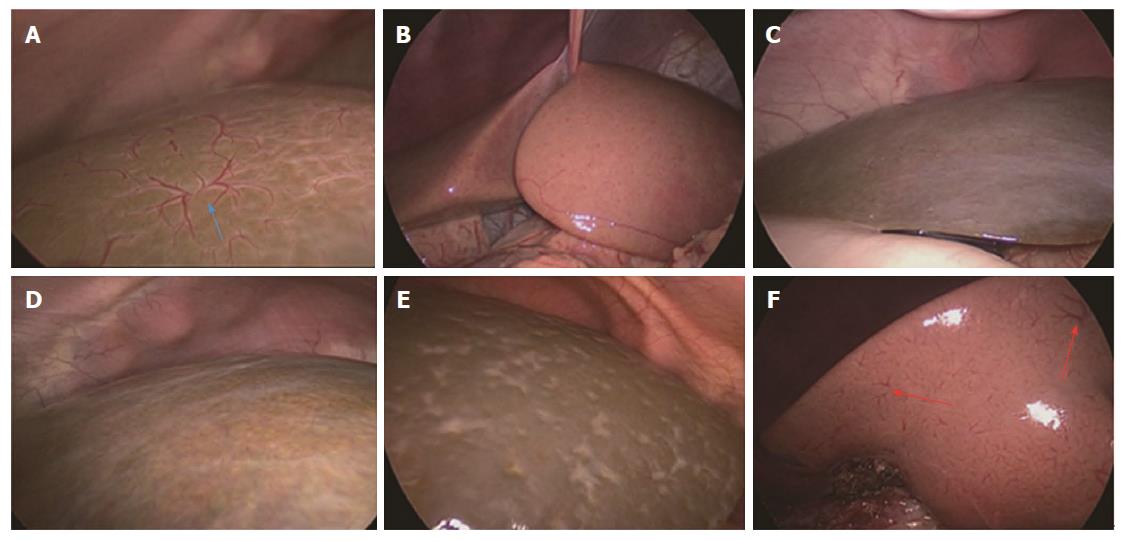
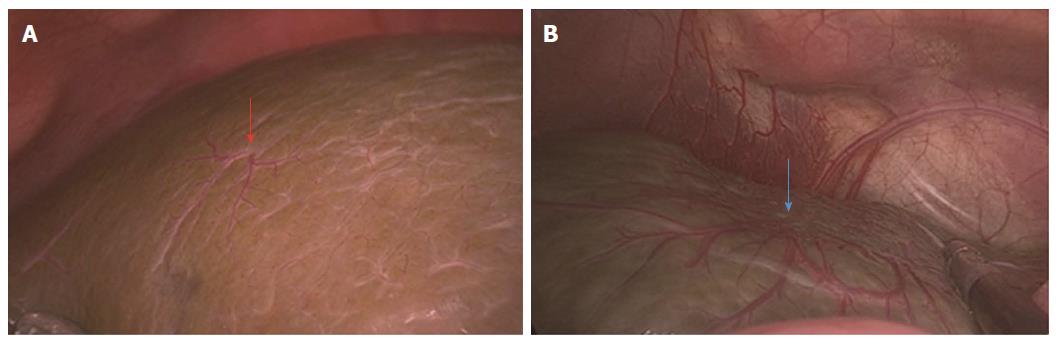
An amplified HSST sign was identified in all patients with BA (Figure 1A) and was not found in any of the control subjects by both observers (Figure 1 B). One observer (Jiang M) determined that there was no HSST sign in 53 (98%) non-BA patients (Figure 1C-E), except for one patient with cytomegalovirus (CMV) hepatitis (Figure 1F). The other observer (Zhou Y) had the opinion that there was no HSST sign in all patients with non-BA. After discussion, the discrepancy was resolved. The sensitivity and specificity of the HSST sign were 100% and 98.1%, respectively (Table 2).
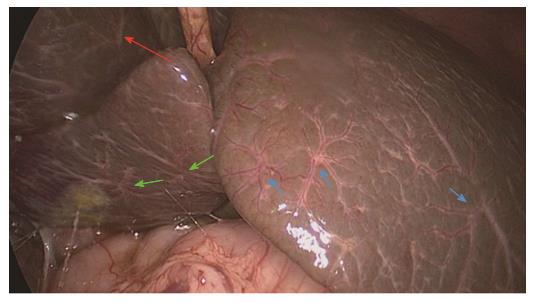
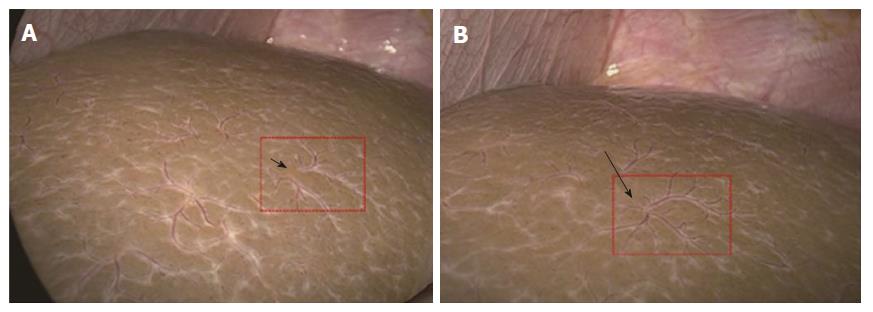
The HSST sign was widely distributed on the surface of both left and right hepatic lobes, including on the visceral and diaphragmatic facies (Figure 2). Thus, we redefined the HSST sign as follows: it is composed of several enlarged tortuous spider-like vascular plexuses with four to eight branches distributed all over the liver surface, which presents as a dispersed type (radiating branches originate from more than one central point, but close to each other to form a spider-like sign) (Figure 3A) or a concentrated type (radiating branches originate from one central point) (Figure 3B).
Logistic regression analysis showed that the presence of the HSST sign was a significant predictor of BA (P < 0.01). In addition, the concordance between the two observers was also robust (κ = 0.94, 95%CI: 0.87–1.0).
Characteristics of patients: The mean age of the BA group was 60 d ± 29 d. The mean age of the non-BA group was 61 d ± 23 d. In the non-BA group, the final diagnoses were infantile hepatitis (n = 43) and biliary hypoplasia (n = 2). As shown in Table 3, there was no significant difference between the BA and non-BA groups in age or male-to-female ratio (P > 0.05 for both). The mean levels of total bilirubin, direct bilirubin, AST and ALT in BA patients had no significant difference compared to those in the non-BA group. The mean level of GGT was significantly higher in the BA group than in the non-BA group (P < 0.001).
| Characteristic | BA (n = 45) | Non-BA (n = 45) | P value |
| Age (d) | 60 ± 29 | 61 ± 23 | 0.90 |
| Male-to-female ratio | 21:24 | 25:20 | 0.40 |
| Total bilirubin (μmol/L) | 192.3 ± 63.5 | 173.6 ± 77.0 | 0.21 |
| Direct bilirubin (μmol/L) | 131.6 ± 140.8 | 104.5 ± 52.3 | 0.23 |
| ALT (U/L) | 172.8 ± 120.9 | 124.3 ± 264.1 | 0.27 |
| AST (U/L) | 255.2 ± 162.1 | 188.7 ± 193.9 | 0.08 |
| GGT (U/L) | 661.8 ± 466.3 | 320.7 ± 384.1 | <0.001 |
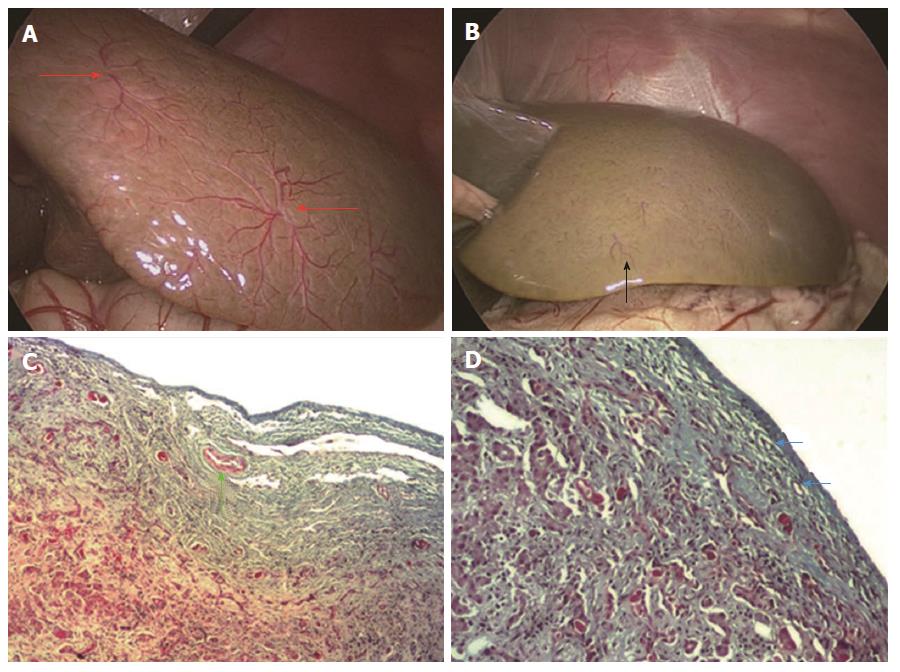
Diagnostic performance of the HSST sign: On high-definition laparoscopic images (5 mm or 10 mm optical laparoscopic, pediatric HOPKINS II 26003BA, STORZ, Germany), the HSST sign in all BA patients was easily observed (Figure 4A). There were no HSST sign observed in non-BA patients, except for one patient (Figure 4B). The patient was considered to have BA due to the presence of the HSST sign, whose final diagnosis was biliary hypoplasia. The sensitivity, specificity, PPV and NPV of the HSST sign were 100%, 97.8%, 97.8% and 100%, respectively.
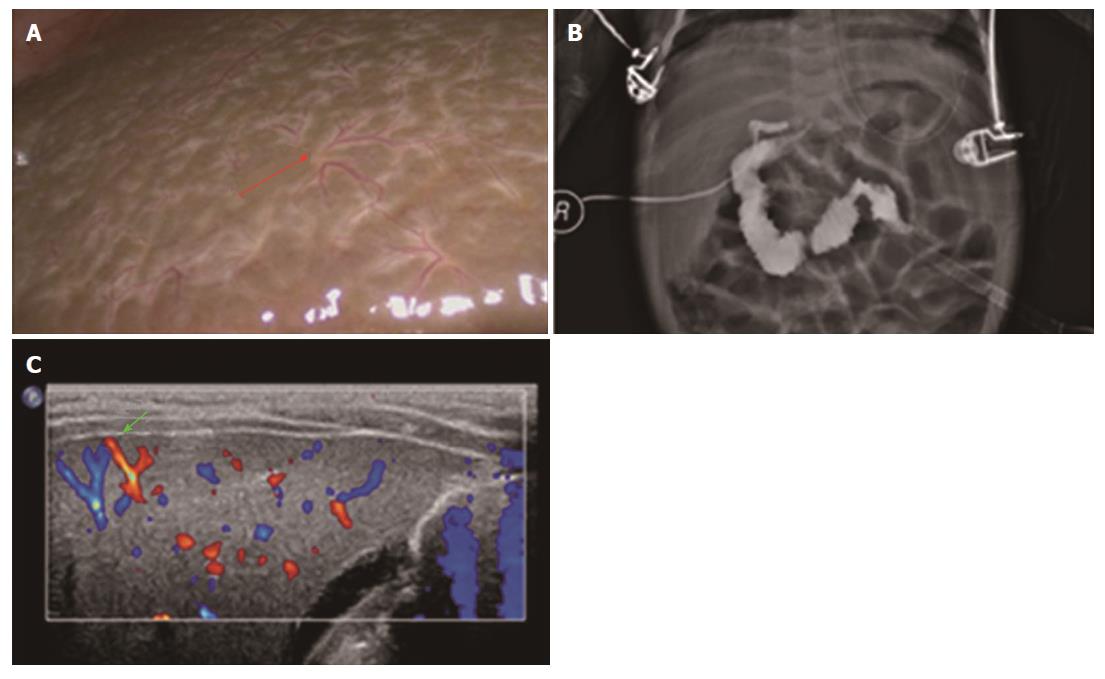
Pathologic evaluation: The hepatic subcapsular spider-like vessels were clearly identified in the stained tissue. The vessels in the BA group were revealed as dilated branches of hepatic arteries in the hepatic subcapsular area (Figure 4C) and the vessels in hypoplasia were revealed as hyperplastic capillaries in the hepatic subcapsular area (Figure 4D).
A large sample study focused on GGT showed that it was higher in the BA group than in other cholestatic disorders for all age groups[22], which was coincident with our results. In our validation set, we found that the area under the receiver operating characteristic curve was 0.745 and GGT at a cut-off value of > 404 U/L had a 64.4% sensitivity and 84.4% specificity in discriminating BA. A level of 300 U/L was also previously reported by Liu et al[16] with 85% accuracy. However high it may be, we cannot confirm BA directly by GGT alone.
In the entire study, there were 14 BA patients (6 in the retrospective study and 8 in the validation set) below the age of 40 d (with the minimum age of 20 d), in whom the HSST sign was observed but there was no liver cirrhosis at gross inspection (Figure 5A). The HSST sign was also found in 10 patients with BA (8 in the retrospective study and 4 in the validation set) who were older than 90 d (91-163 d) (Figure 5B). Based on the above data, the presence of the HSST sign may not have a close relationship with hepatic fibrosis or cirrhosis. It is known that CMV infection was one causative factor of BA and may result in a delayed diagnosis and surgical treatment[23,24]. Mohanty et al[25] reported two cases of CMV infection, initially presenting with intrahepatic cholestasis, who subsequently developed BA. Whether the pathological change in the perivascular arterial tufts is the causative factor or results from the developmental of BA is still unknown and needs further investigation.
Ultrasonography (US) has been widely used in differentiating BA from other causes of jaundice. Hepatic subcapsular flow (HSF) in color Doppler US was documented as being useful to predict BA[8,15,18,26] and had the highest performance in discriminating BA from non-BA conditions among several parameters[18]. However, most of the neonates or infants were uncooperative during color Doppler US examination and other factors, such as the doctor’s experience, machines, and patients’ respiration intensities, also played an important role. Additionally, Takamizawa et al[9] debated that US alone could not rule out BA and laparotomy remained mandatory if BA was suspected. Therefore, we conducted this study and finally determined that the HSST sign was useful for predicting BA with a 100% sensitivity and 97.8% specificity. Here, we had a 53-day-old patient with a pre-operative icteric index of 149.9 but unobvious yellowing of the skin. As shown in the picture, the laparoscopic HSST sign (Figure 6A) and the result of LAC (Figure 6B) were easier to identify than distinguishing HSF in a color Doppler US image (Figure 6C).
A similar phenomenon was also observed in Lee’s[8] and Ramesh’s[27] studies. These authors reported that patients with BA who had hepatic subcapsular flow on color Doppler US images also had subcapsular telangiectatic vessels on the liver surface at the time of the Kasai procedure. In these reports, four patients with TPN-induced cholestasis and two with CMV hepatitis were considered as having HSF[8,15,18]. In our retrospective study, three patients with TPN-induced cholestasis cirrhosis were encountered, but no HSST sign was detected (Figure 2E). In our validation set, there were also two cases of infantile hepatitis who were considered as having HSF, while the HSST sign did not exist. The laparoscopic amplification effect on an intuitive view of the liver surface and ultrasonic artifacts may be the cause of this difference. Moreover, better concordance (κ = 0.937) was achieved through the amplified intuitive view.
Although the signal of hepatic subcapsular vessels was found with US, these vessels on laparoscopic images had their own characteristics: (1) intuitive and stable view of liver surface that was not influenced by interference (machines, doctors’ experiences, thickness of the abdominal wall, etc.); (2) the HSST sign could be observed not only on the diaphragmatic surface but also on the visceral surface; and (3) images were magnified 4-8 times, which made the view of liver surface clear and vessels easily identified.
Microscopic examinations revealed dilated small arteries in the hepatic subcapsular area in patients with BA, which is consistent with Lee’s[8] and El-Guindi’s[15] studies. These vessels were branches of hepatic arteries, instead of capillaries in the hepatic capsule. Until now, this phenomenon was not mentioned for other liver diseases. The findings of arterial hyperplasia or hypertrophy in liver specimens and the enlargement of the hepatic artery on color Doppler US imaging in BA patients were also documented[15]. It has also been reported that the arterial changes might be driven by primary growth signals, or represent a response to a diseased micro-environment that might be rich in growth promoting signals that favor angiogenesis[28-32]. Similarly, the HSST sign is one of the arterial changes in an intuitive view. As with the HSST sign in images 1-5, they were not quite the same and this difference might help in predicting prognoses in the future. Whether it is a primary change of BA or secondary to the bile duct inflammation is unknown and will be our next focus of study.
At present, the procedure for addressing BA patients is a routine examination followed by laparotomy or laparoscopic cholangiography and a laparotomy Kasai operation. For the laparoscopic cholangiography, two or three 5-mm trocars and 30-45 min are needed with one or two instances of radiation exposure. However, with the application of the HSST sign, the procedure for addressing BA may become a routine examination followed by laparoscopic exploration for the HSST sign and a laparotomy Kasai operation in the future, which would shorten the operative time and radiation exposure and only needs one 5-mm trocar. The advantage is obvious especially for non-BA patients. In addition, it reflects the diagnostic function of laparoscopy in BA, similar to a laparoscopic diagnosis of hermaphroditism and inguinal hernias.
In conclusion, the HSST sign was preliminarily found to have a good performance in diagnosing or excluding BA with a 100% sensitivity and 97.8% specificity. According to an early laparoscopic exploration of the HSST sign, we can rapidly differentiate BA from non-BA cases and potentially further prevent the delay of a diagnosis of BA. However, given the limited sample size included in the analysis, the value of the HSST sign should be further confirmed in the future. Along with the further research, the HSST sign might be widely applied in clinical practice through a multicenter and large sample study in the future.
As a common neonatal cholangiopathy that leads to cirrhosis, biliary atresia (BA) is the most common indication for liver transplantation in infants. This disease is characterized by a progressive fibro-obliterative obstruction of extrahepatic bile ducts. A younger age (in the first 2 mo of life) at the time of Kasai portoenterostomy has been found to be life-saving in restoring bile flow and preventing the worsening of liver function. Nevertheless, it is still challenging to differentiate BA from other cholestatic diseases as there are no characteristic manifestations found in the early stage of BA. The demand for a specific and reliable test to diagnose BA has increased.
Color Doppler ultrasound finding of hepatic subcapsular flow has shown much potential for discriminating BA. While in clinical practice, we have noticed a similar but more intuitive phenomenon - a laparoscopic finding of hepatic subcapsular spider-like telangiectasis (HSST) sign, which may be a specific marker for BA. This has not been published in previous literature.
According to these preliminary investigation, the HSST sign showed promising diagnostic performance for differentiating BA from any other cholestasis diseases, such as infantile cholestasis and cholestatic syndrome. The authors found that the sensitivity and specificity of the HSST sign were each generally close to 100%.
With the application of the HSST sign, the procedure for addressing BA may become a routine examination followed by laparoscopic exploration for the HSST sign and a laparotomy Kasai operation in the future, which would shorten the operative time and radiation exposure.
The HSST sign is composed of several enlarged tortuous spider-like vascular plexuses with four to eight branches distributed all over the liver surface, which presents as a dispersed type (radiating branches originate from more than one central point, but close to each other to form a spider-like sign) or a concentrated type (radiating branches originate from one central point).
Very interesting findings. It is worth to be published because of new findings of laparoscopic view for the surface of liver in biliary atresia
We thank Professor Ping Ying for the statistical evaluation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): A
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chan KWEE, Han SJ S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46:566-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 638] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 3. | de Carvalho E, Ivantes CA, Bezerra JA. Extrahepatic biliary atresia: current concepts and future directions. J Pediatr (Rio J). 2007;83:105-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Ohi R. Biliary atresia. A surgical perspective. Clin Liver Dis. 2000;4:779-804. [PubMed] |
| 5. | Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37:4-21. [PubMed] |
| 6. | Ramachandran P, Safwan M, Reddy MS, Rela M. Recent Trends in the Diagnosis and Management of Biliary Atresia in Developing Countries. Indian Pediatr. 2015;52:871-879. [PubMed] |
| 7. | Tan Kendrick AP, Phua KB, Ooi BC, Subramaniam R, Tan CE, Goh AS. Making the diagnosis of biliary atresia using the triangular cord sign and gallbladder length. Pediatr Radiol. 2000;30:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Lee MS, Kim MJ, Lee MJ, Yoon CS, Han SJ, Oh JT, Park YN. Biliary atresia: color doppler US findings in neonates and infants. Radiology. 2009;252:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Takamizawa S, Zaima A, Muraji T, Kanegawa K, Akasaka Y, Satoh S, Nishijima E. Can biliary atresia be diagnosed by ultrasonography alone? J Pediatr Surg. 2007;42:2093-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Kim WS, Cheon JE, Youn BJ, Yoo SY, Kim WY, Kim IO, Yeon KM, Seo JK, Park KW. Hepatic arterial diameter measured with US: adjunct for US diagnosis of biliary atresia. Radiology. 2007;245:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Tiao MM, Chuang JH, Huang LT, Hsieh CS, Lee SY, Liang CD, Chen CL. Management of biliary atresia: experience in a single institute. Chang Gung Med J. 2007;30:122-127. [PubMed] |
| 12. | Park WH, Choi SO, Lee HJ. The ultrasonographic ‘triangular cord’ coupled with gallbladder images in the diagnostic prediction of biliary atresia from infantile intrahepatic cholestasis. J Pediatr Surg. 1999;34:1706-1710. [PubMed] |
| 13. | Nemati M, Rafeey M, Shakeri AB. Ultrasound findings in biliary atresia: the role of triangular cord sign. Pak J Biol Sci. 2009;12:95-97. [PubMed] |
| 14. | Zhou L, Shan Q, Tian W, Wang Z, Liang J, Xie X. Ultrasound for the Diagnosis of Biliary Atresia: A Meta-Analysis. AJR Am J Roentgenol. 2016;206:W73-W82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | El-Guindi MA, Sira MM, Konsowa HA, El-Abd OL, Salem TA. Value of hepatic subcapsular flow by color Doppler ultrasonography in the diagnosis of biliary atresia. J Gastroenterol Hepatol. 2013;28:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Liu CS, Chin TW, Wei CF. Value of gamma-glutamyl transpeptidase for early diagnosis of biliary atresia. Zhonghua Yi Xue Za Zhi (Taipei). 1998;61:716-720. [PubMed] |
| 17. | Siles P, Aschero A, Gorincour G, Bourliere-Najean B, Roquelaure B, Delarue A, Petit P. A prospective pilot study: Can the biliary tree be visualized in children younger than 3 months onMagnetic Resonance Cholangiopancreatography? Pediatr Radiol. 2014;44:1077-1084. |
| 18. | El-Guindi MA, Sira MM, Sira AM, Salem TA, El-Abd OL, Konsowa HA, El-Azab DS, Allam AA. Design and validation of a diagnostic score for biliary atresia. J Hepatol. 2014;61:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Fawaz R, Baumann U, Ekong U, Fischler B, Hadzic N, Mack CL, McLin VA, Molleston JP, Neimark E, Ng VL. Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2017;64:154-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 314] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 20. | Tang ST, Li SW, Ying Y, Mao YZ, Yong W, Tong QS. The evaluation of laparoscopy-assisted cholangiography in the diagnosis of prolonged jaundice in infants. J Laparoendosc Adv Surg Tech A. 2009;19:827-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857-872. [PubMed] |
| 22. | Chen X, Dong R, Shen Z, Yan W, Zheng S. Value of Gamma-Glutamyl Transpeptidase for Diagnosis of Biliary Atresia by Correlation With Age. J Pediatr Gastroenterol Nutr. 2016;63:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Saito T, Terui K, Mitsunaga T, Nakata M, Ono S, Mise N, Yoshida H. Evidence for viral infection as a causative factor of human biliary atresia. J Pediatr Surg. 2015;50:1398-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Oliveira NL, Kanawaty FR, Costa SC, Hessel G. Infection by cytomegalovirus in patients with neonatal cholestasis. Arq Gastroenterol. 2002;39:132-136. [PubMed] |
| 25. | Mohanty S, Shah I, Bhatnagar S. Evolving biliary atresia with cytomegalovirus. Indian Pediatr. 2011;48:644-646. [PubMed] |
| 26. | Kim SS, Kim MJ, Lee MJ, Yoon CS, Han SJ, Koh H. Ultrasonographic findings of type IIIa biliary atresia. Ultrasonography. 2014;33:267-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Ramesh RL, Murthy GV, Jadhav V, Ravindra S. Hepatic subcapsular flow: An early marker in diagnosing biliary atresia. Indian J Radiol Imaging. 2015;25:196-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Huang FC, Hwang KP. Differential diagnosis of infantile choledochal cyst with or without biliary atresia. Acta Paediatr Taiwan. 2006;47:175-180. [PubMed] |
| 29. | dos Santos JL, da Silveira TR, da Silva VD, Cerski CT, Wagner MB. Medial thickening of hepatic artery branches in biliary atresia. A morphometric study. J Pediatr Surg. 2005;40:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Ho CW, Shioda K, Shirasaki K, Takahashi S, Tokimatsu S, Maeda K. The pathogenesis of biliary atresia: a morphological study of the hepatobiliary system and the hepatic artery. J Pediatr Gastroenterol Nutr. 1993;16:53-60. [PubMed] |
| 31. | de Souza AF, Meurer L, da Silveira TR, Gregório C, Reus N, Uribe C, Matte U, dos Santos JL. Angiopoietin 1 and angiopoietin 2 are associated with medial thickening of hepatic arterial branches in biliary atresia. Pediatr Res. 2014;75:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Edom PT, Meurer L, da Silveira TR, Matte U, dos Santos JL. Immunolocalization of VEGF A and its receptors, VEGFR1 and VEGFR2, in the liver from patients with biliary atresia. Appl Immunohistochem Mol Morphol. 2011;19:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |