Published online Oct 14, 2017. doi: 10.3748/wjg.v23.i38.7054
Peer-review started: April 25, 2017
First decision: May 12, 2017
Revised: June 18, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: October 14, 2017
Processing time: 175 Days and 9.6 Hours
Only two cases of myofibroblastic sarcoma in the liver have been reported in the literature. Here, we report the case of a male patient with high-grade myofibroblastic sarcoma mimicking echinococcosis in the liver. The 25-year-old male patient complained of right upper quadrant swelling pain for one week and was initially diagnosed with echinococcosis. He was then scheduled for an exploratory laparotomy. During the operation, a huge mass exceeding 16 cm in diameter was found to occupy nearly the entire right trisegment of the liver, with a clear boundary and a round shape, and the mass was resected by right hepatic trisegmentectomy. Immunohistochemical staining revealed that the tumor tissue was positive for desmin, α-smooth muscle actin, CD56, and vimentin and negative for ALK-1, myogenin, calponin, β-catenin, S100, and glypican-3, with a Ki-67 (MIB-1) index of approximately 20%. Based on the histological manifestations and immunohistochemical staining, a diagnosis of myofibroblastic sarcoma was established. The postoperative recovery was uneventful. There was no evidence of recurrence or metastasis through the last follow-up, 6 mo after surgery, despite a lack of postoperative chemotherapy or radiotherapy. To the best of our knowledge, the present case is the first reported case of high-grade myofibroblastic sarcoma in the liver, and it is also the first reported case in a male patient.
Core tip: The development of myofibroblastic sarcoma in the liver is exceedingly rare. We present a case of high-grade myofibroblastic sarcoma in a male patient. The 25-year-old male patient complained of right upper quadrant swelling pain for one week and was diagnosed with high-grade myofibroblastic sarcoma after right hepatic trisegmentectomy based on the histological manifestations and immunohistochemical staining. There was no evidence of recurrence or metastasis through the last follow-up, 6 mo after surgery, despite a lack of postoperative chemotherapy or radiotherapy. The case presented is the first reported case of high-grade myofibroblastic sarcoma in the liver.
- Citation: Wen J, Zhao W, Li C, Shen JY, Wen TF. High-grade myofibroblastic sarcoma in the liver: A case report. World J Gastroenterol 2017; 23(38): 7054-7058
- URL: https://www.wjgnet.com/1007-9327/full/v23/i38/7054.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i38.7054
Myofibroblastic sarcoma is a rare disease that was initially identified by Mentzel et al[1] in 1998, and all its reports are case reports or case series. It is widely accepted that myofibroblastic sarcoma predominantly develops in the head and neck region, followed by the extremities and then the trunk, with the abdominal cavity being rarely involved. The development of myofibroblastic sarcoma in the liver is exceedingly rare, and there have been only two relevant case reports to date[2,3]. Although there is generally a slight male predominance[1,4], both of the case reports described female patients. In the present report, we present a case of high-grade myofibroblastic sarcoma in a male patient. The clinical manifestations, blood test results, imaging findings, histological and immunohistochemical findings, and treatment are discussed here.
A 25-year-old male patient was referred to the liver surgery department because of right upper quadrant swelling pain for one week without any concomitant symptoms. His medical history was unremarkable, with no hepatitis or surgical history. No family or genetic history was claimed either, and a general physical examination showed no abnormal signs.
Hematological and blood biochemical tests revealed no abnormalities. Serum tumor markers were within the normal limits: α-fetoprotein (AFP), 4.14 ng/mL; carcinoembryonic antigen (CEA), 1.30 ng/mL; and carbohydrate antigen (CA19-9), 6.06 U/mA. Among all of the hepatitis B markers, only surface antibody was positive.
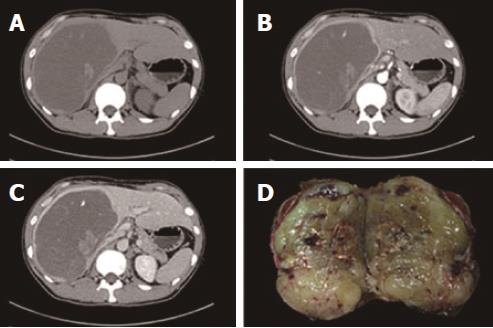
Abdominal color ultrasound revealed a heteroechoic mass in the right trisegment of the liver, measuring 18 cm × 15 cm × 11 cm, with a clear boundary but with no remarkable blood signal. Abdominal contrast-enhanced color ultrasound revealed a heteroechoic mass showing peripheral hyper-enhancement in the arterial phase and peripheral hypo-enhancement in the portal phase, whereas the central portion showed no enhancement throughout the two phases. Dynamic contrast-enhanced computed tomography (CT) imaging revealed a cyst-like lesion with an obvious capsule located in the right trisegment of the liver; this lesion contained a stripe-like, slightly hyperdense area and patchy hypodense areas that were hyper-enhanced in the arterial phase and hypo-enhanced in the portal phase, whereas the central portion remained unenhanced throughout the arterial and portal phases (Figure 1).
Based on the aforementioned findings, liver echinococcosis was suspected. For treatment, a right hepatic trisegmentectomy was scheduled.
Upon laparotomy, a huge solid mass exceeding 16 cm in diameter with a clear boundary was found to occupy nearly the entire right trisegment of the liver. The remnant liver had a normal appearance, showing no cirrhosis. There was also no ascites or peritoneal dissemination, and enlarged lymph nodes were not found in the peritoneal cavity. Intraoperative ultrasound showed that the right hepatic vein was occluded, whereas the middle hepatic vein was pushed away by the mass. In addition, no disseminated lesions were found to be located in the remnant liver. The resected tumor measured 19 cm × 16 cm × 11 cm and weighed 2400 g. Sections through the tumor disclosed grayish-yellow, solid tissue that showed relatively homogenous internal structures (Figure 1).
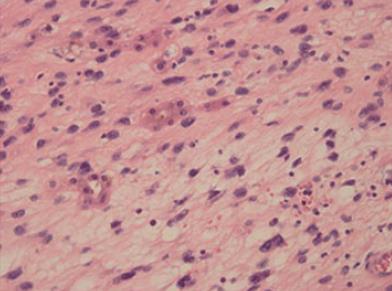
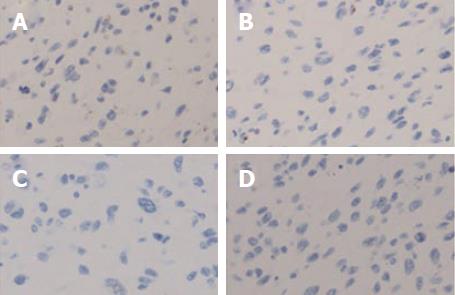
Histologically, the tumor cells were spindle shaped and located in a loose myxoid background and contained small-to medium-sized nucleoli with readily identified mitotic figures (Figures 2 and 3). Histological images revealed high mitotic activity, with more than 19 mitoses per 10 high-power fields (HPFs), along with certain small necrotic areas (< 50%). Immunohistochemical staining showed that the tumor tissue was positive for desmin (Figure 4), α-smooth muscle actin (α-SMA), CD56, and vimentin and negative for ALK-1, myogenin, calponin, β-catenin, CD34, CD10, S100, glypican-3, CDK4, PCK, and CD117, with a Ki-67 (MIB-1) index of approximately 20%. For differential diagnosis, the tumor tissue was negative for CD31 (Figure 5), Factor VIII (to deny vascular tumor, such as angiosarcoma or epithelioid hemangioendothelioma), Melan A or HMB45 (to deny angiomyolipoma). Based on the histological and immunohistochemical findings, a diagnosis of myofibroblastic sarcoma was established, and this sarcoma was further classified as grade 3 according to the French Federation of Cancer Centers Sarcoma Group (FNCLCC) system[5].
The postoperative recovery was uneventful. The patient refused postoperative chemotherapy and radiotherapy; fortunately, however, there was no evidence of recurrence through the last follow-up, 6 months after surgery, despite the lack of postoperative chemotherapy or radiotherapy.
Since Mentzel et al[1] first defined a distinctive clinicopathological entity now known as low-grade myofibroblastic sarcoma in 1998, myofibroblastic sarcoma has been increasingly investigated, with most reports consisting of case reports or case series[2,3,6-11]. Although the World Health Organization (WHO) included low-grade myofibroblastic sarcoma, when characterized reproducibly, in the WHO Classification of Tumors in 2002[12], the definition of intermediate- and high-grade myofibroblastic sarcoma with less reproducible characteristics has not reached a consensus, even in the latest edition of the WHO Classification of Tumors of Soft Tissue and Bone[4].
Given the rare incidence of myofibroblastic sarcoma, there have been few reports describing its etiology. Although a slight male predominance was suggested in the latest WHO classification[4], Chan et al[11] found that the majority of cases involve female patients. To date, the present case is the first reported case of high-grade myofibroblastic sarcoma in the liver and also the first case reported in a male patient, whereas the two existing case reports described female patients[2,3]. Painless swelling or an enlarging mass is the most common complaint[4], with other symptoms depending on the location; for example, obstruction is typical when the lesion develops in the nasal cavity[7]. In the present case, the chief complaint was right upper quadrant swelling pain, which was also present in a report by Yi et al[3], whereas Pan et al[2] reported no pain but abnormal liver function. In the present case and the report by Yi et al[3], the lesion showed a well-defined growth pattern, whereas this type of tumor is commonly reported to display an infiltrative and destructive growth pattern[4,7].
According to the WHO classification, ultrasound examination should be conducted first, but CT or magnetic resonance imaging (MRI) is recommended for further investigation, with equivalent value[4]. Imaging by dynamic contrast-enhanced CT in the report by Yi et al[3] was in close accordance with what was found in the present case. The lesion, which was well defined and round shaped and showed hypodensity on plain CT scans, was peripherally enhanced, whereas the central portion remained unenhanced throughout the arterial and portal phases. Although these characteristics have been reported in only two cases, they constitute a “consensus” that could be referred to when myofibroblastic sarcoma in the liver is suspected. Yi et al[3] also reported a honeycomb appearance on T2WI images and a progressive enhancement pattern as characteristic manifestations on MRI.
The presence of ultrastructural features, such as fibronexus (microtendons) and stress fibers, is no longer emphasized as important for the diagnosis of myofibroblastic sarcoma[13]. Instead, the diagnosis of myofibroblastic sarcoma is now established by histopathology and immunohistochemical staining[13]. Although there is no constant immunophenotype, myofibroblastic sarcoma stains positively for muscle actin, α-SMA, and/or desmin in general, but it is typically negative for S100 protein, epithelial markers, nuclear β-catenin, and h-caldesmon[1,4,13].
Because high-grade myofibroblastic sarcoma was not included in the latest WHO classification[1,4,13], the two grading systems proposed by the National Cancer Institute (NCI) and the FNCLCC are the most widely used[4,5]. The FNCLCC system, which is precisely defined and more reproducible, with better performance in prognostic prediction, is preferred over the NCI system and other systems[14,15]. The present case, which showed high mitotic activity (more than 19 mitoses/10 HPFs) and certain small necrotic areas (< 50%), was classified as grade 3 according to the FNCLCC system[5]. Although low-grade myofibroblastic sarcomas have a local recurrence rate of 5%-10% when treated by wide-margin excision, they rarely metastasize. In fact, Fisher C reported 13 (33%) recurrences and 3 (8%) metastases in 39 published cases with follow-up information[16]. In contrast, high-grade myofibroblastic sarcoma shows a high rate of metastasis, along with recurrence; Fisher C reported 7 (32%) recurrences and 15 (68%) metastases among 22 ultrastructurally confirmed cases[16].
Surgery is the first choice for treatment, with the role of chemotherapy and radiotherapy being controversial. Generally, a wide margin of at least 1-2 cm is recommended because microscopically positive surgical margins are associated with a high risk of local recurrence, distant metastasis and death[4]. Retroperitoneal sarcomas show a much lower survival rate than extremity soft-tissue sarcomas, and high-grade myofibroblastic sarcomas are more aggressive[4]. However, little is known about myofibroblastic sarcoma, especially high-grade myofibroblastic sarcoma, in the liver. There were no signs of recurrence or metastasis in the reported intermediate-grade case[3] or in the high-grade myofibroblastic sarcoma case described in the present report 6 mo after surgery, despite the fact that both patients refused further treatment. Unfortunately, the patient in the report by Pan et al[2] was lost to followup 3 mo after discharge from the hospital. Meng et al[7] reported that myofibroblastic sarcoma of the nasal cavity and paranasal sinus exhibited diverse histological characteristics and strongly aggressive behavior, indicating that myofibroblastic sarcomas with different anatomic distributions may have diverse histological characteristics, biological behaviors and prognoses. We hypothesize that as long as it is resected with a sufficient margin, irrespective of grading, myofibroblastic sarcoma in the liver probably has an optimistic prognosis, as indicated by the report by Yi et al[3] and the present case. However, long-term follow-up and more patient data are needed before we can draw reliable conclusions.
Myofibroblastic sarcoma is a rare disease. The present case is the first reported case of high-grade myofibroblastic sarcoma in the liver, and it is also the first reported case in a male patient. The diagnosis of myofibroblastic sarcoma was established by histopathology and immunohistochemical staining. With a well-defined growth pattern and resection with a sufficient margin, regardless of its grade, the myofibroblastic sarcoma in the liver showed no signs of recurrence or metastasis 6 mo after surgery.
A 25-year-old male patient complained of right upper quadrant swelling pain for 1 week without any concomitant symptoms.
Liver echinococcosis was suspected.
Liver echinococcosis, gastrointestinal stromal tumor, vascular tumor, such as angiosarcoma or epithelioid hemangioendothelioma, angiomyolipoma.
All laboratory tests were within normal limits.
CT imaging found a cyst-like lesion with an obvious capsule located in the right trisegment of the liver.
The diagnosis of high-grade myofibroblastic sarcoma was established by histopathology and immunohistochemical staining.
The patient successfully underwent a right hepatic trisegmentectomy.
There have been no case reports on high-grade myofibroblastic sarcoma in the liver. Only two reports were published reporting intermediate-grade myofbroblastic sarcoma or myofibroblastic sarcoma. The diagnosis was established on the basis of morphology and immunohistochemistry features. Surgical resection was the treatment of choice with gratifying results.
The diagnosis of myofibroblastic sarcoma is established by histopathology and immunohistochemical staining. Surgical resection is the treatment of choice with gratifying results.
The authors report a case of myofibroblastic sarcoma of the liver. The manuscript is well-written and the case is pathologically interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good):
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kai K S- Editor: Gong ZM L- Editor: Wang TQ
E- Editor: Ma YJ
| 1. | Mentzel T, Dry S, Katenkamp D, Fletcher CD. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol. 1998;22:1228-1238. [PubMed] |
| 2. | Pan Y, Wu X, Liu J, Muheremu A. Abnormal liver function induced by myofibroblastic sarcoma infiltrating the liver: A case report. Oncol Lett. 2015;9:798-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Yi X, Xiao D, Long X. Myofibroblastic sarcoma in liver: a case report. Int J Clin Exp Pathol. 2015;8:1073-1076. [PubMed] |
| 4. | Fletcher CD, Bridge J, Hogendoorn P, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. Lyon: IARC Press 2013; 15-18. |
| 5. | Neuville A, Chibon F, Coindre JM. Grading of soft tissue sarcomas: from histological to molecular assessment. Pathology. 2014;46:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Kolli S, Ziaee A, Lee R, Lim MJ, Levy B, Labovitz AJ. Myofibroblastic sarcoma of mitral valve: a case report. J Am Soc Echocardiogr. 2005;18:285-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Meng GZ, Zhang HY, Bu H, Yang GH, Zhang XL, Yang G. Myofibroblastic sarcoma of the nasal cavity and paranasal sinus: a clinicopathologic study of 6 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Morii T, Mochizuki K, Sano H, Fujino T, Harasawa A, Satomi K. Occult myofibroblastic sarcoma detected on FDG-PET performed for cancer screening. Ann Nucl Med. 2008;22:811-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Takácsi-Nagy Z, Muraközy G, Pogány P, Fodor J, Orosz Z. Myofibroblastic sarcoma of the base of tongue. Case report and review of the literature. Strahlenther Onkol. 2009;185:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Miyazawa M, Naritaka Y, Miyaki A, Asaka S, Isohata N, Yamaguchi K, Murayama M, Shimakawa T, Katsube T, Ogawa K. A low-grade myofibroblastic sarcoma in the abdominal cavity. Anticancer Res. 2011;31:2989-2994. [PubMed] |
| 11. | Chan JY, Gooi Z, Wong EW, Ng SK, Tong MC, Vlantis AC. Low-grade myofibroblastic sarcoma: A population-based study. Laryngoscope. 2017;127:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Fletcher CD, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. 3rd ed. Lyon:. IARC Press. 2002;94-96. |
| 13. | Mentzel T. Myofibroblastic sarcomas: a brief review of sarcomas showing a myofibroblastic line of differentiation and discussion of the differential diagnosis. Current Diagnostic Pathology. 2001;7:17-24. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V, Leroux A. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15:350-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 610] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 15. | Deyrup AT, Weiss SW. Grading of soft tissue sarcomas: the challenge of providing precise information in an imprecise world. Histopathology. 2006;48:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Fisher C. Myofibroblastic malignancies. Adv Anat Pathol. 2004;11:190-201. [PubMed] |