Published online Oct 14, 2017. doi: 10.3748/wjg.v23.i38.7037
Peer-review started: August 8, 2017
First decision: August 31, 2017
Revised: September 11, 2017
Accepted: September 19, 2017
Article in press: September 19, 2017
Published online: October 14, 2017
Processing time: 69 Days and 19.3 Hours
To determine whether hepatitis B virus (HBV)-testing could serve as a gateway to vaccinate non-immunized individuals in a low-prevalent country.
Non-immunized subjects participating in a multi-center, HBV-testing campaign in Paris, France were identified and contacted via telephone 3-9 mo after testing in order to determine vaccination status. Vaccination coverage was evaluated in per-protocol (for all respondents) and intent-to-treat analysis (assuming all non-responders did not vaccinate).
In total, 1215/4924 (24.7%) enrolled subjects with complete HBV serology were identified as non-immunized and eligible for analysis. There were 99/902 successfully contacted subjects who had initiated HBV vaccination after screening: per-protocol, 11.0% (95%CI: 9.0-13.2); intent-to-treat, 8.2% (95%CI: 6.7-9.8). In multivariable analysis, vaccination was more likely to be initiated in individuals originating from moderate or high HBV-endemic countries (P < 0.001), patients with limited healthcare coverage (P = 0.01) and men who have sex with men (P = 0.02). When asked about the reasons for not initiating HBV vaccination, the most frequent response was “will be vaccinated later” (33.4%), followed by “did not want to vaccinate” (29.8%), and “vaccination was not proposed by the physician” (21.5%). Sub-group analysis indicated a stark contrast in vaccination coverage across centers, ranging from 0%-56%.
HBV-vaccination after HBV screening was very low in this study, which appeared largely attributed to physician-patient motivation towards vaccination. Increased vaccination coverage might be achieved by emphasizing its need at the organizational level.
Core tip: Testing for hepatitis B virus (HBV) not only serves as a means to identify HBV-infected individuals, but also those who are non-immunized and could further benefit from HBV vaccination. In this mass HBV-screening study within the Paris metropolitan region, vaccine uptake was achieved in 11% of non-immunized patients and was deemed poor. Strategies to increase vaccination after testing need to be considered.
- Citation: Boyd A, Bottero J, Carrat F, Gozlan J, Rougier H, Girard PM, Lacombe K. Testing for hepatitis B virus alone does not increase vaccine coverage in non-immunized persons. World J Gastroenterol 2017; 23(38): 7037-7046
- URL: https://www.wjgnet.com/1007-9327/full/v23/i38/7037.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i38.7037
Hepatitis B virus (HBV) is one of the most frequent chronic infectious diseases in Western countries[1], with a prevalence of roughly one million in the United States[2] and 14 million in Europe[3]. The effectiveness of HBV vaccination in reducing infection incidence has been demonstrated in several countries with varying HBV prevalence[4-6], making it the single most effective tool for prevention. Nevertheless, coverage rates remain insufficient despite the wide availability of vaccines[3,7] and are particularly low in certain at-risk infection groups[8,9].
In France, the proportion of vaccinated individuals is surprisingly modest compared to other industrialized countries[10], even though vaccination is reimbursed in part by the national healthcare system. Recent epidemiological studies have estimated vaccine coverage at 50%[6], compared to 83% worldwide and higher than 90% in the Western Pacific region[1]. Prevalence of individuals with hepatitis B surface antigen (HBsAg) positive serology has remained exceedingly low over the past decade (0.7%)[11]. Nevertheless, 2500 persons are acutely infected each year, with the majority belonging to high-risk groups[12]. Vaccination campaigns targeted at these risk groups could help curb incident infections.
Accordingly, in current national guidelines, vaccination of non-immunized individuals who fall into these high-risk categories is strongly recommended[13]. Considering that HBV testing is necessary in determining those eligible for vaccination and HBV tests are given frequently in France (at almost 3.4 million tests per year), HBV screening could serve as a wide-reaching gateway to vaccination. Unfortunately, vaccination practices, specifically following HBV-testing, are poorly understood. The objective of this study was then to describe rates of vaccination among non-immunized individuals during a mass-screening program. We also intended to evaluate the reasons for not initiating HBV vaccination after testing.
Participants were selected from the multi-center OPTISCREEN-B study, which took place in the Paris, France metropolitan region from 2011-2012[14,15]. Briefly, the study was conducted in two phases. The first phase was a cross-sectional study aimed at evaluating several rapid test candidates that could detect serological markers commonly used in HBV screening. The second phase was a parallel-group, randomized trial aimed at comparing the use of standard HBV serological tests versus rapid tests (NCT01767597). Participant recruitment occurred at 10 primary healthcare centers, with various objectives related to HBV screening, vaccination, and care - three were sexually transmitted disease (STD) clinics, three were clinics with predominately general practitioners, three focused on immigrant health, and one on incarcerated individuals. The study procedures of both phases have been described in detail[14,15]. The study was approved by the Hôtel-Dieu Hospital Ethics Committee (Paris, France) in accordance with the Helsinki Declaration.
Volunteers were asked to participate during their regular consultations if ≥ 18 years old and could be available for further contact and medical follow-up at a single university teaching hospital. For both studies, only individuals eligible for HBV screening were invited to participate. Individuals were not included for the following reasons - for both phases: unwilling to participate; for Phase II only: already participated in the OPTISCREEN-B Phase I validation study or were not covered under the national healthcare system. Signed written informed consent was obtained for all eligible participants.
In this sub-study, we restricted our sample population to participants who were identified as non-immunized by complete HBV serological testing [i.e. HBsAg, anti-hepatitis B core (HBc) antibody, and anti-hepatitis B surface (HBs) antibody negative serology].
During the study visit, roughly 10 mL of blood was drawn and then tested for HBsAg, anti-HBs antibody, and anti-HBc antibody. Serostatus was determined using a commercially-available enzyme-linked immunoassay assay (MONOLISA AgHBs Ultra, anti-HBs plus, anti-hepatitis B core antibody-anti-HBc-plus; Bio-Rad, Hercules, California, United States of Americ). Results were provided 7-14 d after testing and, depending on the study center, were either mailed to the participants or left at the study center for collection.
Questionnaires were administered by a trained clinical research assistant during a face-to-face interview with the participant. Questions were asked in lay terms on a variety of sociodemographic characteristics, healthcare coverage, HBV transmission risk-factors, as well as other potential risk-factors. HBV-endemicity of birth country was established according to World Health Organization classification (prevalence of HBsAg-positive individuals): high (8%), intermediate (2%-8%), and low (2%).
Non-immunized individuals were identified by serology (HBsAg, anti-HBs antibody and anti-HBc antibody negative) and then contacted via telephone roughly 6-9 and 3-6 mo after testing in Phase I and II, respectively. If a participant could not be contacted after the third attempt, they were considered as having “no reply”. Subjects were asked whether or not they initiated immunization against HBV and at which dates their injection(s) were administered. As we conducted follow-up during a telephone interview, physical verification of the participants’ vaccination card was not performed. If they indicated no vaccination, they were asked to choose one or several reason(s) from a list for not initiating HBV immunization.
The primary outcome in per-protocol analysis was defined as the proportion of non-immunized participants initiating vaccination during follow-up who were able to be contacted. In intent-to-treat analysis, the outcome was defined as the proportion of non-immunized participants initiating vaccination assuming that those who were unable to be contacted did not initiate or complete vaccination. Clopper-Pearson 95% confidence intervals (CI) were calculated for these proportions.
In order to evaluate the determinants of HBV vaccination during follow-up (defined by per-protocol analysis), we used random-effects logistic regression to estimate univariate odds ratios (OR) of various risk-factors and their 95% CIs while accounting for within-center correlation. A multivariable model was then constructed in a backwards-stepwise fashion. A full model containing risk-factors with a P-value ≤ 0.10 in univariable analysis was initially selected and covariates with a P-value > 0.10, as tested using a likelihood ratio test, were sequentially excluded from the model.
All statistical analysis was performed using STATA (v13.1, College Station, TX, United States of America) and reviewed by two biomedical statisticians. Significance was determined using a P-value < 0.05.
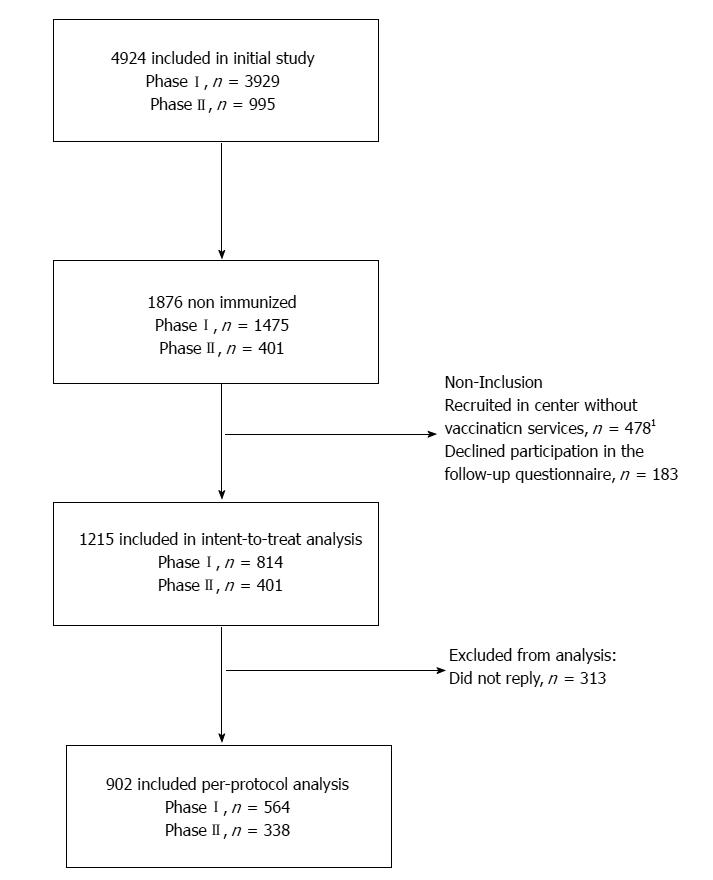
The flow of study participants is represented in Figure 1. A total of 4924 participants were included in the OPTISCREEN-B study, 1876 (38.1%) of whom were non-immunized and considered for the present sub-study. Of them, 661 were not included because they sought care at a center where, at the time of Phase I, did not offer vaccination services (n = 478) or declined participation in the follow-up questionnaire (n = 183). In total, 1215 participants were included in analysis.
Table 1 provides a summary of the study population. The majority of individuals was male, coming from a country of low HBV-prevalence, and covered under the French national healthcare system. Included participants were born in the following geographical regions: West/East Europe, n = 710 (58.4%); Mediterranean, n = 183 (15.1%); Asia, n = 90 (7.4%); Sub-Saharan Africa, n = 135 (11.1%); South America, n = 43 (3.5%); other, n = 54 (4.4%). A total of 1043 (85.8%) of participants were indicated for vaccination according to French recommendations, the most frequent reasons for which were multiple lifetime sexual partners, originating from a moderate/high HBV-endemic country and traveling to high endemic countries. A smaller proportion was men who had sex with men (MSM) or partook in nasal or intravenous drug-use. Characteristics of study participants were compered between enrollment phases in Supplementary Table 1.
| Total (n = 1215) | Follow-up contact | P value | ||
| Reply (n = 902) | No reply (n = 313) | |||
| Male | 702 (57.8) | 511 (56.7) | 191 (61.0) | 0.18 |
| Age (yr), mean ± SD | 36 ± 15 | 37 ± 15 | 34 ± 14 | 0.02 |
| HBV prevalence of birth country | < 0.001 | |||
| Low (< 2.0%) | 670 (55.1) | 527 (58.4) | 143 (45.7) | |
| Intermediate (2.0%-8.0%) | 331 (27.2) | 222 (24.6) | 109 (34.8) | |
| High (> 8.0%) | 214 (17.6) | 153 (17.0) | 61 (19.5) | |
| Parents from high endemic region | 276 (22.7) | 205 (22.7) | 71 (22.7) | 0.9 |
| Traveled to high endemic region1 | 284 (23.4) | 204 (22.6) | 80 (25.6) | 0.3 |
| Healthcare in high endemic region | 177 (14.6) | 124 (13.8) | 53 (16.9) | 0.17 |
| Health insurance plan | < 0.001 | |||
| Social security/CMU2 | 913 (75.2) | 719 (79.7) | 194 (62.0) | |
| CMUc3 | 61 (5.0) | 47 (5.2) | 14 (4.5) | |
| AME4/Other/ None | 241 (19.8) | 136 (15.1) | 105 (33.6) | |
| Received transfusion | 48 (4.0) | 33 (3.7) | 15 (4.8) | 0.4 |
| Received tattoos | 151 (12.4) | 100 (11.1) | 51 (16.3) | 0.02 |
| Received piercing | 510 (42.0) | 387 (42.9) | 123 (39.3) | 0.3 |
| Close contact with HBV+ individual | 75 (6.2) | 57 (6.4) | 18 (5.8) | 0.7 |
| Number of life-time sexual partners | 0.001 | |||
| 0-1 | 181 (14.9) | 118 (13.1) | 63 (20.1) | |
| 2-9 | 560 (46.1) | 409 (45.3) | 151 (48.2) | |
| ≥ 10 | 474 (39.0) | 375 (41.6) | 99 (31.6) | |
| > 1 sexual partner within 12 mo | 593 (48.8) | 447 (49.6) | 146 (46.7) | 0.4 |
| Men who have sex with men | 93 (8.9) | 73 (8.1) | 20 (6.4) | 0.3 |
| Nasal drug-use | 145 (11.9) | 108 (12.0) | 37 (11.8) | 0.9 |
| Intravenous drug-use | 5 (0.4) | 5 (0.6) | 0 | 0.3 |
| Long-term stay at a medical center | 50 (4.1) | 38 (4.2) | 12 (3.8) | 0.8 |
| Previously incarcerated | 87 (7.2) | 63 (7.0) | 24 (7.7) | 0.7 |
Among the 1215 participants included in analysis, 99 (8.2%) claimed to have initiated HBV vaccination, while the first injection was given a median 31 d (IQR = 12-55) after testing; 803 (66.1%) did not initiate vaccination; and 313 (25.7%) did not respond after the third contact attempt. Participants who did not respond were significantly younger (P = 0.02), more likely to come from an intermediate HBV-endemic country (P < 0.001), did not have any healthcare coverage (P < 0.001), and had a lower number of lifetime sexual partners (P = 0.001) (Table 1).
In per-protocol analysis (Table 2), immunization coverage was 11.0% (95%CI 9.0-13.2) during follow-up. This proportion varied substantially across centers (0%-55.6%), with the lowest vaccine coverage observed in STD clinics (7.5%) and centers with mainly general practitioners (6.3%) and the highest rates in immigrant-health clinics (46.8%). Assuming those with no reply did not initiate HBV vaccination (intent-to-treat analysis), immunization coverage was 8.2% (95%CI 6.7-9.8), also with high between-center variability (Table 2).
| n | No reply n (%) | Immunization coverage | |||
| Vaccinatedn3 | Per protocol1(%) | ITT2(%) | |||
| Total | 1215 | 313 (25.8) | 99 | 11 | 8.2 |
| General practitioner | 418 | 85 (20.3) | 21 | 6.3 | 5 |
| Policlinique Saint Antoine | 165 | 47 (28.5) | 10 | 8.6 | 6.1 |
| Travel clinic Saint Antoine | 31 | 5 (16.1) | 3 | 11.5 | 10 |
| CPAM | 222 | 33 (14.9) | 8 | 4.2 | 3.6 |
| STD clinics | 635 | 166 (26.1) | 35 | 7.5 | 5.5 |
| CDAG Saint Antoine | 51 | 10 (19.6) | 0 | 0 | 0 |
| CDAG Figuier | 275 | 78 (28.4) | 22 | 11.2 | 8 |
| CDAG Belleville | 309 | 78 (25.2) | 13 | 5.6 | 4.2 |
| Immigrant-health clinics | 140 | 61 (43.6) | 37 | 46.8 | 26.4 |
| Croix-Rouge Moulin Joly | 9 | 0 (0) | 5 | 55.6 | 55.6 |
| CRAMIF | 18 | 0 (0) | 9 | 50 | 50 |
| Médecin du Monde | 113 | 61 (54.0) | 23 | 44.2 | 20.4 |
| Prison (UCSA Paris) | 22 | 0 (0) | 6 | 27.3 | 27.3 |
Table 3 describes the per-protocol analysis of risk-factors associated with HBV vaccination. Of note, there was no association between vaccination and study phase (Phase II versus I, OR = 0.66, 95%CI 0.27-1.62, P = 0.4). In multivariable analysis, vaccination was more likely to be initiated in MSM (P = 0.02), participants born in intermediate/high endemic zones (P < 0.001) and those without national healthcare coverage (P = 0.006). When replacing gender with MSM in the multivariable model, there was no significant difference between males and females (adjusted - OR = 0.66, 95%CI 0.40-1.10).
| Risk-factor | Univariate | Multivariable5 | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Female vs male | 0.61 (0.37-0.99) | 0.04 | ||
| Age (per yr) | 1.00 (0.98-1.02) | 0.8 | ||
| Parents from high endemic region | 1.52 (0.89-2.58) | 0.12 | ||
| Traveled to high endemic region1 | 1.43 (0.83-2.45) | 0.19 | ||
| Care in high endemic region | 1.45 (0.79-2.67) | 0.2 | ||
| Transfusion | 0.28 (0.03-2.28) | 0.2 | ||
| Tattoos | 1.26 (0.63-2.55) | 0.5 | ||
| Piercing | 1.05 (0.66-1.67) | 0.8 | ||
| Close contact with HBV+ individual | 1.14 (0.48-2.70) | 0.8 | ||
| Men who have sex with men | 2.05 (0.97-4.34) | 0.06 | 2.53 (1.14-5.60) | 0.02 |
| Nasal drug-use | 0.37 (0.13-1.07) | 0.07 | 0.41 (0.14-1.21) | 0.1 |
| Intravenous drug-use | 3.42 (0.41-28.40) | 0.3 | ||
| Long-term stay at a medical center | 1.02 (0.33-3.14) | 0.9 | ||
| Previously incarcerated | 0.71 (0.24-2.06) | 0.5 | ||
| HBV-prevalence of birth region | ||||
| Low (< 2.0%) | 1 | 1 | ||
| Intermediate (2.0%-8.0%) | 4.39 (2.35-8.19) | < 0.001 | 3.58 (1.85-6.95) | < 0.001 |
| High (> 8.0%) | 4.43 (2.24-8.75) | < 0.001 | 3.44 (1.65-7.15) | 0.001 |
| Health insurance plan | ||||
| Social security or CMU2 | 1 | 1 | ||
| CMUc3 | 1.13 (0.35-3.62) | 0.8 | 0.76 (0.23-2.58) | 0.7 |
| AME4, Other, or none | 4.54 (2.21-9.30) | < 0.001 | 2.60 (1.23-5.52) | 0.01 |
| Number of life-time sexual partners | ||||
| 0-1 | 1 | |||
| 2-9 | 0.93 (0.47-1.87) | 0.8 | ||
| ≥ 10 | 0.75 (0.35-1.60) | 0.5 | ||
In the 803 non-vaccinees who were able to be contacted, Table 4 summarizes their reasons for not vaccinating. Roughly one-third stated that they would vaccinate at a later time. Other reasons included no desire to vaccinate (29.8%), vaccination was not suggested by their physician (21.5%), or the participant did not return to obtain their serological results confirming non-immunized status (14.0%). Participants who preferred not to vaccinate were significantly more likely to be male (P = 0.002), older (P < 0.001), come from a low endemic country (P < 0.001), be covered under the French national healthcare system (P < 0.001), and have higher number of lifetime sexual partners (P = 0.004) (Supplementary Table 2).
| Reason | n (%) |
| Serology results were never obtained | 112 (14.0) |
| Vaccination was not proposed by physician | 173 (21.5) |
| Vaccination was not indicated (according to physician) | 26 (3.2) |
| Not at risk | 14 (1.7) |
| Vaccination was already done1 | 12 (1.5) |
| Participant did not want to be vaccinated | 239 (29.8) |
| Not perceived to be at risk | 95 (11.8) |
| Vaccination was already done | 21 (2.6) |
| Not receptive to vaccinations in general | 44 (5.5) |
| Not receptive to HBV vaccination | 89 (11.1) |
| Will be vaccinated later | 268 (33.4) |
HBV testing has been proposed as a useful gateway to identify non-immunized individuals and encourage them to initiate vaccination[16], yet data regarding the extent of how successful this strategy would be, if applied, are sparse. Previous studies in France and California have shown that vaccine coverage ranges from 8.6%-11.2% after testing for HBV in the context of STD clinics[17,18]; however, these results were obtained from mostly younger, sexually-active individuals or more specifically, among adults diagnosed with chlamydia, gonorrhoae and/or syphilis who were still susceptible to HBV infection. In the OPTISCREEN-B studies, serving as a testing campaign against HBV infection in a population with diverse at-risk characteristics, we similarly observed a low vaccination rate of 11.0% in per-protocol and 8.2% in intent-to-treat analysis.
The reasons for low vaccine coverage are somewhat difficult to pinpoint. While examining individual characteristics, it was interesting to find that those originating from regions of moderate or high HBV endemicity and those in difficult socioeconomic situations, based on their healthcare plan, were significantly more likely to initiate HBV vaccination after testing. These data are relieving considering that these two risk-factor groups constitute the largest number of newly identified infections in France[12]. However, it should be mentioned that these groups also had the highest proportions of non-response, particularly individuals without healthcare or from intermediate HBV-endemic countries. MSM were also observed to have a significantly higher vaccination initiation rates, while this key population has shown rather high vaccine uptake in previous intervention studies[19,20]. Unfortunately, two groups with high HBV-infection risk, namely persons having close contact with an infected individual or engaging in nasal or intravenous drug use, were not more likely to become vaccinated.
When addressing the reasons for not vaccinating, approximately one-third responded that they did not want to be vaccinated, mainly because they were not receptive to HBV vaccination or not perceived to be at risk. Vaccine hesitancy has already been illustrated in a study from a French STD clinic, where most non-immunized persons did not trust the safety of HBV vaccines or viewed it as “not well characterized”[21]. We also observed that individuals covered under the French national healthcare plan or those coming from a low HBV-endemic country were more likely not to vaccinate. Their lack of motivation could stem from a lower perception of HBV-transmission risk, which has been previously evidenced in persons with higher socioeconomic status in France[22].
Another one-fifth of non-vaccinated individuals claimed that their physician did not propose vaccination. This result is rather surprising given that professionals have been recommended to initiate vaccination for all non-immunized persons susceptible to transmission since 1995. Few (3.2%) participants stated that they were not indicated for vaccination by their physician, implying that recommendation criteria did not play a major role in the low vaccine coverage observed with this group. One striking feature of our data, however, was that post-testing vaccination rates varied from 0% to 56% across study centers, largely implicating organizational factors in promoting HBV vaccination.
These results support the need for structural reorganization in testing facilities as a means to encourage vaccination. Promising data on interventions at the organization level have been presented in other research, in which increased vaccination rates were achieved after providing focused training to healthcare professionals and individuals seeking testing[23] or when free vaccinations were offered directly after HBV serological results were returned[17]. Other interventions involving financial incentives have also shown substantial effects in at-risk populations, such as intravenous drug-users[24,25]. These strategies would certainly be helpful in counterbalancing vaccine hesitancy, which has been an emerging topic over the past decades[10], notably among health-care providers in general and in France[26-28]. Without these interventions, the proportion of individuals at risk of HBV-infection could rebound and persist as a public health issue[6].
Nevertheless, many of the participants in this analysis came from difficult socioeconomic backgrounds. Past research has underscored the lack of access to preventative health services especially among migrants[29], certain racial/ethnic groups[30], and homeless individuals[31]. Since the majority of vaccination occurs within the context of these services, social inclusion of disadvantaged groups, particularly at primary care centers, would probably be necessary in order to achieve greater vaccine outreach. Although the French healthcare system attempts at addressing care in these groups[32], further improvements could be made with other strategies, such as mobile clinics providing vaccinations[33] or culturally-adapted text message reminders[34]. The services provided at these healthcare centers should also emphasize education on HBV disease and the benefits of vaccination, possibly in combination with care related to other health issues.
There were several noteworthy parallels with the results from this study compared to previous analyses in the OPTISCREEN-B program. The profile of participants accepting vaccination (i.e. from regions of moderate to high HBV endemicity, without health care coverage) were also those who more frequently tested for HBV in the past[35], which are perhaps indicators of effective prevention efforts in these at-risk groups. In addition, the between-center variability in the proportion receiving vaccination mirrored that of the proportion of physicians who would propose HBV testing[35]. The similar variation offers evidence that any organizational changes to promoting vaccination would have to include a more comprehensive HBV prevention strategy. Finally, our study group has observed in a past evaluation that roughly 20%-50% participants newly diagnosed with HBV returned for further consultation at a referent center[15], reflecting the potential for inadequate transition to “appropriate care”[36]. The increased need to foster individuals into appropriate care appears to be shared among non-immunized patients.
Several methodological limitations need to be noted when interpreting the results of our study. First, we used a convenience sample combining participants from two studies. Certain risk-factor groups, such as intravenous drug-users and sex-workers, might not be adequately represented and could have different vaccine coverage than those reported herein. In addition, the lack in numbers may have resulted in inadequate power in order to establish whether they were less likely to complete vaccination. Second, the time-frame of follow-up was relatively short and perhaps some participants received vaccination 6-9 mo or more after their interview. Non-immunized patients did vaccinate a median 30 d, suggesting that few individuals would have initiated vaccination after the end of follow-up and vaccine coverage would have been similar if follow-up were prolonged. Nevertheless, roughly one-third of unvaccinated individuals stated that they would do so in the future. Third, the short time-frame also made it impossible to ascertain whether participants completed HBV vaccination, as the date of last vaccination would have fallen outside the follow-up period. Lastly, the proportion with no reply, which was comparable to other studies[17], could have biased results.
Furthermore, we should highlight that individuals with low levels of anti-HBs antibodies (> 0-10 mIU/mL), who could possibly benefit from booster HBV vaccination, were not included in this analysis. The results from our study might not be generalizable to these individuals.
In conclusion, HBV vaccination after screening was very low in this study, implying that HBV testing alone cannot encourage non-immunized individuals to vaccinate. The fact that large discrepancies in vaccine coverage were observed across centers and that both physician and participant motivation towards vaccination was rather limited provides evidence that involvement at the health structure level is crucial in increasing vaccination coverage. Future interventions and campaigns should work at increasing local awareness of low vaccine coverage rates, especially in groups with poor access to health services, and the need to vaccinate non-immunized individuals as soon as they are identified.
Testing campaigns for hepatitis B virus (HBV) have often focused on identifying infected patients [i.e. those with hepatitis B surface antigen (HBsAg)-positive serology] and increasing disease-status awareness. Few evaluations have been performed on how testing campaigns can impact vaccination uptake after receiving non-immunized status.
Since France has a low proportion of vaccinated individuals compared to other Western countries, interventions to increase HBV vaccination rates need to be evaluated in this population. This analysis addressed vaccination initiation among non-immunized participants in a large-scale screening campaign within the Paris metropolitan region. These individuals reflect a wide range of groups at-risk of HBV infection, allowing potential identification of subgroups with low vaccination coverage.
We aimed to describe rates of vaccination among non-immunized individuals during a mass-screening program. We also intended to evaluate the reasons for not initiating HBV vaccination after testing. This allows a first-hand account of why individuals do not vaccinate and helps tailor the needs for future intervention campaigns.
Participants were recruited from two large phases of a multi-center, HBV-testing campaign in Paris, France. Non-immunized subjects were identified and contacted via telephone 3-9 mo after testing in order to determine whether they initiated vaccination. We considered vaccination coverage of all respondents (in a per-protocol analysis) and the overall non-immunized study population while assuming no vaccination in non-responders (in an intent-to-treat analysis).
Overall vaccination uptake was low with 11% of respondents declaring HBV vaccination initiation within 3-6 mo of testing. Few risk-factors for increased vaccination initiation were identified: from moderate or high HBV-endemic countries, with limited healthcare coverage, and men who have sex with men. Compelling differences were observed between centers with vaccination coverage ranging from 0%-56%.
Given the low vaccine uptake in individuals at potential risk of HBV-infection, there is a major concern in the cascade of care among non-immunized individuals. The contrasting vaccination rates between centers indicate that the challenge to increase vaccination initiation lies within center-specific practices. At the individual level, increasing motivation to vaccinate among physicians and non-immunized persons alike should be stressed.
We wish to thank all study participants as well as all medical and paramedical centers participating in the study, Tabassome Simon and nurses from the CRC-Est, and the data management center, especially Frederic Chau, Frederic Fotre, Sylvain Bitschine, Isabelle Goderel and Gregory Pannetier. We also acknowledge Samia Hicham, Judith Leblanc, Manuela Sébire-Le Cam, Farid Djoumad, Julie Lamarque, and Christelle Pauleau for their extraordinary effort in data collection.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Chen XX, Guo YM, Watson DI S- Editor: Chen K
L- Editor: A E- Editor: Ma YJ
| 1. | World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis[J]. 2016;. |
| 2. | Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW; Centers for Disease Control and Prevention (CDC). Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20. [PubMed] |
| 3. | Hatzakis A, Wait S, Bruix J, Buti M, Carballo M, Cavaleri M, Colombo M, Delarocque-Astagneau E, Dusheiko G, Esmat G. The state of hepatitis B and C in Europe: report from the hepatitis B and C summit conference*. J Viral Hepat. 2011;18 Suppl 1:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Ni YH, Huang LM, Chang MH, Yen CJ, Lu CY, You SL, Kao JH, Lin YC, Chen HL, Hsu HY. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology. 2007;132:1287-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 246] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1-31. [PubMed] |
| 6. | Ramière C, Roche L, Scholtès C, Iwaz J, Saison J, Ecochard R, André P. Evolution of the incidence of hepatitis B virus infection and immunization rates in a large French cohort born between 1960 and 1994. Clin Microbiol Infect. 2016;22:889.e1-889.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Fonteneau L, Urcun JM, Guthmann JP, Collet M, Neulat N, Bristol-Gauzy P, Guignon N, Lévy-Bruhl D, Herbet JB. [Vaccination coverage in 6-year-old preschool children, France, 2005-2006]. Arch Pediatr. 2013;20:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Beck CR, Cloke R, O’Moore É, Puleston R. Hepatitis B vaccination coverage and uptake in prisons across England and Wales 2003-2010: a retrospective ecological study. Vaccine. 2012;30:1965-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Hoover KW, Butler M, Workowski KA, Follansbee S, Gratzer B, Hare CB, Johnston B, Theodore JL, Tao G, Smith BD. Low rates of hepatitis screening and vaccination of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2012;39:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine. 2014;32:2150-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1663] [Cited by in RCA: 1329] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 11. | Caisse nationale de l’assurance maladie des travailleurs salariés (France), Meffre C, Centre technique d’appui et de formation des centres d’examens de santé (France), Institut de veille sanitaire (France). Prévalence des hépatites B et C en France en 2004. Saint-Maurice: Institut de veille sanitaire; 2007; . |
| 12. | Antona D, Letort M-J, Lévy-Bruhl D. Estimation du nombre annuel de nouvelles infections par le virus de l’hépatite B en France, 2004-2007. Bull Epidemiol Hebd. 2009;20-21:196-199. |
| 13. | Institut de veille sanitaire. Calendrier des vaccinations et recommandations vaccinales 2016. Bull Epidemiol Hebd. 2016;Hors-série. |
| 14. | Bottero J, Boyd A, Gozlan J, Lemoine M, Carrat F, Collignon A, Boo N, Dhotte P, Varsat B, Muller G. Performance of rapid tests for detection of HBsAg and anti-HBsAb in a large cohort, France. J Hepatol. 2013;58:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Bottero J, Boyd A, Gozlan J, Carrat F, Lemoine M, Rougier H, Varsat B, Boo N, Charlois-Ou C, Collignon A. Effectiveness of hepatitis B rapid tests toward linkage-to-care: results of a randomized, multicenter study. Eur J Gastroenterol Hepatol. 2016;28:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Bottero J, Brouard C, Roudot-Thoraval F, Deuffic-Burban S, Hofliger P, Abergel A, Volant J, Dhumeaux D, Yazdanpanah Y; Viral Hepatitis Testing Experts group. 2014 French guidelines for hepatitis B and C screening: a combined targeted and mass testing strategy of chronic viruses namely HBV, HCV and HIV. Liver Int. 2016;36:1442-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Launay O, Le Strat Y, Tosini W, Kara L, Quelet S, Lévy S, Danan J, Réveillon J, Houdayer J, Bouvet E. Impact of free on-site vaccine and/or healthcare workers training on hepatitis B vaccination acceptability in high-risk subjects: a pre-post cluster randomized study. Clin Microbiol Infect. 2014;20:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Hechter RC, Jacobsen SJ, Luo Y, Nomura JH, Towner WJ, Tartof SY, Tseng HF. Hepatitis B testing and vaccination among adults with sexually transmitted infections in a large managed care organization. Clin Infect Dis. 2014;58:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | van Rijckevorsel G, Whelan J, Kretzschmar M, Siedenburg E, Sonder G, Geskus R, Coutinho R, van den Hoek A. Targeted vaccination programme successful in reducing acute hepatitis B in men having sex with men in Amsterdam, the Netherlands. J Hepatol. 2013;59:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Chonwattana W, Raengsakulrach B, Holtz TH, Wasinrapee P, Tongtoyai J, Chaikummao S, Pattanasin S, McNicholl JM, van Griensven F, Curlin ME. Hepatitis B vaccination uptake and correlates of serologic response among HIV-infected and uninfected men who have sex with men (MSM) in Bangkok, Thailand. Vaccine. 2016;34:2044-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Moyroud L, Hustache S, Goirand L, Hauzanneau M, Epaulard O. Negative perceptions of hepatitis B vaccination among attendees of an urban free testing center for sexually transmitted infections in France. Hum Vaccin Immunother. 2017;13:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Brouard C, Gautier A, Saboni L, Jestin C, Semaille C, Beltzer N; KABP France group. Hepatitis B knowledge, perceptions and practices in the French general population: the room for improvement. BMC Public Health. 2013;13:576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Zacharias T, Wang W, Dao D, Wojciechowski H, Lee WM, Do S, Singal AG. HBV Outreach Programs Significantly Increase Knowledge and Vaccination Rates Among Asian Pacific Islanders. J Community Health. 2015;40:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Weaver T, Metrebian N, Hellier J, Pilling S, Charles V, Little N, Poovendran D, Mitcheson L, Ryan F, Bowden-Jones O. Use of contingency management incentives to improve completion of hepatitis B vaccination in people undergoing treatment for heroin dependence: a cluster randomised trial. Lancet. 2014;384:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Topp L, Day CA, Wand H, Deacon RM, van Beek I, Haber PS, Shanahan M, Rodgers C, Maher L; Hepatitis Acceptability and Vaccine Incentives Trial (HAVIT) Study Group. A randomised controlled trial of financial incentives to increase hepatitis B vaccination completion among people who inject drugs in Australia. Prev Med. 2013;57:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 562] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 27. | Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The State of Vaccine Confidence 2016: Global Insights Through a 67-Country Survey. EBioMedicine. 2016;12:295-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 691] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 28. | Verger P, Collange F, Fressard L, Bocquier A, Gautier A, Pulcini C, Raude J, Peretti-Watel P. Prevalence and correlates of vaccine hesitancy among general practitioners: a cross-sectional telephone survey in France, April to July 2014. Euro Surveill. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Rosano A, Dauvrin M, Buttigieg SC, Ronda E, Tafforeau J, Dias S. Migrant’s access to preventive health services in five EU countries. BMC Health Serv Res. 2017;17:588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Kim HS, Rotundo L, Yang JD, Kim D, Kothari N, Feurdean M, Ruhl C, Unalp-Arida A. Racial/ethnic disparities in the prevalence and awareness of Hepatitis B virus infection and immunity in the United States. J Viral Hepat. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | van Laere IR, de Wit MA, Klazinga NS. Pathways into homelessness: recently homeless adults problems and service use before and after becoming homeless in Amsterdam. BMC Public Health. 2009;9:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Nay O, Béjean S, Benamouzig D, Bergeron H, Castel P, Ventelou B. Achieving universal health coverage in France: policy reforms and the challenge of inequalities. Lancet. 2016;387:2236-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Whelan C, Chambers C, Chan M, Thomas S, Ramos G, Hwang SW. Why do homeless people use a mobile health unit in a country with universal health care? J Prim Care Community Health. 2010;1:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Phillips AL, Kumar D, Patel S, Arya M. Using text messages to improve patient-doctor communication among racial and ethnic minority adults: an innovative solution to increase influenza vaccinations. Prev Med. 2014;69:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Bottero J, Boyd A, Lemoine M, Carrat F, Gozlan J, Collignon A, Boo N, Dhotte P, Varsat B, Muller G. Current state of and needs for hepatitis B screening: results of a large screening study in a low-prevalent, metropolitan region. PLoS One. 2014;9:e92266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Bottero J, Boyd A, Gozlan J, Carrat F, Nau J, Pauti MD, Rougier H, Girard PM, Lacombe K. Simultaneous Human Immunodeficiency Virus-Hepatitis B-Hepatitis C Point-of-Care Tests Improve Outcomes in Linkage-to-Care: Results of a Randomized Control Trial in Persons Without Healthcare Coverage. Open Forum Infect Dis. 2015;2:ofv162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |