Published online Oct 14, 2017. doi: 10.3748/wjg.v23.i38.7016
Peer-review started: July 27, 2017
First decision: August 10, 2017
Revised: August 24, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: October 14, 2017
Processing time: 84 Days and 22.7 Hours
To evaluate the risk factors for postoperative recurrence after primary bowel resection in a cohort of Korean Crohn’s disease (CD) patients.
This study included 260 patients with no history of previous bowel surgery who underwent primary surgery for CD between January 2000 and December 2010 at Asan Medical Center (Seoul, South Korea). The median follow-up period was 101 mo.
During the follow-up period, 66 patients (25.4%) underwent a second operation for disease recurrence. At 1, 5 and 10 years after the first operation, the cumulative rate of surgical recurrence was 1.1%, 8.3% and 35.9% and clinical recurrence occurred in 1.2%, 23.6% and 68.1%, respectively. In multivariate analysis, undergoing an emergency operation was a significant risk factor for surgical recurrence-free survival (SRFS) [HR = 2.431, 95%CI: 1.394-4.240, P = 0.002], as were the presence of perianal disease after the first operation (HR = 1.715, 95%CI: 1.005-2.926, P = 0.048) and history of smoking (HR = 1.798, 95%CI: 1.088-2.969, P = 0.022). The postoperative use of anti-tumor necrosis factor (TNF) agents reduced SRFS risk (HR = 0.521, 95%CI: 0.300-0.904, P = 0.02).
History of smoking, postoperative perianal disease and undergoing an emergency operation were independent risk factors for surgical recurrence. Using anti-TNF agents may reduce surgical recurrence.
Core tip: Our study demonstrated the clinical feature and long-term prognosis of Crohn’s disease (CD) in a large cohort of non-Caucasian patients. We investigated risk factors for the postoperative recurrence of CD in a patient population from a single tertiary referral center. History of smoking, postoperative perianal disease and undergoing an emergency operation were found to be independent risk factors for surgical recurrence in CD. Using anti-TNF agents for these patients may reduce surgical recurrence. These findings indicate that affected patients with CD may benefit from close postoperative surveillance and probably from the early administration of anti-TNF agents.
- Citation: Yang KM, Yu CS, Lee JL, Kim CW, Yoon YS, Park IJ, Lim SB, Park SH, Ye BD, Yang SK, Kim JC. Risk factors for postoperative recurrence after primary bowel resection in patients with Crohn’s disease. World J Gastroenterol 2017; 23(38): 7016-7024
- URL: https://www.wjgnet.com/1007-9327/full/v23/i38/7016.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i38.7016
Crohn’s disease (CD) is a chronic inflammatory gastrointestinal disease with a heterogeneous disease course. Some patients experience low morbidity, whereas others need repeated operations[1]. Conservative medical treatment is regarded as the principal therapeutic approach to treat patients with CD, but the lifetime risk of surgical resection for patients with CD can be 70% to 80%[2,3]. Approximately 10% to 30% of patients who undergo an operation experience clinical recurrence during the first postoperative year, and more than 60% experience during the first postoperative decade. Surgical recurrence rates at 5 and 10 years have been reported as 20% to 25% and 34% to 49%, respectively[4,5]. Thus, to provide optimal patient care, it is important to be able to predict which patients have a higher risk of recurrence.
A range of possible risk factors thought to impact the surgical recurrence rate have been investigated: age at diagnosis, sex, smoking behavior, duration of disease before surgery, family history, behavior of disease (perforating or non-perforating disease), history of medication (use of 5-aminosalicylic acid, corticosteroids, immunomodulators or anti-tumor necrosis factor (anti-TNF) agents), priority of operation (emergency or elective), presence of granulomas in the resected bowel specimen, length of resected bowel, technique of anastomosis used in the surgery and postoperative complications[6,7]. However, such studies on risk factors have largely been inconclusive owing to different patient populations and clinical manifestations.
In this study, we investigated risk factors for the postoperative recurrence of CD, as determined by the need for an additional operation, in a patient population from a single tertiary care center.
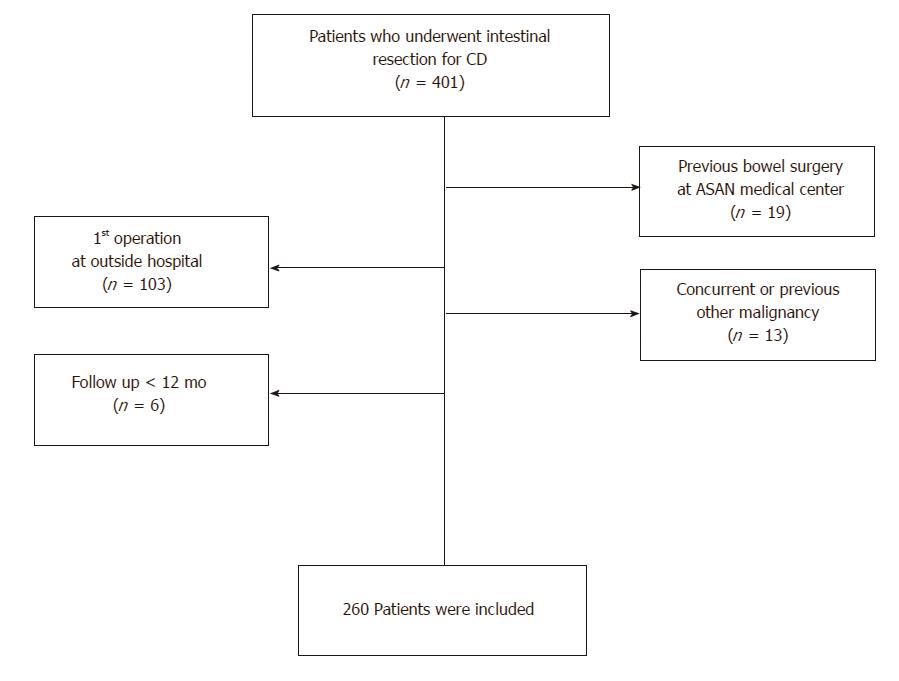
Patients who underwent intestinal resection for CD at Asan Medical Center (Seoul, Korea) between January 2000 and December 2010 were enrolled in this retrospective study. A total of 401 patients underwent intestinal resection for pathologically confirmed CD during the study period. Exclusion criteria included a history of previous bowel surgery, such as small or large bowel resection or strictureplasty, a concurrent or previous history of other malignancies, and patients who have had follow up for a short period of time (< 12 mo). A total of 260 patients were included in our analysis (Figure 1). The following variables were retrospectively reviewed from the prospectively collected database for these patients: demographics, preoperative disease characteristics, disease phenotype (Montreal classification)[8], any history of perianal disease (e.g., an abscess or fistula), the presence of extraintestinal manifestations, a history of smoking, comorbidity, the operative approach (open or laparoscopy), operative indications (perforating or non-perforating), the rate of stoma formation, the reoperation rate, the duration of follow-up, postoperative complications and regimens of pre- and post-medical treatment.
The study protocol was approved by the institutional review board of Asan Medical Center (registration no: 2016-1139), in accordance with the Declaration of Helsinki.
CD distribution was assessed based on pathological and intraoperative findings, radiologic imaging using a small bowel series (SBS), abdomen and pelvis computed tomography (CT), CT enterography, magnetic resonance imaging (MRI) enterography and colonofiberscopy (CFS). Perianal disease included stricture, fistula or an abscess in the perianal area, anal canal or perirectal space at any time during the course of CD. After primary intestinal resection, reassessment was performed by CT enterography, MRI enterography or CFS after 6 to 12 mo. Surgical recurrence was defined as a repeated operation for pathologically confirmed CD on any part of the bowel or a repeated operation for pathologically confirmed anastomotic disease, including instances where the small bowel was diseased at the anastomosis or the stoma site. Clinical recurrence was defined as the recurrence of CD-related symptoms, confirmed by objective signs of radiology or endoscopy findings. As this was a retrospective study the Crohn’s disease Activity Index (CDAI) and Rutgeerts’ score were not available.
Our medical treatment policy for treating CD was based on a step-up approach, with more potent therapies added if patients became unresponsive to first-line or less toxic agents, as described in a previous report from our institution[9]. The use of anti-TNF agents in this study was less frequent than in Western studies because of the strict Korean government health insurance reimbursement policy during our study period. During this period, two anti-TNF agents (infliximab and adalimumab) were available in Korea.
Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. Multivariate analyses for survival that included the variables that showed statistical significance in the log-rank test were performed using Cox proportional hazards model with a 95%CI to assess the risk factors associated with surgical recurrence-free survival (SRFS) and clinical recurrence-free survival (CRFS). Statistical significance was defined as P < 0.05; all analyses were performed using SPSS software version 21 (IBM Corp., Armonk, NY).
The clinicopathological characteristics of the patients are summarised in Tables 1 and 2. The median follow-up time for the 260 patients was 101 mo (range, 12-193 mo). The interval between the first intestinal resection and clinical recurrence was 64 mo (range, 12-148 mo) and the interval between the first intestinal resection and surgical recurrence was 77.5 mo (range, 15-149 mo).
| Variables | Number of patients |
| (n = 260) | |
| SEX | |
| Male | 187 (71.9) |
| Female | 73 (28.1) |
| Follow-up period, month | 101 (12-193) |
| Age at diagnosis | 23 (12-74) |
| Age at 1st operation | 28 (15-74) |
| Age at 2nd operation | 34 (22-59) |
| Montreal classification | |
| Age at diagnosis, yr | |
| A1 ( ≤ 16) | 25 (9.6) |
| A2 (17-40) | 215 (82.7) |
| A3 ( > 40) | 20 (7.7) |
| Location | |
| L1 (Terminal ileum) | 113 (43.5) |
| L2 (Colon) | 38 (14.6) |
| L3 (Ileocolon) | 107 (41.2) |
| L4 (Isolated upper disease) | 2 (0.8) |
| Behavior | |
| B1 (Inflammatory) | 6 (2.3) |
| B2 (Stricturing) | 80 (30.8) |
| B3 (Penetrating) | 174 (66.9) |
| Family history (Crohn's disease) | 7 (2.7) |
| Presence of extraintestinal manifestation | 20 (7.7) |
| Perianal disease | |
| at diagnosis | 122 (46.9) |
| after 1st operation | 62 (23.8) |
| History of smoking | 70 (26.9) |
| Current smoker | 12 (4.6) |
| Ex-smoker | 58 (22.3) |
| Non-smoker | 190 (73.1) |
| Preoperative use of medication | |
| Anti-TNF | 27 (10.4) |
| 5-ASA | 223 (85.8) |
| Steroid | 147 (56.5) |
| Immunomodulator1 | 128 (49.2) |
| Antibiotics2 | 128 (49.2) |
| Malignancy | 163 (62.7) |
| Colorectal cancer | 3 (1.2) |
| Lung cancer | 1 (0.4) |
| Variables | Number of patients |
| (n = 266) | |
| Operative approach | |
| Open | 215 (82.7) |
| Laparoscopy | 45 (17.3) |
| Type of operation | |
| Elective | 210 (80.8) |
| Emergency | 50 (19.2) |
| Indication for 1st operation | |
| Perforating | 167 (64.2) |
| Non-perforating | 93 (35.8) |
| OP name | |
| ICR | 85 (32.7) |
| RHC | 72 (27.7) |
| SB R&A | 49 (18.8) |
| TC/STC | 32 (12.3) |
| TPC/APR | 19 (7.3) |
| Others | 3 (1.2) |
| Type of anastomosis | |
| Handsewn | 5 (1.9) |
| Stapled | 225 (86.5) |
| Permanent stomy | 28 (10.8) |
| Postoperative use of medication | |
| Anti-TNF | 95 (36.5) |
| 5-ASA | 244 (93.8) |
| Steroid | 82 (31.5) |
| Immunomodulator | 220 (84.6) |
| Presence of complication | 58 (22.3) |
| Wound infection | 17 (6.5) |
| Anastomosis leakage | 12 (4.6) |
| Intra-abdominal abscess | 11 (4.2) |
| Entero-cutaneous fistula | 7 (2.7) |
| Bleeding | 4 (1.5) |
| Ileus | 3 (1.2) |
| Others | 4 (1.5) |
| Time to clinical recurrence, mo | 64 ± 29.8 |
| Time to surgical recurrence, mo | 77.5 ± 30.8 |
Elective operations were performed for 210 (80.8%) patients; the remaining 50 (19.2%) patients underwent emergency operations because of generalised peritonitis or severe obstruction. Among 260 patients, 122 (46.9%) patients had perianal disease before first surgery. Of these, 42 (34.4%) patients had perianal disease after first operation. Because of the strict Korean government health insurance reimbursement policy, only 27 (10.4%) patients treated with anti-TNF agents before first surgery, and 17 (63%) patients continued anti-TNF agents after first operation. Antibiotics such as ciprofloxacin or metronidazole were administered to 163 patients (62.7%) in case of infectious complications such as intra-abdominal abscess, perianal abscess or fistula before first surgery. All the patients who underwent first surgery used antibiotics for a certain period of time. During the follow-up period, three patients (1.5%) experienced colorectal cancer, one patient experienced lung cancer (0.4%).
The majority (215/260, 82.7%) of the patients were diagnosed between the ages of 17 and 40 years, with 9.6% experiencing childhood onset of CD (≤ 16 years) and 7.7% diagnosed as older adults (> 40 years). Montreal classifications were distributed as follows: 43.5% exhibited disease in the terminal ileum region (L1), 41.2% in the ileocolon (L3) and 14.6% in the colon region (L2); 67% had penetrating disease (B3, fistula, abscess or perforation), 31% had stricturing disease (B2) and 2% had inflammatory disease (B1, non-stricturing and non-penetrating). Because these patients were treated with surgical intervention, proportion of disease behavior was different from the whole patients with CD in Korea. Disease behavior of whole CD patients at diagnosis was inflammatory (B1) in 1591 patients (77.1%), stricturing (B2) in 174 (10.3%), and penetrating (B3) in 211 (12.6%)[9].
Clinical recurrence occurred in 1.9%, 24.2% and 68.3% of the patients at 1, 5 and 10 years, respectively, after the first intestinal resection (Figure 2). In multivariate analysis (Table 3), history of smoking, presence of perianal disease after the first operation and undergoing an emergency operation were associated with CRFS.
| Univariate | Multivariate | |||
| P value | HR | 95%CI | P value | |
| Clinical recurrence free survival | ||||
| Type of operation | 0.001a | 0.002a | ||
| Elective | 1 | |||
| Emergency | 1.856 | 1.246-2.763 | ||
| Perianal disease after the 1st operation | 0.077 | 1.446 | 1.005-2.081 | 0.047a |
| History of smoking | 0.001a | 1.727 | 1.220-2.444 | 0.002a |
| Surgical recurrence free survival | ||||
| Age at diagnosis, yr | 0.097 | 0.065 | ||
| A1 ( ≤ 16) | 3.512 | 0.944-13.068 | ||
| A2 ( 17-40) | 1.547 | 0.475-5.036 | ||
| A1 ( > 40) | ||||
| Type of operation | 0.001a | 0.002a | ||
| Elective | 1 | |||
| Emergency | 2.431 | 1.394-4.240 | ||
| Perianal disease after the 1st operation | 0.056 | 1.715 | 1.005-2.926 | 0.048a |
| History of smoking | 0.047a | 1.798 | 1.088-2.969 | 0.022a |
| Postoperative use of anti-TNF | 0.016a | 0.521 | 0.300-0.904 | 0.02a |
During the follow-up period, 66 patients (25.4%) underwent a second intestinal resection for disease recurrence. Among these, 13 (20%) patients underwent a third intestinal resection, four patients (31%) a fourth and one patient (25%) a fifth. The cumulative rate of surgical recurrence was 1.1%, 9.2% and 36.4% at 1, 5 and 10 years, respectively, after the first operation (Figure 1). In univariate analysis, the presence of perianal disease after the first operation, undergoing an emergency operation and postoperative use of anti-TNF agents were all associated with SRFS.
Multivariate analysis revealed the following risk factors for SRFS (Table 3): undergoing the first resection as an emergency operation (HR = 2.431, 95%CI: 1.394-4.240, P = 0.002), history of smoking (HR: 1.798, 95%CI: 1.088-2.969, P = 0.022) and the presence of perianal disease after the first intestinal resection (HR = 1.715, 95%CI: 1.005-2.926, P = 0.048); in addition, the risk was reduced with the postoperative use of anti-TNF agents (HR = 0.521, 95%CI: 0.300-0.904, P = 0.020). Younger age at diagnosis seemed to be associated with an increased risk of surgical recurrence, but it was not statistically significant (HR = 3.512, 95%CI: 0.944-13.068, P = 0.065). However, no impact on SRFS was found for disease location, presence of postoperative complications, length of resected small bowel, perforating disease or preoperative duration of the disease. Azathioprine and 6-mercaptopurine did not demonstrate a clear clinical effect for the prevention of surgical recurrence after the first intestinal resection.
The increasing tendency in Korea over the past few decades calls for more attention to be paid to the natural course of CD in these newly developed disease population. Our study demonstrated the clinical feature and long-term prognosis of CD in a large cohort of non-Caucasian patients.
Korean patients with CD differ from Western patients in sex distribution (a predominance of men), disease location (a lower proportion of isolated colonic disease) and the occurrence of perianal fistula (a higher incidence)[10,11]. Although the pathogenesis of CD is largely unknown, differences have been reported in susceptibility genes and phenotypic features of CD between Asian and Western populations. For example, NOD2 or ATG16L1, which are regarded as well-established CD susceptibility genes among Caucasians, are not replicated in Asians, whereas the association of TNFSF15 with CD is stronger in Koreans and Japanese than in Caucasians[12,13].
The surgical recurrence rate was 25% to 45% 10 years after the first intestinal resection[14,15], although postoperative clinical recurrence occurred earlier during the disease course. Our study found that cumulative surgical and clinical recurrence rates were 36.4% and 68.4%, respectively, at 10 years after the first intestinal resection.
Our study demonstrated that patients with CD who had a higher risk of surgical recurrence after primary bowel surgery were those who underwent the first resection as an emergency operation, those with history of smoking and those with postoperative perianal disease. The use of postoperative anti-TNF agents seemed to reduce surgical recurrence.
Korean patients with CD show higher incidence of perianal disease than Western patients[9]. Previous study in our center[11], perianal fistulas occurred in 46.8% of patients. Also, the cumulative frequency of perianal fistula was 54.3% after 15 years. These results are in contrast to those of Western studies in which the cumulative frequency of perianal fistula in patients with CD attending referral centers was reported to be 13%-38%[16]. Furthermore, the presence of perianal disease was one of the predictors of postoperative recurrence, as has been suggested by previous studies in Asian population[17-19]. Gao et al[17] also assessed risk factors for surgical recurrence and advocated perianal CD correlated with a higher clinical recurrence rate. Korean CD patients with perianal disease had a higher risk of surgical recurrence[18,20]. The present study showed that patients with CD who developed perianal disease after the first resection had a high risk of surgical and clinical recurrence, indicating that these patients had a poor disease outcome after the primary operation. Similarly, despite a wide variation on reported risk of recurrence, the presence of perianal disease is thus considered to be a risk factor for postoperative recurrence in Caucasian population[3,21,22]. The presence of perianal disease is a sign of an increased inflammatory process and this may also influence intestinal CD.
Most patients with CD can be operated on electively, with only few patients requiring acute surgical intervention. In the present study, an indication for an emergency first intestinal resection was significantly associated with surgical recurrence. Riss et al[23] reported that an urgent indication for surgery was found to be significantly associated with the rate of surgical recurrence. Another retrospective study of 116 consecutive patients undergoing their first ileocolectomy at a large Austrian referral centre between 1997 and 2006 demonstrated that urgent index ileocolectomy increased the risk of repeat surgery approximately six-fold[24]. An emergency presentation could be a sign of a significant lack of an immune response to the on-going inflammation, making an emergency operation necessary. Furthermore, preoperative work up and intraoperative evaluation were limited in emergency situation and an emergency operation was usually performed by less experienced surgeons. As a consequence, these patients may be prone to developing early recurrence after the surgical treatment.
Smoking is the most widely recognized environmental risk factor for the initiation and recurrence of Crohn’s disease[25]. Sutherland et al[26], reported that the 5- and 10-year recurrence rates were significantly higher in smokers (36% and 70%) than in non-smokers (20% and 41%; OR = 2.1, P = 0.007). Indeed, smoking is a significant predictive risk factor for all types of recurrences: endoscopic, clinical and surgical[6,27]. Cottone et al[25], conducted multivariate analyses to examine predictive factors for three types of recurrence (clinical, endoscopic and surgical recurrences) after primary surgery. They found that smoking was an independent significant factor for clinical, endoscopic and surgical recurrence. In the present study, history of smoking was significant risk factor for clinical and surgical recurrence.
Another important finding was that postoperative exposure to anti-TNF agents significantly reduced the likelihood of surgical recurrence. The introduction of anti-TNF agents has significantly changed the treatment strategy for CD. Recent studies have shown that anti-TNF agents reduced postoperative recurrence[28-30]. In the present study, postoperative use of anti-TNF agents seemed to reduce the risk of reoperation, indicating that medical treatment is one of the important factors affecting surgical recurrence[31]. A randomised controlled trial was able to demonstrate clearly an advantage of anti-TNF agents in maintaining clinical remission in patients with CD[32].
Immunomodulators such as azathioprine or 6-MP has been widely used, and several studies[33,34] showed that immunomodulators could reduce rate of surgery. Similarly, previous study [9] in our center revealed that early use of immunomodulators was independently related to the decreased surgery rate (P = 0.01). However, because immunomodulators were generally used after first surgery, it is difficult to determine their impact on decreasing the postoperative recurrence. In present study, 85% of patients used azathioprine or 6-MP after first surgery and use of immunomodulator showed no significant difference in postoperative recurrence. More recently, these drugs are used in combination with anti-TNF drugs to decrease their immunogenicity and increase anti-TNF drug concentrations.
Antibiotics have not been shown to be effective for induction and maintenance of remission for active CD. The best data for antibiotics are in the short-term treatment of perianal disease and in combination with anti-TNF[35]. Aside from these indications, antibiotics should really be used to treat the inflammatory complications of CD such as enteric fistula or abscess. The main limitations to using these agents long standing are antibiotic resistance and side effect.
Surgical recurrence rates differ immensely between studies. One reason could be that there is crossover with a period of rapidly changing management options, both medical and surgical. Stapled anastomosis has now virtually replaced the hand-sewn technique in the majority of centres performing surgery for CD. Stapled anastomosis following ileocecal resection is an example, as it has been reported to be safe and feasible in patients with CD[36], with a few studies demonstrating a lower incidence of anastomotic recurrence after stapled functional rather than hand-sewn end-to-end anastomosis[37]. The vast majority of patients in the present study (225/230, 97.8%) received a stapled anastomosis. Among them, 210 patients (93.3%) received a functional end-to-end anastomosis with linear stapler, with 15 patients (6.7%) receiving an end-to-end or a side-to-end anastomosis with circular stapler. Only 5 patients (2.2%) received a hand-sewn anastomosis because of severe bowel edema or dilatation.
Several other risk factors for postoperative recurrence have been found over the past decades, such as a family history of CD, perforating type, younger age at diagnosis, the type of anastomotic configuration, sex, disease location, and the presence of postoperative complications[15,38,39], but none of these factors was associated with an increased risk for repeat surgery in the present analysis.
The present study had several limitations. First, the number of patients was relatively small. Owing to this limited study population, we were unable to demonstrate an increased risk of recurrence when there were known risk factors such as a perforating type disease, family history and a presence of complication. As this study was retrospective in design, there was no standardised follow-up protocol of endoscopy or image study for the patients who underwent primary bowel resection.
However, relatively large cohort of patients in Korea was included in this study with near complete follow up, so that these results showed a clinical importance in Asian population.
In conclusion, our present referral center study of long-term course of CD in Korea revealed a several factors that were significantly associated with a higher risk for surgical recurrence: the initial surgery as an emergency, history of smoking and the presence of perianal disease after the first intestinal resection. Anti-TNF agents seemed to reduce surgical recurrence. These findings indicate that affected patients with CD may benefit from close postoperative surveillance and probably from the early administration of anti-TNF agents.
Majority of Crohn’s disease (CD) patients experienced recurrence after their first bowel resection and postoperative recurrence affects the quality of patients’ lives. Thus, to provide optimal patient care, it is important to be able to predict which patients have a higher risk of recurrence. They investigated risk factors for the postoperative recurrence of CD in a patient population from a single tertiary referral center in Korea.
The increasing tendency in Korea over the past few decades calls for more attention to be paid to the natural course of CD in these newly developed disease population. This study demonstrated the clinical feature and long-term prognosis of CD in a large cohort of non-Caucasian patients. In this study, history of smoking, postoperative perianal disease and undergoing an emergency operation were found to be independent risk factors for surgical recurrence in CD. The postoperative use of anti-TNF agents reduced surgical recurrence.
This study evaluated the risk factors which contribute the surgical recurrence and showed that Korean CD patients with postoperative perianal disease had increased risk of surgical recurrence.
Presence of postoperative perianal disease associated with surgical recurrence and using anti-TNF agents for patients with risk factors may reduce surgical recurrence. Physicians should consider aggressive and early top-down therapy for perianal CD patients.
Postoperative perianal disease: Presence of perianal disease after first bowel resection.
This is an interesting study in which the investigators evaluated the risk factors for postoperative recurrence after primary bowel resection in a cohort of Korean CD patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chowdhury P, Fernandez-Rodriguez CM, Lihaug Hoff DA, S- Editor: Qi Y L- Editor: A E- Editor: Ma YJ
| 1. | Munkholm P, Langholz E, Davidsen M, Binder V. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol. 1995;30:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 345] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Freeman HJ. Long-term natural history of Crohn’s disease. World J Gastroenterol. 2009;15:1315-1318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn’s disease. Br J Surg. 2000;87:1697-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 251] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Michelassi F, Balestracci T, Chappell R, Block GE. Primary and recurrent Crohn’s disease. Experience with 1379 patients. Ann Surg. 1991;214:230-238; discussion 238-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 176] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Post S, Herfarth C, Böhm E, Timmermanns G, Schumacher H, Schürmann G, Golling M. The impact of disease pattern, surgical management, and individual surgeons on the risk for relaparotomy for recurrent Crohn’s disease. Ann Surg. 1996;223:253-260. [PubMed] |
| 6. | Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008;23:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Simillis C, Yamamoto T, Reese GE, Umegae S, Matsumoto K, Darzi AW, Tekkis PP. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol. 2008;103:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2352] [Article Influence: 123.8] [Reference Citation Analysis (2)] |
| 9. | Park SH, Yang SK, Park SK, Kim JW, Yang DH, Jung KW, Kim KJ, Ye BD, Byeon JS, Myung SJ. Long-term prognosis of crohn’s disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis. 2014;20:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Yang SK, Yun S, Kim JH, Park JY, Kim HY, Kim YH, Chang DK, Kim JS, Song IS, Park JB. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 381] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 11. | Ye BD, Yang SK, Cho YK, Park SH, Yang DH, Yoon SM, Kim KJ, Byeon JS, Myung SJ, Yu CS. Clinical features and long-term prognosis of Crohn’s disease in Korea. Scand J Gastroenterol. 2010;45:1178-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Yang SK, Park M, Lim J, Park SH, Ye BD, Lee I, Song K. Contribution of IL23R but not ATG16L1 to Crohn’s disease susceptibility in Koreans. Inflamm Bowel Dis. 2009;15:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Yang SK, Lim J, Chang HS, Lee I, Li Y, Liu J, Song K. Association of TNFSF15 with Crohn’s disease in Koreans. Am J Gastroenterol. 2008;103:1437-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Heimann TM, Greenstein AJ, Lewis B, Kaufman D, Heimann DM, Aufses AH Jr. Comparison of primary and reoperative surgery in patients with Crohns disease. Ann Surg. 1998;227:492-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 475] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 16. | American Gastroenterological Association Clinical Practice Committee. American Gastroenterological Association medical position statement: perianal Crohn’s disease. Gastroenterology. 2003;125:1503-1507. [PubMed] |
| 17. | Gao X, Yang RP, Chen MH, Xiao YL, He Y, Chen BL, Hu PJ. Risk factors for surgery and postoperative recurrence: analysis of a south China cohort with Crohn’s disease. Scand J Gastroenterol. 2012;47:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Han YM, Kim JW, Koh SJ, Kim BG, Lee KL, Im JP, Kim JS, Jung HC. Patients with perianal Crohn’s disease have poor disease outcomes after primary bowel resection. J Gastroenterol Hepatol. 2016;31:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Yang CH, Ding J, Gao Y, Chen X, Yang ZB, Xiao SD. Risk factors that predict the requirement of aggressive therapy among Chinese patients with Crohn’s disease. J Dig Dis. 2011;12:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Lee JL, Yu CS, Lim SB, Park IJ, Yoon YS, Kim CW, Yang SK, Kim JC. Surgical Treatment of Crohn Colitis Involving More Than 2 Colonic Segments: Long-Term Outcomes From a Single Institution. Medicine (Baltimore). 2016;95:e3793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Parente F, Sampietro GM, Molteni M, Greco S, Anderloni A, Sposito C, Danelli PG, Taschieri AM, Gallus S, Bianchi Porro G. Behaviour of the bowel wall during the first year after surgery is a strong predictor of symptomatic recurrence of Crohn’s disease: a prospective study. Aliment Pharmacol Ther. 2004;20:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, Guslandi M, Oldenburg B, Dotan I, Marteau P, Ardizzone A, Baumgart DC, D’Haens G, Gionchetti P, Portela F, Vucelic B, Söderholm J, Escher J, Koletzko S, Kolho KL, Lukas M, Mottet C, Tilg H, Vermeire S, Carbonnel F, Cole A, Novacek G, Reinshagen M, Tsianos E, Herrlinger K, Oldenburg B, Bouhnik Y, Kiesslich R, Stange E, Travis S, Lindsay J; European Crohn’s and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Special situations. J Crohns Colitis. 2010;4:63-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 547] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 23. | Riss S, Schuster I, Papay P, Mittlböck M, Stift A. Repeat intestinal resections increase the risk of recurrence of Crohn’s disease. Dis Colon Rectum. 2013;56:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Riss S, Schuster I, Papay P, Herbst F, Mittlböck M, Chitsabesan P, Stift A. Surgical recurrence after primary ileocolic resection for Crohn’s disease. Tech Coloproctol. 2014;18:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Cottone M, Rosselli M, Orlando A, Oliva L, Puleo A, Cappello M, Traina M, Tonelli F, Pagliaro L. Smoking habits and recurrence in Crohn’s disease. Gastroenterology. 1994;106:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 275] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Sutherland LR, Ramcharan S, Bryant H, Fick G. Effect of cigarette smoking on recurrence of Crohn’s disease. Gastroenterology. 1990;98:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 230] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Connelly TM, Messaris E. Predictors of recurrence of Crohn’s disease after ileocolectomy: a review. World J Gastroenterol. 2014;20:14393-14406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Sorrentino D, Terrosu G, Avellini C, Maiero S. Infliximab with low-dose methotrexate for prevention of postsurgical recurrence of ileocolonic Crohn disease. Arch Intern Med. 2007;167:1804-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, Harrison J, Plevy SE. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology. 2009;136:441-450.e1; quiz 716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 432] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 30. | Yoshida K, Fukunaga K, Ikeuchi H, Kamikozuru K, Hida N, Ohda Y, Yokoyama Y, Iimuro M, Takeda N, Kato K. Scheduled infliximab monotherapy to prevent recurrence of Crohn’s disease following ileocolic or ileal resection: a 3-year prospective randomized open trial. Inflamm Bowel Dis. 2012;18:1617-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Bouguen G, Siproudhis L, Gizard E, Wallenhorst T, Billioud V, Bretagne JF, Bigard MA, Peyrin-Biroulet L. Long-term outcome of perianal fistulizing Crohn’s disease treated with infliximab. Clin. Gastroenterol Hepatol. 2013;11:975-981.e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 771] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 33. | Lakatos PL, Golovics PA, David G, Pandur T, Erdelyi Z, Horvath A, Mester G, Balogh M, Szipocs I, Molnar C. Has there been a change in the natural history of Crohn’s disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977-2009. Am J Gastroenterol. 2012;107:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 34. | Peyrin-Biroulet L, Oussalah A, Williet N, Pillot C, Bresler L, Bigard MA. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut. 2011;60:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 35. | Schwartz DA, Loftus EV Jr, Tremaine WJ, Panaccione R, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 712] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 36. | Riss S, Bittermann C, Zandl S, Kristo I, Stift A, Papay P, Vogelsang H, Mittlböck M, Herbst F. Short-term complications of wide-lumen stapled anastomosis after ileocolic resection for Crohn’s disease: who is at risk? Colorectal Dis. 2010;12:e298-e303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Scarpa M, Angriman I, Barollo M, Polese L, Ruffolo C, Bertin M, D’Amico DF. Role of stapled and hand-sewn anastomoses in recurrence of Crohn’s disease. Hepatogastroenterology. 2004;51:1053-1057. [PubMed] |
| 38. | Unkart JT, Anderson L, Li E, Miller C, Yan Y, Gu CC, Chen J, Stone CD, Hunt S, Dietz DW. Risk factors for surgical recurrence after ileocolic resection of Crohn’s disease. Dis Colon Rectum. 2008;51:1211-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Yamamoto T. Factors affecting recurrence after surgery for Crohn’s disease. World J Gastroenterol. 2005;11:3971-3979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 174] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |