Published online Oct 14, 2017. doi: 10.3748/wjg.v23.i38.6973
Peer-review started: April 27, 2016
First decision: June 22, 2017
Revised: July 20, 2017
Accepted: September 5, 2017
Article in press: September 5, 2017
Published online: October 14, 2017
Processing time: 182 Days and 9.1 Hours
To investigate whether mesenchymal stem cells (MSCs) from adipose-derived stromal cells (ADSCs) and bone marrow stromal cells (BMSCs) have similar hepatic differentiation potential.
Mouse ADSCs and BMSCs were isolated and cultured. Their morphological and phenotypic characteristics, as well as their multiple differentiation capacity were compared. A new culture system was established to induce ADSCs and BMSCs into functional hepatocytes. Reverse transcription polymerase chain reaction, Western blot, and immunofluorescence analyses were performed to identify the induced hepatocyte-like cells. CM-Dil-labeled ADSCs and BMSCs were then transplanted into a mouse model of CCl4-induced acute liver failure. Fluorescence microscopy was used to track the transplanted MSCs. Liver function was tested by an automatic biochemistry analyzer, and liver tissue histology was observed by hematoxylin and eosin (HE) staining.
ADSCs and BMSCs shared a similar morphology and multiple differentiation capacity, as well as a similar phenotype (with expression of CD29 and CD90 and no expression of CD11b or CD45). Morphologically, ADSCs and BMSCs became round and epithelioid following hepatic induction. These two cell types differentiated into hepatocyte-like cells with similar expression of albumin, cytokeratin 18, cytokeratin 19, alpha fetoprotein, and cytochrome P450. Fluorescence microscopy revealed that both ADSCs and BMSCs were observed in the mouse liver at different time points. Compared to the control group, both the function of the injured livers and HE staining showed significant improvement in the ADSC- and BMSC-transplanted mice. There was no significant difference between the two MSC groups.
ADSCs share a similar hepatic differentiation capacity and therapeutic effect with BMSCs in an acute liver failure model. ADSCs may represent an ideal seed cell type for cell transplantation or a bio-artificial liver support system.
Core tip: We investigated whether mesenchymal stem cells from adipose-derived stromal cells (ADSCs) and bone marrow stromal cells (BMSCs), have similar hepatic differentiation potential. We found that adipose-derived stromal cells resemble bone marrow stromal cells in their hepatocyte differentiation potential in vitro and in vivo. Because ADSCs are obtained more easily and less invasive than BMSCs, ADSCs might be more suitable seed cells for cell transplant or liver tissue engineering. We also developed a new protocol of preparing mouse BMSCs and established a new hepatic induction system.
- Citation: Xu LJ, Wang SF, Wang DQ, Ma LJ, Chen Z, Chen QQ, Wang J, Yan L. Adipose-derived stromal cells resemble bone marrow stromal cells in hepatocyte differentiation potential in vitro and in vivo. World J Gastroenterol 2017; 23(38): 6973-6982
- URL: https://www.wjgnet.com/1007-9327/full/v23/i38/6973.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i38.6973
Liver transplantation remains the only effective treatment for liver failure. However, its clinical application is limited by donor organs and immune rejection. To this end, hepatocyte transplantation and a bio-artificial liver support system are two potential surrogate complementary therapies for patients with liver failure. Hepatocyte-like cells can be induced from mesenchymal stem cells (MSCs) for xenotransplantation and have been demonstrated to perform hepatocyte functions in preclinical animal studies[1,2]. However, the best type of MSC has not yet been investigated, and therefore it is important to screen for an ideal seed cell type for cell transplantation or a bio-artificial liver support system.
MSCs are non-hematopoietic multipotent stem cells that can be isolated from multiple tissues, such as bone marrow, adipose tissue, cord blood, and amniotic fluid[3-5]. Bone marrow stromal cells (BMSCs) are the most extensively studied and fertile stem cell source used in regenerative medicine and liver tissue engineering[6,7]. More recently, adipose-derived stromal cells (ADSCs) were identified as another promising and extensively studied stem cells for use, especially in liver tissue engineering[8]. Although both ADSCs and BMSCs can be induced into hepatocyte-like cells[9-12], there is no uniform culture system to evaluate their hepatic differentiation potential. Therefore, it is necessary to establish an ideal system to improve the efficiency of hepatic induction.
In this study, we developed a novel culture protocol for mouse BMSCs and optimized the hepatic differentiation system. We compared the morphological and phenotypic characteristics, as well as the multi-differentiation capacity of BMSCs and ADSCs in vitro. We also compared therapeutic effect of BMSCs and ADSCs following transplantation into a mouse model of acute liver failure.
Male BALB/c mice (3 d old and 6 wk old; Charles River, Beijing, China) were purchased from the Laboratory Animal Center of the Academy of Military Medical Sciences of China (Beijing). All studies were conducted after approved by the Ethics Committee of the Animal Facility of Chinese PLA General Hospital and complied with the guidelines for the care of laboratory animals. Mice were housed in cages in a controlled environment (25 °C and a 12 h light/dark cycle) and fed standard mouse chow and tap water, and were observed every day in our animal facility.
The isolation and purification of ADSCs were performed as previously described[13]. Fibrous tissue was excluded and adipose tissue was minced into pieces of < 1 mm3 and digested in 1 mg/mL collagenase I for 1 h at 37 °C. The cell suspension was filtered through a 100 µmol/L cell strainer and centrifuged at 300 × g for 5 min. ADSCs were plated at a density of 5 × 105/cm2 with alpha minimal essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin, and cultured in a humidified incubator at 37 °C and 5% CO2. Cells were harvested after reaching a 90% confluence with 0.25% trypsin-EDTA (Gibco, American). Cells in passages 2-4 were used for subsequent experiments.
A new method was established to isolate mouse BMSCs as follows: 3-d-old male BALB/c mice were sacrificed by cervical dislocation and soaked in 75% alcohol for 5 min. The tibia and fibula were isolated under sterile conditions and washed twice with phosphate-buffered saline (PBS) containing 5% penicillin/streptomycin. Muscle and fibrous tissue were excluded. Tibias and fibulas were minced into pieces of < 1 mm3 and washed once with α-MEM, cultured directly by incubation with α-MEM supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified incubator at 37 °C and 5% CO2. After 72 h, half of the medium was changed and the bone chips were kept. After reaching a 50% confluence, cells were harvested with 0.25% trypsin-EDTA and seeded as the first passage. At each passage, cells were diluted 1:3-4 every two days. BMSCs at passages 2-4 were used for subsequent experiments.
For the cell proliferation assay, 2 × 103 viable ADSCs and BMSCs were seeded in triplicate onto a 96-well plate. Cell proliferation was measured using a Cell Counting Kit-8 (CCK-8; Beyotime, China). Plates were placed in a humidified incubator at 37 °C until the cells adhered to the plate. Next, 10 µL of the CCK-8 solution was added to each well and plates were incubated for another 2 h at 37 °C prior to reading the absorbance at 450 nm on a microplate reader. The assay was repeated every day at the same time for 10 d.
Passage 2 and 3 ADSCs and BMSCs were trypsinized and incubated with fluorescein isothiocyanate-conjugated CD45 and CD90, and phycoerythrin-conjugated CD11b and CD29 antibodies for 30 min at 4 °C, followed by two washes with PBS. Fluorescent-labeled cells were analyzed on a flow cytometer.
For adipogenic differentiation, cells were seeded at 1 × 104/cm2 on 12-well plates. When cells adhered to the plate, the expansion medium (α-MEM supplemented with 10% FBS and 1% penicillin/streptomycin) was replaced with adipogenic induction medium containing 10−6 mmol/L dexamethasone (Dex), 0.5 µmol/L isobutylmethylxanthine, 200 µmol/L indomethacin, and 5 µg/mL (wt/v) insulin, and the cells were incubated for 8 d. Cells cultured in a base medium of α-MEM supplemented with 10% (v/v) FBS served as a negative control. Adipogenic differentiation was assessed by Oil-Red-O staining.
For osteogenic differentiation, cells were seeded at 5 × 103/cm2 on 12-well plates. When cells adhered to the plate, the expansion medium was replaced with osteogenic induction medium containing 10−7 mmol/L Dex, 10 mmol/L β-glycerol phosphate, and 50 µmol/L ascorbate-2-phosphate. Cells cultured in a base medium of α-MEM supplemented with 10% FBS were used as a negative control. Cells were incubated for 3 wk and osteogenic differentiation was assessed by Alizarin Red staining.
Hepatic differentiation was achieved following a one-step procedure using mouse ADSCs and BMSCs. ADSCs and BMSCs (passage 3) were seeded at 5 × 103/cm2 onto 24-well culture dishes in expansion medium. When cells adhered to the plate, the expansion medium was replaced with hepatocyte culture medium (HCM; DMEM containing 10% FBS) supplemented with 50 ng/mL hepatocyte growth factor (HGF), 25 ng/mL fibroblast growth factor 4 (FGF4), 30 ng/mL oncostatin M (OSM), 20 ng/mL epidermal growth factor (EGF), 25 ng/mL acidic fibroblast growth factor (aFGF), 10 ng/mL basic fibroblast growth factor (bFGF), 10-6 mmol/L Dex, 1 × insulin-transferrin-selenium (ITS), 2 mmol/L ascorbic acid (Vc), and 50 μmmol/L nicotinamide (Vpp). Differentiation medium (1 mL) was added to each 24-well culture dish and changed every 3 d. Afterwards, cells were cultured for 10 d in HCM. Undifferentiated cells served as negative controls and the HepG2 cell line served as a positive control.
On day 10 of hepatic differentiation, total RNA was isolated from ADSCs and BMSCs with Trizol reagent (Sigma-Aldrich, St. Louis, MO, United States), reverse-transcribed into first-strand cDNA using oligo (dT) primer, and amplified with 35 cycles (95 °C, 10 min; 58 °C, 1 min; and 72 °C, 5 min) of PCR using 10 pmmol/L of specific primers. Upon completion of the PCR, products were examined by 2% agarose gel electrophoresis. Actin was used as an internal standard (30 cycles of amplification were performed). To compare the differences in expression levels of albumin (ALB), alpha fetoprotein (AFP), cytokeratin 18 (CK-18), CK-19, and glucose-6-phosphate (G-6-P) between undifferentiated and differentiated ADSCs and BMSCs, the products were quantified on an image analyzer (Uvitec, Warwickshire, United Kingdom). The primer sequences used are listed in Supplement Table 1.
Western blot analysis was performed as previously described[6]. In brief, total cellular proteins were prepared and quantified by the Bradford method. A total of 80 µg of lysates was electrophoresed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto a nitrocellulose membrane (Immoblin-P, Millipore, Bedford, MA, United States). Membranes were blocked with 5% fat free milk powder at room temperature for 2 h and incubated overnight with polyclonal rabbit anti-mouse CYP1A1 or CK-19, goat anti-mouse ALB, AFP, or CK-18 (1:1000; Santa Cruz Biotechnology, Dallas, TX, United States), or anti-β-actin antibody (Santa Cruz Biotechnology) at 4 °C overnight. After three washes of 15 min in Tris-buffered saline containing Tween 20 (TBST), the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody, HRP-conjugated rabbit anti-goat IgG antibody (Zhongshan Jinqiao, Beijing, China), or goat anti-mouse IgG antibody for 2 h at room temperature. The membranes were washed again in TBST. Enhanced chemiluminescence reagent was added and monitored for color development.
Cultured cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 30 min at room temperature, washed twice with PBS, and permeabilized with 1% Triton X-100 (Sigma-Aldrich) for 20 min at room temperature. Cells were then incubated with blocking solution consisting of PBS and 10% normal goat serum NGS at room temperature for 2 h. For immunofluorescence staining, primary antibodies (1:200; Santa Cruz Biotechnology) against ALB, AFP, CK-18, CK-19, or CYP1A1 were used. Following incubation with the primary antibodies overnight at 4 °C, cells were incubated with secondary antibodies for 2 h at 37 °C. Subsequently, cells were stained with 4’,6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature and photographed with a structured illumination fluorescence microscope.
Six-week-old BALB/c mice were used for the transplantation experiment. An acute liver failure model was established by administering one dose of CCl4. Mice received intraperitoneal injection of 100 μL/20 g body weight of olive oil containing 10 μL CCl4 24 h before MSC transplantation. On day 0, mice underwent MSC transplantation at a concentration of 1 × 106 cells per mouse (0.2 mL cell suspension was injected via the tail vein). As a control, PBS-transplanted CCl4-treated mice and non-transplanted olive oil-treated mice were used. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured on days 1, 2, 3, and 7 after transplantation. Liver tissue samples were harvested at the indicated time points after cell transplantation, fixed with 4% paraformaldehyde, embedded in paraffin, and stained with hematoxylin and eosin (HE). Frozen sections (8 μm thick) were used for fluorescence observations.
Data are presented as the mean ± SD. ALT and AST levels were analyzed by Student’s t test using SPSS software version 19.0 (SPSS Inc., Chicago, IL, United States). Significance for all statistical analyses was defined as P < 0.05.
Cells were observed under an inverted microscope. On the second day of primary culture, few ADSCs were observed. On the third day, the number of cells increased (the cell density was approximately 60%) and, on the fourth day, the cells exhibited a spiral-shaped structure and reached a 90% confluence. Cells were passaged at 1:3 (Supplement Figure 1A). On the second day of primary culture (BMSCs), few fibroblast-like cells had “climbed out” around the bone fragments. The number of cells increased over time and, on the fourth day, BMSCs reached a 50% confluence and were passaged at 1:1 (Supplement Figure 1A).
From passages 1-4, the two types of MSCs were long and spindle-shaped and showed no difference in morphology (Supplement Figure 1B).
The cell number of the second and third generations of the two cell types was determined over time and a growth curve was drawn. The two cell types showed active proliferative capacity, with an S-shaped growth curve. During the first three days, cells grew slowly. Between days 4 and 7, the two cell types grew rapidly and entered a logarithmic growth period. Cells stopped growing at day 8 and entered the growth plateau phase (Supplement Figure 1C). There was no significant difference between the two types of adult stem cells in the same generation, and there was no significant difference between the same kinds of adult stem cells in the different generation (Supplement Table 1).
ADSC and BMSC cell surface markers were analyzed by flow cytometry. Both ADSCs and BMSCs expressed the stem cell-associated surface markers CD90 and CD29, but did not express CD11b or CD45 (Supplement Table 2). The two cell types had a similar proliferative ability and stem cell capacity, consistent with our previous study.
The adipogenic and osteogenic differentiation of ADSCs and BMSCs was evaluated at passages 2 and 3. Small and round vacuoles began to appear in the cytoplasm on the third day of induction. On the 8th day, a large number of lipid droplets appeared in the majority of the induced cells and the cells became round, oval, or polygonal. Adipogenic differentiation was identified by Oil-Red-O staining, which stained the lipid vacuoles bright red.
After three weeks of induction, cells aggregated in some areas and formed a multilayered, nodular structure known as a bone nodule. Osteogenic differentiation was identified by Alizarin Red staining, which stained the bone nodules red. Oil-Red-O and Alizarin Red images of such cells were shown in our previous study.
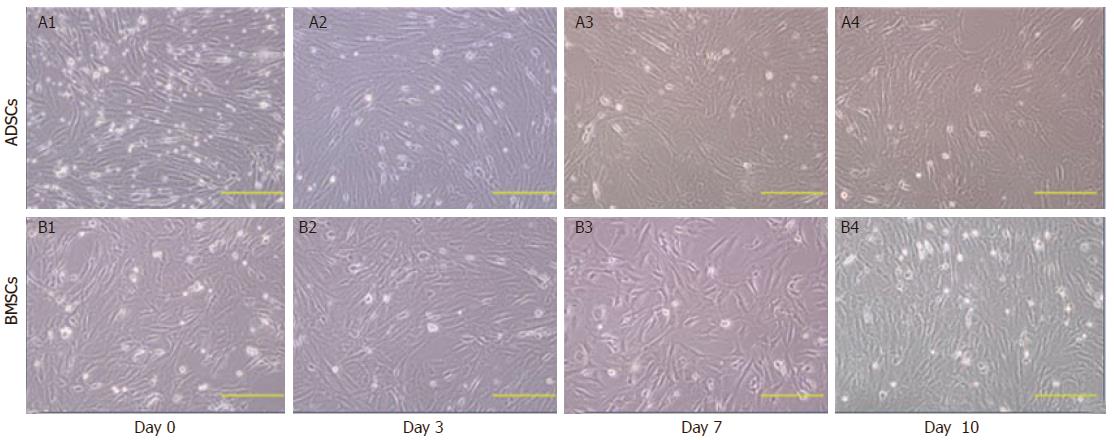
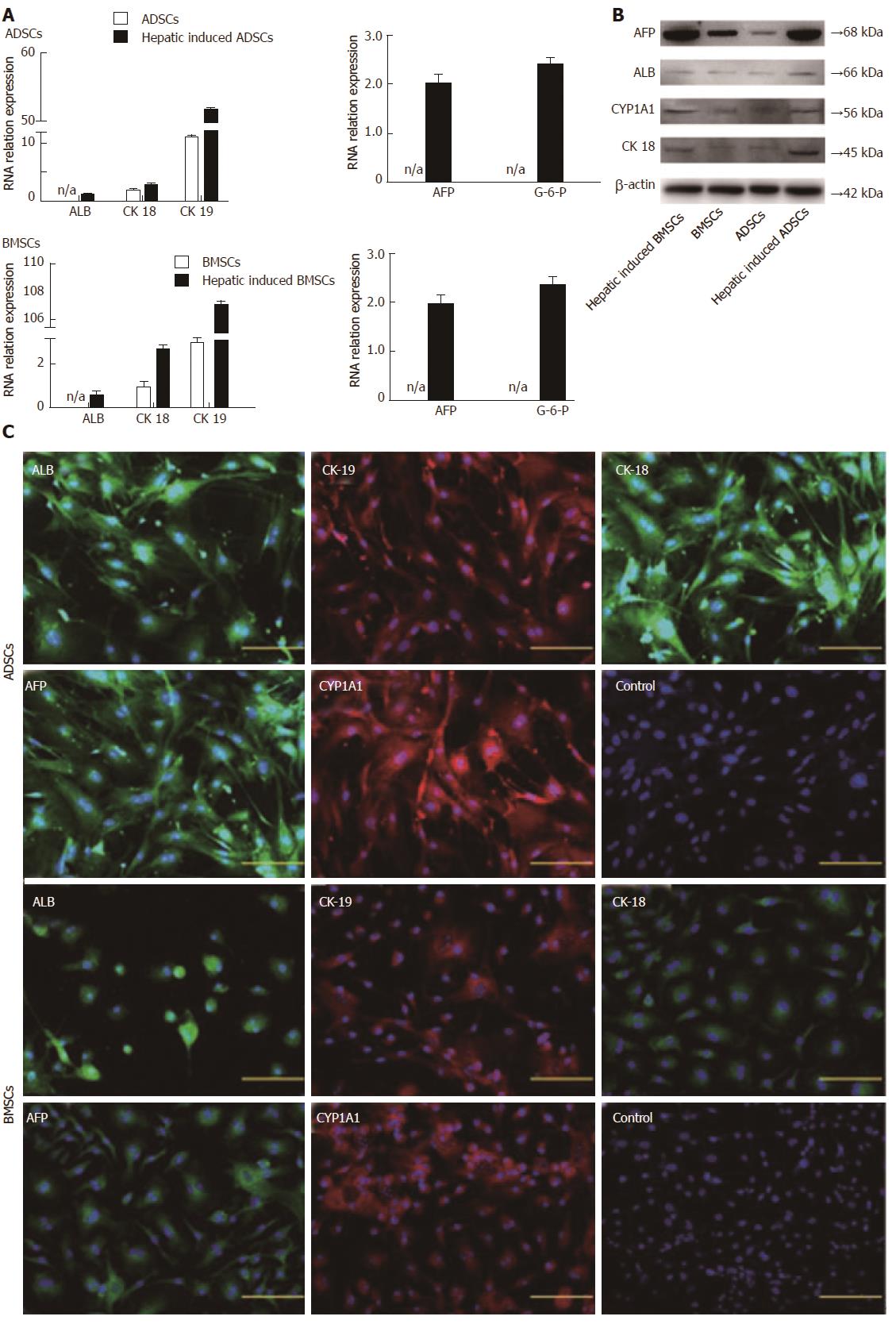
Before differentiation, both ADSCs and BMSCs exhibited fibroblast morphology with spindle cell bodies. After differentiation, the cell morphology changed dramatically. On day 4 after hepatic differentiation, cells became round in shape. On day 7, the number of oval and polygonal cells increased, and on day 10, the induced cells exhibited a clear polygonal shape (Figure 1). The expression of several hepatic genes was examined after hepatic differentiation by RT-PCR. Uninduced cells served as a negative control. On day 10 after hepatic differentiation, the expression of ALB, AFP, CK-18, CK-19, and G-6-P was significantly enhanced (Figure 2A).
The expression of ALB, AFP, CYP1A1, and CK-18 in hepatocyte-like cells was confirmed by Western blot analysis (Figure 2B). The expression of ALB, AFP, CYP1A1, and CK-18 increased continuously in the two types of hepatocyte-like cells compared with undifferentiated ADSCs and BMSCs, suggesting that these two types of MSCs were successfully induced into hepatocyte-like cells in vitro.
On day 10 after differentiation, immunofluorescence staining showed that the differentiated hepatocyte-like cells expressed the hepatocyte markers ALB, CK-19, CK-18, AFP, and CYP1A1 (Figure 2C), whereas the undifferentiated ADSCs and BMSCs (Figure 2C) did not express the hepatocyte-related markers.
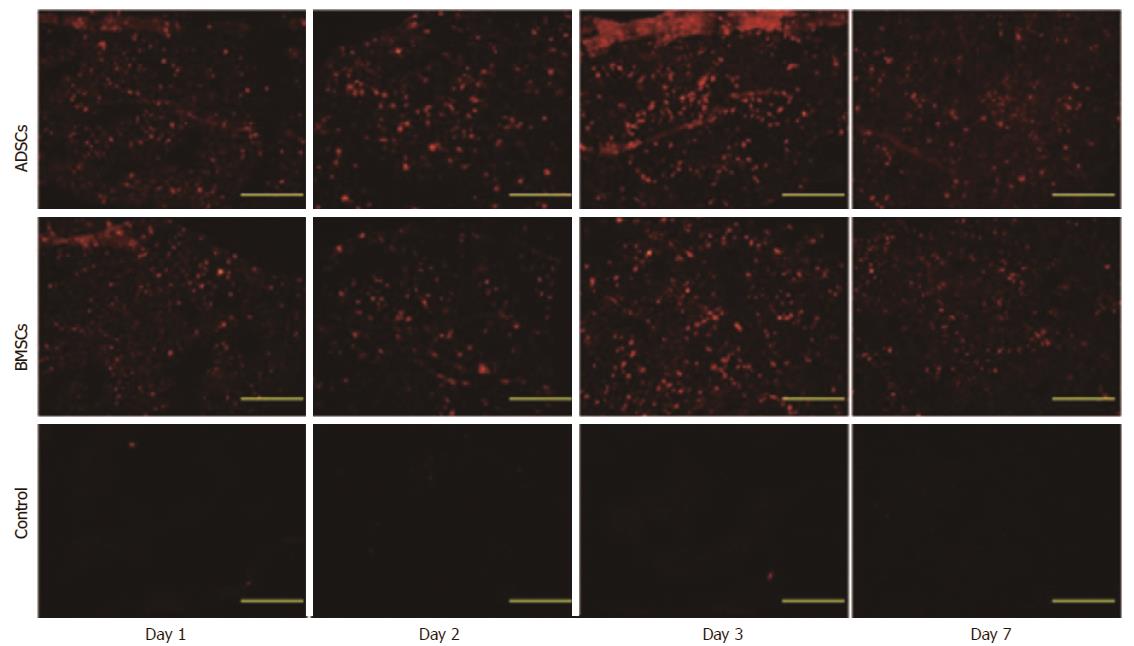
To investigate whether the CM-Dil-labeled MSCs localized to the injured liver, animals were sacrificed on days 1, 2, 3 and 7 following treatment. In the ADSC and BMSC groups, red fluorescent cells were detected in the injured livers. In addition, the largest number of cells was detected on day 3 after transplantation and gradually decreased on day 7 (Figure 3). These results suggest that cells transplanted via the intravenous route can be integrated in the liver parenchyma during the early stage of transplantation.
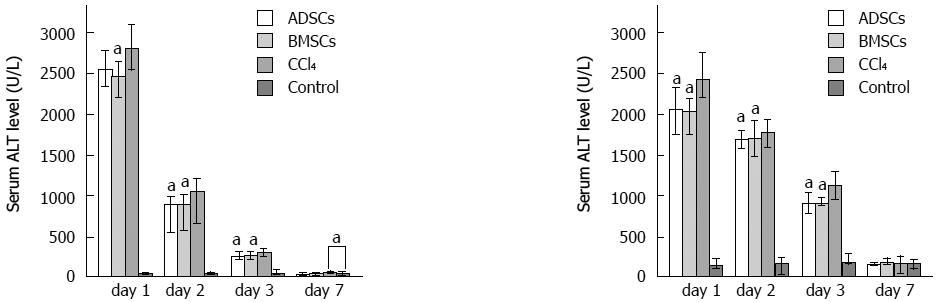
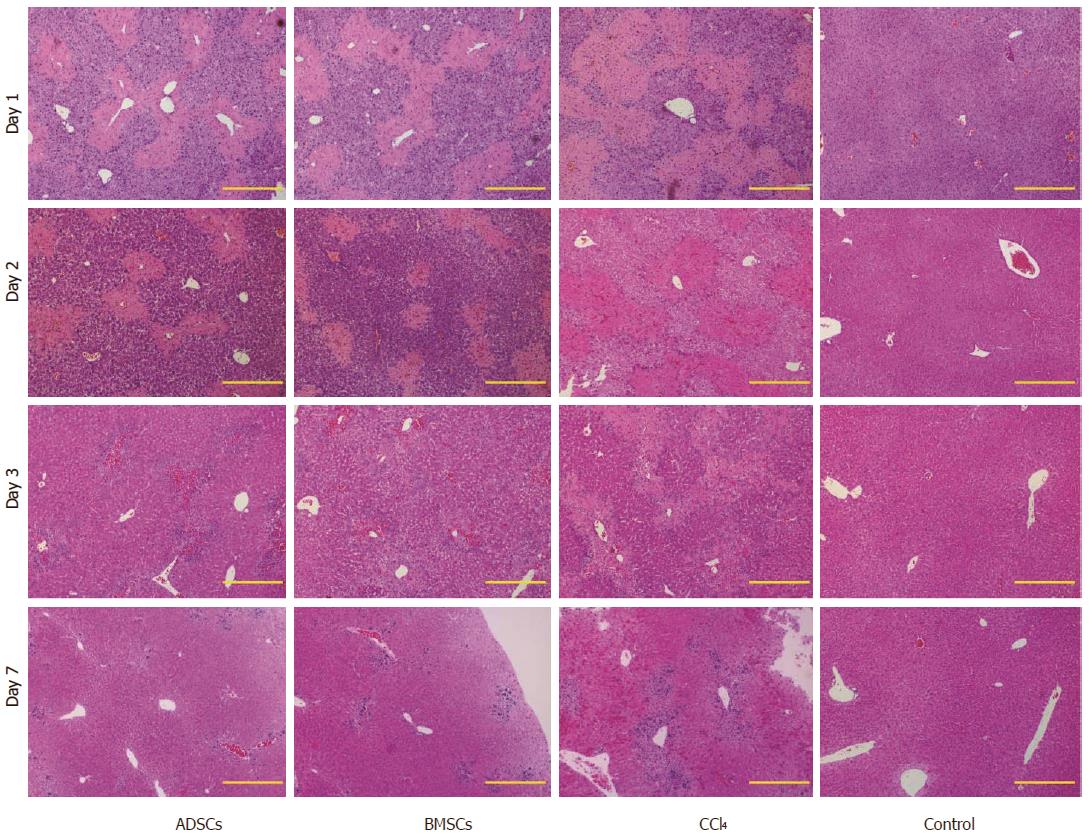
To address whether ADSCs and BMSCs can regenerate the injured mouse liver, these cell types were transplanted into liver-injured mice. Twenty-four hours after CCl4 injection, mice exhibited serious liver injury. Biochemical parameters, including ALT and AST, were measured and stem cells were transplanted. The therapeutic abilities of the two experimental groups were compared at different time points. Following transplantation, the ALT and AST levels of the experimental groups decreased significantly compared with the control group (Figure 4). HE staining revealed that the degree of injury was much weaker in the injured transplanted mice than in the injured, non-transplanted mice (Figure 5). On day 7, all of the experimental groups recovered histologically. However, damaged areas were still observed in the CCl4 group. These observations indicated that ADSCs and BMSCs possessed similar repairing abilities against CCl4-induced liver injury.
Availability of a large number of functional hepatocytes is essential for cytotherapy. However, primary hepatocyte cultures have been hindered by their short life span and the rapid loss of hepatic function in vitro[14]. MSCs have become a promising type of seed cell for liver transplantation for their multiple differentiation potential, high proliferation capacity, and plastic adherence properties. However, it is difficult to determine the ideal seed cells from different types of MSCs. In the present study, we established a novel isolation and culture protocol for mouse BMSCs that could provide a large number of BMSCs in a short period of time and share a similar multiple differentiation ability with ADSCs. We also established a new HCM that not only shortens the induction time but also produces a large number of hepatocyte-like cells. With the same induction system, ADSCs resembled BMSCs in term of hepatocyte differentiation potential both in vitro and in vivo.
A large quantity of ADSCs can be easily obtained by digesting adipose tissue derived from mice, rats, and humans with collagenase I[15]. However, it is difficult to obtain a large quantity of pure mouse BMSCs. We analyzed the conditioned medium method of Sun et al[16] and the compact bone method of Zhu et al[17], and developed a novel and reliable isolation and culture method that provides a large number of BMSCs in a short period of time. Compared to the method of Sun et al[16], the period required to obtain purified cells was shortened to 10-11 d. Compared to the method of Zhu et al[17], we omitted the digestion step of collagenase II. Additionally, the passage time was shortened to 2 d (compared to Zhu’s twice per week). Compared to the method for the isolation and culture of mouse BMSCs from bone marrow[18], our protocol not only shows less contamination of hematopoietic lineage cells, but also obtains a large number of BMSCs in a short period of time. The number of BMSCs obtained from five mice can reach 107 in 10-11 d. We believe that the reason for the rapid proliferation of cells is that neonatal mice exhibit better cell viability. Therefore, we developed a novel and easy method to isolate mouse BMSCs from tibia and fibula bone fragments.
Among the various hepatic differentiation systems, a one-step induction method has been most extensively applied[19]. Various cytokines and chemicals can be used to induce MSCs into hepatocyte-like cells. Current studies have reported that HGF, FGF4, EGF, OSM, Dex, aFGF, bFGF, DMSO, Vpp, and Vc can be applied for the hepatic differentiation of ADSCs[10,20]. Additionally, HGF, FGF4, EGF, OSM, Dex, ITS, trichostatin A (TSA), and L-glutamine have been applied for the hepatic differentiation of BMSCs[21-24]. In the present study, we used multiple factors, including HGF, FGF4, OSM, aFGF, bFGF, Dex, Vpp, ITS, and Vc, to develop a new induction system to promote the differentiation of MSCs. This hepatic induction system induced ADSCs and BMSCs into hepatocyte-like cells in only 10 d. However, a previously reported one-step induction system usually took more than two weeks[25-26]. This hepatic induction system shortens the induction time compared to the previously reported one-step system. Therefore, we consider it as an optimized one-step hepatic induction system.
We also identified induced hepatocyte-like cells from ADSCs and BMSCs in terms of morphology, as well as gene and protein expression. Our results revealed a similar hepatic differentiation potential of these two cell types in vitro. In vivo, we established an animal model of acute liver failure and proved that ADSCs and BMSCs engrafted into the injured liver showed similar therapeutic effects in the improvement of liver function. The results in our study suggest that BMSCs and ADSCs may serve as ideal seed cells for treating liver injury.
In conclusion, we developed a new protocol for the preparation of mouse BMSCs and established a new hepatic induction system. Our findings proved that ADSCs resembled BMSCs in the hepatic differentiation potential in vitro and in vivo. ADSCs can be obtained more easily and are less invasive than BMSCs. Therefore, ADSCs may be a more suitable seed cell type for use in cell transplantion or liver tissue engineering. In future experiments, we will explore the roles of ADSCs in liver tissue engineering.
Liver transplantation remains the only effective treatment for liver failure. However, its clinical application is limited by donor organs and immune rejection. To this end, hepatocyte transplantation and a bio-artificial liver support system are two potential surrogate complementary therapies for patients with liver failure. Hepatocyte-like cells can be induced from mesenchymal stem cells (MSCs) for xenotransplantation and have been demonstrated to perform hepatocyte functions in preclinical animal studies. However, the best type of MSCs has not yet been investigated and therefore it is important to screen for an ideal seed cell type for use in cell transplantation or a bio-artificial liver support system.
MSCs are non-hematopoietic multipotent stem cells that can be isolated from multiple tissues, such as bone marrow, adipose tissue, cord blood, and amniotic fluid. Bone marrow stromal cells (BMSCs) are the most extensively studied and fertile stem cell source used in regenerative medicine and liver tissue engineering. More recently, adipose-derived stromal cells (ADSCs) were identified as another promising and extensively studied stem cells for use, especially in liver tissue engineering. Although both ADSCs and BMSCs can be induced into hepatocyte-like cells, there is no uniform culture system to evaluate their hepatic differentiation potential. Therefore, it is necessary to establish an ideal system to improve the efficiency of hepatic induction.
A new protocol for the preparation of mouse BMSCs and a new hepatic induction system were established. This study proved that ADSCs resembled BMSCs in the hepatic differentiation potential in vitro and in vivo. ADSCs can be obtained more easily and are less invasive than BMSCs. Therefore, ADSCs may serve as a more suitable seed cell type for use in cell transplantion or liver tissue engineering.
ADSCs are likely to serve as suitable seed cells for use in cell transplantion or liver tissue engineering.
MSC transplant is a promising alternative therapy for some liver diseases. Availability of large number of functional hepatocytes is essential for cytotherapy. Both ADSCs and BMSCs can be induced into hepatocyte-like cells. Therefore, both ADSCs and BMSCs may be suitable seed cells for use in cell transplantion or liver tissue engineering.
In this paper, the authors investigated a novel method of generating differentiated hepatocytes from two sources of mesenchymal stem cells and tested their ability to recover liver damage in an experimental mouse model. The paper thus adressed an important question as stem cell therapy could help overcome current limitations of treatment for metabolic liver diseases and liver injury.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ocker M S- Editor: Wei LJ L- Editor: Ma JY
E- Editor: Ma YJ
| 1. | Lee SY, Kim HJ, Choi D. Cell sources, liver support systems and liver tissue engineering: alternatives to liver transplantation. Int J Stem Cells. 2015;8:36-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Hu C, Li L. In vitro culture of isolated primary hepatocytes and stem cell-derived hepatocyte-like cells for liver regeneration. Protein Cell. 2015;6:562-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Kellner J, Sivajothi S, McNiece I. Differential properties of human stromal cells from bone marrow, adipose, liver and cardiac tissues. Cytotherapy. 2015;17:1514-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Park KM, Hussein KH, Ghim JH, Ahn C, Cha SH, Lee GS, Hong SH, Yang S, Woo HM. Hepatic differentiation of porcine embryonic stem cells for translational research of hepatocyte transplantation. Transplant Proc. 2015;47:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Yang L, Wang Y, Wang X, Liu Y. Effect of allogeneic umbilical cord mesenchymal stem cell transplantation in a rat model of hepatic cirrhosis. J Tradit Chin Med. 2015;35:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH, Du HT, Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013;19:4702-4717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Zheng Z, Li X, Li Z, Ma X. Artificial and bioartificial liver support systems for acute and acute-on-chronic hepatic failure: A meta-analysis and meta-regression. Exp Ther Med. 2013;6:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Yang D, Wang ZQ, Deng JQ, Liao JY, Wang X, Xie J, Deng MM, Lü MH. Adipose-derived stem cells: A candidate for liver regeneration. J Dig Dis. 2015;16:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Yang T, Wang Y, Jiang S, Liu X, Yu Z. Hepatocyte growth factor-induced differentiation of bone mesenchymal stem cells toward hepatocyte-like cells occurs through nuclear factor-kappa B signaling in vitro. Cell Biol Int. 2016;40:1017-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kato T, Okochi H, Ochiya T. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Deng L, Kong X, Liu G, Li C, Chen H, Hong Z, Liu J, Xia J. Transplantation of Adipose-Derived Mesenchymal Stem Cells Efficiently Rescues Thioacetamide-Induced Acute Liver Failure in Mice. Transplant Proc. 2016;48:2208-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Huang B, Cheng X, Wang H, Huang W, la Ga Hu Z, Wang D, Zhang K, Zhang H, Xue Z, Da Y. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med. 2016;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Xu L, Wang S, Sui X, Wang Y, Su Y, Huang L, Zhang Y, Chen Z, Chen Q, Du H. Mesenchymal Stem Cell-Seeded Regenerated Silk Fibroin Complex Matrices for Liver Regeneration in an Animal Model of Acute Liver Failure. ACS Appl Mater Interfaces. 2017;9:14716-14723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Kim BK, Chung KW, Sun HS, Suh JG, Min WS, Kang CS, Sim SI, Shin WS, Kim CC. Liver disease during the first post-transplant year in bone marrow transplantation recipients: retrospective study. Bone Marrow Transplant. 2000;26:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Feng Z, Li C, Jiao S, Hu B, Zhao L. In vitro differentiation of rat bone marrow mesenchymal stem cells into hepatocytes. Hepatogastroenterology. 2011;58:2081-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang PH, Mao N. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, Zhang Y, Mao N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 410] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 18. | Kitano Y, Radu A, Shaaban A, Flake AW. Selection, enrichment, and culture expansion of murine mesenchymal progenitor cells by retroviral transduction of cycling adherent bone marrow cells. Exp Hematol. 2000;28:1460-1469. [PubMed] |
| 19. | Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 393] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Dong J. Direct comparison of different coating matrix on the hepatic differentiation from adipose-derived stem cells. Biochem Biophys Res Commun. 2015;456:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Xin J, Ding W, Hao S, Jiang L, Zhou Q, Wu T, Shi D, Cao H, Li L, Li J. Human bone marrow mesenchymal stem cell-derived hepatocytes express tissue inhibitor of metalloproteinases 4 and follistatin. Liver Int. 2015;35:2301-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Lu T, Yang C, Sun H, Lv J, Zhang F, Dong XJ. FGF4 and HGF promote differentiation of mouse bone marrow mesenchymal stem cells into hepatocytes via the MAPK pathway. Genet Mol Res. 2014;13:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Ye D, Li T, Heraud P, Parnpai R. Effect of Chromatin-Remodeling Agents in Hepatic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells In Vitro and In Vivo. Stem Cells Int. 2016;2016:3038764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev. 2011;7:103-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Stock P, Brückner S, Winkler S, Dollinger MM, Christ B. Human bone marrow mesenchymal stem cell-derived hepatocytes improve the mouse liver after acute acetaminophen intoxication by preventing progress of injury. Int J Mol Sci. 2014;15:7004-7028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Zhang YN, Lie PC, Wei X. Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy. 2009;11:548-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |