Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6482
Peer-review started: March 3, 2017
First decision: June 3, 2017
Revised: June 22, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: September 21, 2017
Processing time: 204 Days and 1.9 Hours
To evaluate the accuracy and best cut-off value of fecal calprotectin (FC) and fecal lactoferrin (FL) to predict disease recurrence in asymptomatic patients presenting with anastomotic strictures.
This was a longitudinal single tertiary center study based on prospectively collected data (recorded in a clinical database created for this purpose) performed between March 2010 and November 2014. Crohn’s disease (CD) patients with anastomotic stricture who submitted to postoperative endoscopic evaluation were included. Stools were collected on the day before bowel cleaning for FC and FL. Endoscopic balloon dilation (EBD) was performed if the patient presented an anastomotic stricture not traversed by the colonoscope, regardless of patients’ symptoms. Successful dilation was defined as passage of the colonoscope through the dilated stricture into the neotermimal ileum. Postoperative recurrence was defined as a modified Rutgeerts score of ≥ i2b.
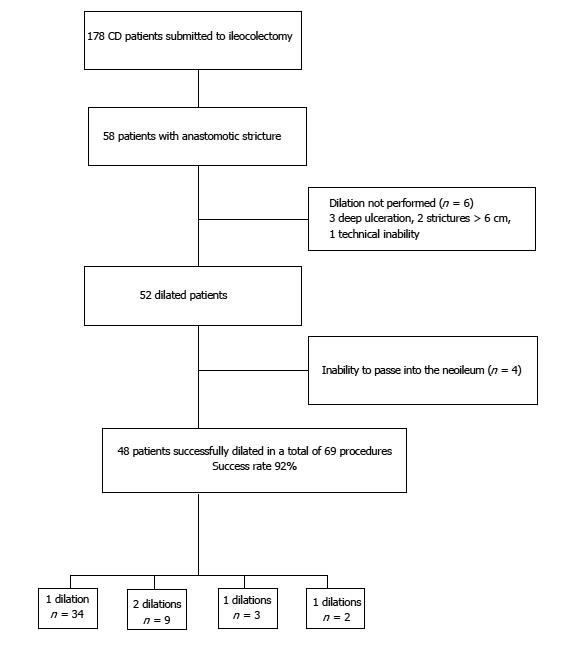
In a total of 178 patients who underwent colonoscopy, 58 presented an anastomotic stricture, 86% were asymptomatic, and 48 (54% male; median age of 46.5 years) were successfully dilated. Immediate success rate was 92% and no complications were recorded. FC and FL levels correlated significantly with endoscopic recurrence (P < 0.001) with an optimal cut-off value of 90.85 µg/g (sensitivity of 95.5%, specificity of 69.2%, positive predictive value (PPV) of 72.4%, negative predictive value (NPV) of 94.7% and accuracy of 81%] for FC and of 5.6 µg/g (sensitivity of 77.3%, specificity of 69.2%, PPV of 68%, NPV of 78.4% and accuracy of 72.9%) for FL.
Fecal markers are good predictors of CD endoscopic recurrence in patients with asymptomatic anastomotic stricture. FC and FL may guide the need for EBD in this context.
Core tip: This longitudinal study evaluated the accuracy of fecal calprotectin (FC) and fecal lactoferrin (FL) to predict disease recurrence in postoperative Crohn’s disease asymptomatic patients with an anastomotic stricture. FC and FL levels accurately predicted en-doscopic recurrence in the presence of anastomotic stricture and thus may guide the need for endoscopic balloon dilation (EBD) in this context. A normal value of fecal markers can reassure clinicians and be safely used to avoid balloon dilation if we only aim to diagnose recurrence. A high value of fecal markers has a high likelihood of recurrence so EBD should be performed in order to provide adequate endoscopic therapy and adjust or optimize medical therapy.
- Citation: Lopes S, Andrade P, Rodrigues-Pinto E, Afonso J, Macedo G, Magro F. Fecal marker levels as predictors of need for endoscopic balloon dilation in Crohn’s disease patients with anastomotic strictures. World J Gastroenterol 2017; 23(35): 6482-6490
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6482.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6482
Crohn’s disease (CD) is a chronic inflammatory disorder with progression to penetrating or stricturing phenotype as part of the natural history. More than half of CD patients experience these complications during disease lifetime and will need surgery[1,2]. It is well known that disease recurrence proximal or at the anastomosis is almost universal, with progression to luminal narrowing and stricturing behavior[3]. It is known that despite the permanent reduction of luminal caliber and disease progression, the majority of patients remain asymptomatic, with normal biomarkers. As the primary therapeutic goal of CD has shifted from clinical remission to achieving mucosal healing[4,5], it may be important to access the mucosa proximal to strictures to evaluate disease recurrence and escalate therapy if needed. Over the last decade there is increasing evidence for endoscopic balloon dilation (EBD) as a safe and minimally invasive effective method for the treatment of stricturing disease[6-14]. Median technical success has been reported as 90%, with major complication rate around 3%-10%[6,9,10]. Symptomatic recurrence is common, with a reported frequency ranging from 13% to 100%[12]. However, it has been shown that repeated dilations do not reduce the efficacy of the procedure and may prevent surgery in compliant patients[8-12].
Fecal markers, namely fecal calprotectin (FC) and fecal lactoferrin (FL), have proved to be useful and accurate non-invasive tools in evaluating disease activity in CD. Recent works have also demonstrated their validity in diagnosing recurrence in the postoperative setting, suggesting that normal values of fecal biomarkers can obviate the need for endoscopic evaluation[15-18]. In patients with elevated levels of fecal biomarkers, endoscopy should be performed in order to confirm recurrence and escalate therapy if indicated[19]. To our knowledge there are no studies evaluating the performance of FC and FL in patients with asymptomatic anastomotic CD strictures and limited data is available on the long-term effect of medical therapy escalation after balloon dilation of anastomotic strictures.
The aims of this study were, therefore, to evaluate the accuracy of FC and FL in the diagnosis of recurrent disease in the neoterminal ileum in asymptomatic/mild disease patients that had undergone bowel resection for CD and present with stricture of the anastomosis, to evaluate the best cut-off value of FC and FL to diagnose recurrence, and to evaluate the immediate technical success and safety rate of EBD.
A longitudinal single tertiary center study based on prospectively collected data (recorded in a clinical database created for this purpose), was performed between March 2010 and November 2014. All patients gave informed written consent to participate in the study that was approved by the Ethics Committee of our Institution. From a cohort of consecutive CD patients who submitted to postoperative endoscopic evaluation after ileocolectomy, we selected the group of patients with anastomotic stricture. All patients were followed at our inflammatory bowel disease (IBD) outpatient clinic and were referred for endoscopic evaluation at our institution.
Inclusion criteria were definitive diagnosis of CD established by standard clinical, radiographic, endoscopic and histological criteria[20], previous ileocolectomy, and existence of an anastomotic stricture. Exclusion criteria were: age less than 18-years-old; strictures length greater than 6 cm; fistulae or deep ulceration of the strictured segment; technical impossibility of passing the catheter/balloon through the strictures; and active disease in the colon or upper digestive tract.
Clinical disease activity was assessed on the day of endoscopic examination, according to the clinical criteria of the Harvey-Bradshaw index (HBI)[21,22]. Clinically inactive disease was defined as HBI < 5. All procedures were performed under propofol sedation, with CO2 insufflation, by a single senior endoscopist (SL) and on an outpatient basis. Mechanical intestinal bowel preparation was done the day before colonoscopy with polyethylene glycol bowel preparation solution (Klean Prep®; Helsinn Birex Pharmaceuticals, Dublin, Ireland). Stool samples were collected the day before beginning bowel preparation (preferably from the first stool in the morning) and then kept in the refrigerator until being brought to the hospital. For FC, within a maximum 7 d after collection, stools were extracted in accordance with the manufacturer’s instructions, using the Fecal Sample Preparation Kit (Roche Diagnostics, Mannheim, Germany) and analyzed using immunoassay (EliA Calprotectin®; Thermo Fisher Scientific, Freiburg, Germany). For FL evaluation, stools were stored at -80 °C upon arrival at the laboratory, and samples were thawed and analyzed using a commercially available quantitative enzyme-linked immunoassay test (IBD-Scan®; Tech-Lab, Blacksburg, VA, United States). The techniques for measurement of fecal markers followed the manufacturer´s guidelines.
Postoperative disease activity of the neoterminal ileum was evaluated according to the modified Rutgeerts score[23] (i0: no lesions in the distal ileum; i1: < 5 aphthous lesions in the distal ileum; i2a: lesions confined to the ileocolonic anastomosis, including anastomotic strictures; i2b: > 5 aphthous ulcers or larger lesions, with normal mucosa in between, in the neoterminal ileum, with or without anastomotic lesions; i3: diffuse aphthous ileitis with diffusely inflamed mucosa; i4: large ulcers with diffuse mucosal inflammation or nodules or strictures in the neoterminal ileum). Postoperative recurrence was defined as a modified Rutgeerts score of ≥ i2b.
EBD was performed if the patient presented an anastomotic stricture not traversed by the colonoscope, regardless of the patient’s symptoms. All dilations were performed with the same type of colonoscope (Olympus® CF type H180AL; Tokyo, Japan) under fluoroscopic control to allow the endoscopist to characterize the strictures, exclude peristricture fistulae, evaluate the optimal diameter of the balloon to use and to prompt identify any complication during the procedure. Dilations were performed with a guidewire (Boston Scientific® Jagwire 0.035 in; Marlborough, MA, United States) placed through the strictures [after contrast instillation through a catheter (Olympus® Ball Tip/6Fr) over which a through-the-scope balloon (Cook Medical®, Bloomington, IN, United States) was placed]. The balloon was inflated using contrast agent and the pressure maintained for 2 min, to a maximum diameter of 18 mm.
The procedure could be repeated at the discretion of the endoscopist. Successful dilation was defined as passage of the colonoscope through the dilated stricture into the neotermimal ileum. Only patients with a successful dilation were analyzed, as progression to the neoterminal ileum was mandatory to evaluate disease recurrence. Major complications were defined as major bleeding requiring surgery, blood transfusion or hospital admission and perforation. Minor, self-limited bleeding was not registered as a complication.
SPSS 20.0 for Windows (SPSS, Chicago, IL, United States) was used for statistical analysis. Categorical variables were described as absolute frequencies (n) and relative frequencies (%); continuous variables were described as mean ± SD (parametric distributions) or as median and percentiles (non-parametric distributions). The normality of the continuous variables was tested using the Kolmogorov-Smirnov test and the respective histogram. Student’s t-test was used to compare quantitative variables with a normal distribution, and the Mann-Whitney U test was used to compare the quantitative variables without a normal distribution. Any groups with more than two quantitative variables were compared using the Kruskal-Wallis test. A Pearson χ2 test was used to compare categorical variables. Kaplan-Meier analysis with log rank statistics was used to estimate event-free interval. A logistic regression was performed to assess predictors of disease recurrence and need for dilation. Statistical significance was set at P < 0.05.
One hundred and seventy-eight consecutive CD patients (51.7% male; median age 46.4 years) who previously submitted to right ileocolectomy were evaluated by colonoscopy. At the time of endoscopic evaluation, 31 (17.4%) patients complained of subocclusive symptoms, and 66.3% of patients were being treated with immunomodulators and 38.2% with biologics.
Of the 178 evaluated patients, 58 (32.6%) pr-esented with an anastomotic stricture. The majority were asymptomatic, with only 8 (13.8%) patients presenting with subocclusive symptoms. All patients were in clinical remission (HBI < 5 in 83.3%) or with mild clinical disease (HBI 5-7 in 16.7%). Among the 58 patients presenting with anastomotic stricture, 52 were dilated (6 were excluded due to deep ulceration, stricture size > 6 cm or technical inability). Of the total 52 dilated patients, 4 were excluded as it was not possible to evaluate the neoterminal ileum (Figure 1). Baseline characteristics of the 48 successfully dilated patients are summarized in Table 1. The majority were men (54%) with a median age of 46.5 years. At the time of dilation, 42% of patients were being treated with biologics and 54% with immunomodulators.
| Characterization | n (%) |
| Women, | 22 (45.8) |
| Median time between diagnosis and surgery, mo (IQR) | 36.0 (14.0-120.0) |
| Median time between surgery and endoscopic evaluation, mo (IQR) | 114.5 (60.8-199.0) |
| Median age at endoscopic evaluation, yr (IQR) | 46.5 (39.3-53.4) |
| Montreal classification | |
| Age at diagnosis | |
| A1, ≤ 16 yr | 6 (12.5) |
| A2, 17-40 yr | 33 (68.8) |
| A3, > 40 yr | 9 (18.8) |
| Location | |
| L1: ileal | 30 (62.5) |
| L3: ileocolonic | 17 (35.4) |
| L4: upper gastrointestinal tract | 1 (2.1) |
| Behavior | |
| B1: non-stricturing, non-penetrating | 1 (2.1) |
| B2: stricturing | 27 (56.2) |
| B3: penetrating | 20 (41.7) |
| Perianal disease | 14 (29.2) |
| Smoking | |
| Never | 26 (54.2) |
| Current | 12 (25.0) |
| Past | 10 (20.8) |
| Concomitant treatment | |
| Corticosteroids | 9 (18.8) |
| Immunomodulators (Azathioprine/6MP/ Methotrexate) | 26 (54.2) |
| Biologics (Infliximab, adalimumab) | 20 (41.7) |
| 5-ASA | 17 (35.4) |
| Median fecal calprotectin, μg/g (IQR) | 134.0 (35.3-321.0) |
| Median, fecal lactoferrin, μg/g (IQR) | 6.2 (2.0-22.4) |
| Modified Rutgeerts score | |
| i0, i1, i2a | 26 (54.2) |
| i2b, i3, i4 | 22 (45.8) |
| Subocclusive symptoms | 8 (16.7) |
| Harvey-Bradshaw index | |
| Remission (HBI < 5) | 40 (83.3) |
| Mild disease (HBI 5-7) | 8 (16.7) |
| Need of redilation | 14 (29.2) |
| Surgery after dilation | 2 (1.4) |
| Median follow up, mo (IQR) | 34.0 (27.5-52.5) |
Overall, technical success rate was 92% (48/52) and no major complications were recorded. The 18-mm balloon diameter was used in 69.2% of the patients and the 15-mm diameter in 25.0%. Redilation was required in 14 patients (29.2%) during a median follow-up time of 34 mo (27.5-52.5 mo). During the follow-up period, only 2 patients needed surgery, both cases due to long strictures that did not allow EBD (Table 2).
| Characterization | n (%) |
| Patients with stricture | 58 (32.6) |
| Stricture type | |
| Anastomotic | 58 (100) |
| Dilated patients | 52 (29.2) |
| Causes for non-dilation | |
| Length of stenosis | 2 (33.3) |
| Ulceration of mucosa | 3 (50.0) |
| Technical inability | 1 (16.7) |
| Successful dilated patients | |
| Balloon size, n = 52 | 48 (92.3) |
| 15 mm | 13 (25.0) |
| 16.5 mm | 3 (5.8) |
| 18 mm | 36 (69.2) |
| Complications | 0 (0.0) |
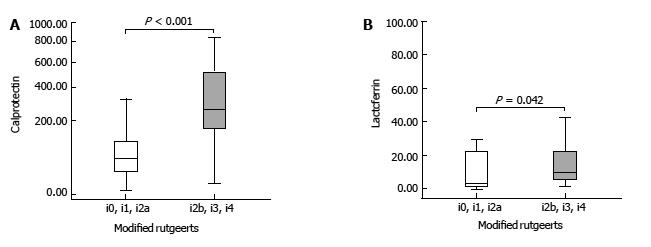
Of the 48 successfully dilated patients, 22 presented with endoscopic recurrence defined as modified Rutgeerts score of ≥ i2b. Of these, 16 patients presented with severe disease (i3 = 3 and i4 = 13). Recurrence was diagnosed only after dilation of the anastomotic stricture and intubation of the neoterminal ileum. Comparing FC and FL levels in patients with endoscopic recurrence and in patients with endoscopic remission we found a significantly higher level in patients with endoscopic recurrence [FC: 257.0 μg/g, interquartile range (IQR): 161.0-565.0 μg/g) vs 53.9 μg/g, IQR: 23.9-146.0 μg/g; P < 0.001 and FL: 9.1 μg/g, IQR: 5.5-27.8 μg/g vs 3.9 μg/g, IQR: 1.5-21.9 μg/g; P = 0.042] (Figure 2). No other clinical variable or biomarker reached statistical difference between the two groups (Table 3).
| Endoscopic recurrence,n = 22 | No endoscopic recurrence,n = 26 | P value | |
| Sex, M:F | 11:11 | 15:11 | 0.404 |
| Median age, yr (IQR) | 47.0 (35.5-53.3) | 46.5 (39.8-54.8) | 0.472 |
| Median hemoglobin, g/dL (IQR) | 13.8 (12.5-15.1) | 13.9 (13.1-15.0) | 0.715 |
| Median albumin, g/L (IQR) | 42.4 (36.1-45.9) | 42.1 (39.8-44.6) | 0.886 |
| Median C-reactive protein, mg/L (IQR) | 5.6 (1.6-8.2) | 2.4 (0.9-9.9) | 0.457 |
| Median fecal calprotectin, μg/g (IQR) | 257.0 (161.0-565.0) | 53.9 (23.9-146.0) | < 0.001 |
| Median fecal lactoferrin, μg/g (IQR) | 9.1 (5.5-27.8) | 3.9 (1.5-21.9) | 0.042 |
| Smoking, yes/no/past | 3/13/6 | 9/13/4 | 0.196 |
| Subocclusive symptoms, yes/no | 2/20 | 6/20 | 0.183 |
| HBI, remission/mild | 17/5 | 24/2 | 0.145 |
| Concomitant treatment | |||
| Anti-TNF- α agents, yes/no | 12/10 | 14/12 | 0.596 |
| Immunomodulators, yes/no | 9/13 | 11/15 | 0.578 |
| Steroids, yes/no | 4/18 | 5/21 | 0.611 |
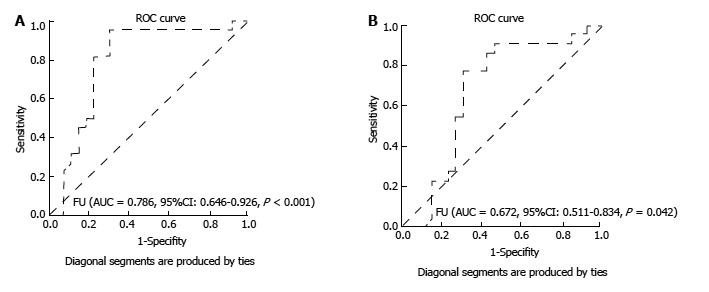
The best calculated cut-off value for FC to predict recurrent disease was 90.85 µg/g, with a sensitivity of 95.5%, a specificity of 69.2%, a positive predictive value (PPV) of 72.4%, a negative predictive value (NPV) of 94.7% and an accuracy of 81%. The area under the ROC curve for diagnosing endoscopic recurrence was 0.786 (95%CI: 0.646-0.926, P < 0.05) for FC. Concerning FL, the best calculated cut-off was 5.6 µg/g, with sensitivity of 77.3%, specificity of 69.2%, PPV of 68%, NPV of 78.4% and accuracy of 72.9%. The area under the ROC curve for diagnosing endoscopic recurrence was 0.672 (95%CI: 0.511-0.834, P = 0.042) (Figure 3).
One of the major drawbacks of CD surgical therapy is the high recurrence rate of the disease at the anastomotic site or in the neoterminal ileum, with the development of stricture and bowel obstruction[8]. As the primary therapeutic goal of CD has shifted from clinical remission to achieving mucosal healing[4,5], it may be important to access the mucosa proximal to strictures to evaluate disease recurrence and escalate therapy if needed. The recently published POCER trial[16] demonstrated that initial postoperative treatment according to clinical risk stratification and early endoscopic evaluation and step-up therapy if there was recurrent disease was superior to standard medical therapy.
In this study, endoscopy was the gold standard method to define recurrent disease. With respect to non-invasive methods, both serum biomarkers and clinical activity indexes have demonstrated a poor correlation with disease activity[24-26]. The use of fecal markers in the postoperative period has been studied in small groups of patients with variable results. Recently, however, some studies evaluated the value of fecal biomarker measurement after surgery for CD. In 2015, Wright et al[15] demonstrated that FC was sensitive enough to diagnosis CD recurrence in 135 patients submitted to bowel resection with a high enough NPV to reassure clinicians that few patients with recurrence would be missed. More recently, Lopes et al[27] showed that both FC and FL strongly correlated with endoscopic findings in the evaluation of CD after surgery and accurately predicted endoscopic recurrence in 99 CD patients who submitted to ile-ocolonic resection.
The results of both of these studies suggests that fecal biomarkers may be incorporated in the postoperative management algorithm, both to diagnose recurrence and to assess response to therapy. In our study, we evaluated if in asymptomatic CD patients with anastomotic strictures not traversed by the colonoscope, fecal markers perform as well as a predictor of disease recurrence, defining groups of patients that will need more invasive methods. In this series of 48 patients with strictures, only 18% complained of subocclusive symptoms. Similarly to what has been published[28-30], we also did not find any correlation between patient symptoms, serum bi-omarkers and HBI and endoscopic or radiographic findings, supporting the belief that using only symptoms or C-reactive protein levels to inform treatment decisions may increase the risk of disease progression and complications.
It is controversial whether or not asymptomatic strictures should be endoscopically treated. Despite being a safe and minimally invasive technique, there is a 3%-10%[6,9,10] described risk of major complications, especially in centers with limited procedural volume per year. Our data confirmed the safety and efficacy of EBD in the context of CD anastomotic strictures, with a technical success rate of 92%. We had no serious complications, which may be explained by several factors: careful patient selection; use of fluoroscopic image to evaluate, in real time, stricture characteristics and the therapeutic procedure indicated; maximum diameter of the balloon used (18 mm); use of carbon dioxide as type of insufflation; and experience of the endoscopist performing the technique with a uniform technical approach. The dilation of the anastomosis allowed the diagnosis of recurrence in 22 patients, that otherwise would have been missed if we only relied on symptoms or biochemical markers.
We used the modified Rutgeerts score to diagnose endoscopic recurrence, although it is not yet validated. Despite being used for several decades in clinical trials and clinical practice, the Rutgeerts score is also an invalidated score. In this study, we chose to use the modified Rutgeerts score, as demonstrated in a previous work[27] as having a better correlation between the modified score and fecal markers to diagnose recurrent disease. Recurrence was defined by a modified Rutgeerts score of i2b, not considering the presence of only anastomotic stricture as a criterion, as many other factors may be implicated in stricture development[1,2]. In that paper[27] the calculated best cut-off level for FC for predicting recurrence was 100 µg/g, with a sensitivity of 74% and a NPV of 91%. This is in accordance with other studies[15] that defined in the post-operative setting a higher cut-off than that of 50 μg/g used to diagnose inflammatory bowel disease[31].
In the present study, both FC and FL were also significantly higher in patients with endoscopic recurrence, with an area under the receiver operating characteristic curve for detection of endoscopic recurrence of 0.786 for FC and of 0.672 for FL. The best cut-off value of FC as predictor of recurrence was 90.85 μg/g, with sensitivity of 95.5%, NPV of 94.7% and accuracy of 81%. If we adopted the commonly used cut-off value of 7.25 μg/g for lactoferrin[32-34], we would have missed patients with recurrence (false negative results). The cut-off value of 5.6 μg/g had sensitivity of 77.3%, NPV of 78.4% and accuracy of 72.9%. Our findings support the potential value of these two noninvasive markers in the monitoring of patients submitted to bowel resection and presenting with an anastomotic stricture.
Indeed, a FC and/or FL concentration lower than 90.85 μg/g and 5.6 μg/g respectively, have high accuracy to exclude disease recurrence, with no need to further therapeutic intervention. This may be of particular interest in centers with low expertise in EBD and/or with a lower procedural volume per year, in order to avoid complications and/or be used as an indication for referring patients to tertiary centers. If these results are reproduced and validated by others, in a large number of patients, this conservative strategy could be adopted, reserving balloon dilation for symptomatic patients and those with high levels of fecal markers, in order to facilitate step-up therapy.
To our knowledge this is the first report on the performance of FC and FL in the context of anastomotic strictures in CD. Our results suggest that low values of fecal markers can predict, with a great amount of certainty, disease remission. This may avoid application of endoscopic dilation in asymptomatic patients in centers with less endoscopic expertise, reassuring physicians that the use of fecal markers serves as a good indicator to monitor disease recurrence. The serial monitoring of FC and FL can help to make decisions in indeterminate results and a persistently elevated value may serve as another useful indicator when considering therapy intensification.
In conclusion, postoperative FC and FL levels accurately predicted endoscopic recurrence in the presence of anastomotic stricture. Considering that a significant number of patients remain asymptomatic, with normal serum biomarkers, despite the permanent reduction of luminal caliber, a normal value of fecal markers can reassure clinicians and be safely used to avoid balloon dilation if we only aim to diagnose recurrence. In asymptomatic patients with a high FC and/or FL level, there is a great chance of having disease activity proximal to the stricture, so EBD should be performed in order to provide adequate endoscopic therapy and adjust or optimize medical therapy.
Recurrent disease in the neoterminal ileum and anastomotic strictures are frequent complications of Crohn´s disease (CD). Despite the permanent reduction of luminal caliber and disease progression, the majority of patients remain asymptomatic. Fecal calprotectin (FC) and lactoferrin have been suggested as surrogate non-invasive markers for diagnosing postoperative disease recurrence. There are no studies evaluating the performance of fecal markers as predictors of disease recurrence in asymptomatic patients with an anastomotic stricture.
The results demonstrated that FC and lactoferrin are good predictors of CD endoscopic recurrence in patients with asymptomatic anastomotic stricture and may guide the need for endoscopic balloon dilation in this context.
A normal value of fecal markers can reassure clinicians and be safely used to avoid balloon dilation if we only aim to diagnose recurrence. A high value of fecal markers has a high likelihood of recurrence, so endoscopic balloon dilation should be performed in order to provide adequate endoscopic therapy and adjust or optimize medical therapy.
Fecal markers may avoid the need of endoscopic balloon dilation in asymptomatic patients with anastomotic stricture if we only aim to diagnose disease recurrence.
Calprotectin is a protein complex, constituting up to 60% of neutrophil cytosol protein that is released upon neutrophil activation. Lactoferrin, an iron-binding protein, is the main component of secondary granules that degranulate during inflammatory process. Both these proteins are remarkably stable and resistant to degradation, easily detected and have been proved to reflect endoscopic disease activity in CD, predicting endoscopic inflammation and being a surrogate marker of mucosal healing.
The authors have performed a very interesting and important study. They concluded that postoperative FC and FL levels accurately predicted endoscopic recurrence in the presence of anastomotic stricture.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Portugal
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lankarani KB, Sun SY S- Editor: Ma YJ
L- Editor: A E- Editor: Ma YJ
| 1. | Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: impact on disease course and insights into the aetiology of its effect. J Crohns Colitis. 2014;8:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | De Cruz P, Kamm MA, Prideaux L, Allen PB, Desmond PV. Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2012;18:758-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 567] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Solberg IC, Lygren I, Jahnsen J. Mucosal healing after initial treatment may be a prognostic marker for long-term outcome in inflammatory bowel disease. Gut. 2008;57:A15. |
| 5. | De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2013;19:429-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 6. | Paine E, Shen B. Endoscopic therapy in inflammatory bowel diseases (with videos). Gastrointest Endosc. 2013;78:819-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Koltun WA. Long-term value of endoscopic dilatation for Crohn’s strictures. Gut. 2010;59:288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Atreja A, Aggarwal A, Dwivedi S, Rieder F, Lopez R, Lashner BA, Brzezinski A, Vargo JJ, Shen B. Safety and efficacy of endoscopic dilation for primary and anastomotic Crohn’s disease strictures. J Crohns Colitis. 2014;8:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Scimeca D, Mocciaro F, Cottone M, Montalbano LM, D’Amico G, Olivo M, Orlando R, Orlando A. Efficacy and safety of endoscopic balloon dilation of symptomatic intestinal Crohn’s disease strictures. Dig Liver Dis. 2011;43:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Hassan C, Zullo A, De Francesco V, Ierardi E, Giustini M, Pitidis A, Taggi F, Winn S, Morini S. Systematic review: Endoscopic dilatation in Crohn’s disease. Aliment Pharmacol Ther. 2007;26:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Mueller T, Rieder B, Bechtner G, Pfeiffer A. The response of Crohn’s strictures to endoscopic balloon dilation. Aliment Pharmacol Ther. 2010;31:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Van Assche G, Vermeire S, Rutgeerts P. Endoscopic therapy of strictures in Crohn’s disease. Inflamm Bowel Dis. 2007;13:356-358; discussion 362-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Hoffmann JC, Heller F, Faiss S, von Lampe B, Kroesen AJ, Wahnschaffe U, Schulzke JD, Zeitz M, Bojarski C. Through the endoscope balloon dilation of ileocolonic strictures: prognostic factors, complications, and effectiveness. Int J Colorectal Dis. 2008;23:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Ferlitsch A, Reinisch W, Püspök A, Dejaco C, Schillinger M, Schöfl R, Pötzi R, Gangl A, Vogelsang H. Safety and efficacy of endoscopic balloon dilation for treatment of Crohn’s disease strictures. Endoscopy. 2006;38:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Wright EK, Kamm MA, De Cruz P, Hamilton AL, Ritchie KJ, Krejany EO, Leach S, Gorelik A, Liew D, Prideaux L. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology. 2015;148:938-947.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 16. | De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, Liew D, Prideaux L, Lawrance IC, Andrews JM. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 439] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 17. | Boschetti G, Laidet M, Moussata D, Stefanescu C, Roblin X, Phelip G, Cotte E, Passot G, Francois Y, Drai J. Levels of Fecal Calprotectin Are Associated With the Severity of Postoperative Endoscopic Recurrence in Asymptomatic Patients With Crohn’s Disease. Am J Gastroenterol. 2015;110:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Qiu Y, Mao R, Chen BL, He Y, Zeng ZR, Xue L, Song XM, Li ZP, Chen MH. Fecal calprotectin for evaluating postoperative recurrence of Crohn’s disease: a meta-analysis of prospective studies. Inflamm Bowel Dis. 2015;21:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Yamamoto T. The clinical value of faecal calprotectin and lactoferrin measurement in postoperative Crohn’s disease. United European Gastroenterol J. 2015;3:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, Kupcinskas L, Mantzaris G, Travis S, Stange E; European Crohn’s and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 792] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 21. | Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2181] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 22. | Best WR. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw Index. Inflamm Bowel Dis. 2006;12:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Gecse K, Lowenberg M, Bossuyt P, D’Haens G. Sa1198 Agreement Among Experts in the Endoscopic Evaluation of Postoperative Recurrence in Crohn’s Disease Using the Rutgeerts Score. Gastroenterology. 2014;146:S227. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Karoui S, Ouerdiane S, Serghini M, Jomni T, Kallel L, Fekih M, Boubaker J, Filali A. Correlation between levels of C-reactive protein and clinical activity in Crohn’s disease. Dig Liver Dis. 2007;39:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 330] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 27. | Lopes S, Andrade P, Afonso J, Rodrigues-Pinto E, Dias CC, Macedo G, Magro F. Correlation Between Calprotectin and Modified Rutgeerts Score. Inflamm Bowel Dis. 2016;22:2173-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Regueiro M, Kip KE, Schraut W, Baidoo L, Sepulveda AR, Pesci M, El-Hachem S, Harrison J, Binion D. Crohn’s disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm Bowel Dis. 2011;17:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF, Florent C, Bouvry M, Mary JY, Modigliani R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 325] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Landi B, Anh TN, Cortot A, Soule JC, Rene E, Gendre JP, Bories P, See A, Metman EH, Florent C. Endoscopic monitoring of Crohn’s disease treatment: a prospective, ran-domized clinical trial. The Groupe d’Etudes Therapeutiques des Affections Inflammatoires Digestives. Gastroenterology. 1992;102:1647-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 181] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 486] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 32. | Lamb CA, Mansfield JC. Measurement of faecal calprotectin and lactoferrin in inflammatory bowel disease. Frontline Gastroenterol. 2011;2:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Zhou XL, Xu W, Tang XX, Luo LS, Tu JF, Zhang CJ, Xu X, Wu QD, Pan WS. Fecal lactoferrin in discriminating inflammatory bowel disease from irritable bowel syndrome: a diagnostic meta-analysis. BMC Gastroenterol. 2014;14:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Wang Y, Pei F, Wang X, Sun Z, Hu C, Dou H. Diagnostic accuracy of fecal lactoferrin for inflammatory bowel disease: a meta-analysis. Int J Clin Exp Pathol. 2015;8:12319-12332. [PubMed] |