Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6467
Peer-review started: June 15, 2017
First decision: July 17, 2017
Revised: July 26, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: September 21, 2017
Processing time: 98 Days and 14.8 Hours
To develop and validate a risk estimation of tumor recurrence following curative resection of operable hepatocellular carcinoma (HCC).
Data for 128 patients with operable HCC (according to Barcelona Clinic Liver Cancer imaging criteria) who underwent preoperative computed tomography (CT) evaluation at our hospital from May 1, 2013 through May 30, 2014 were included in this study. Follow-up data were obtained from hospital medical records. Follow-up data through May 30, 2016 were used to retrospectively analyze preoperative multiphasic CT findings, surgical histopathology results, and serum α-fetoprotein and thymidine kinase-1 levels. The χ2 test, independent t-test, and Mann-Whitney U test were used to analyze data. A P-value of < 0.05 was considered statistically significant.
During the follow-up period, 38 of 128 patients (29.7%) had a postoperative HCC recurrence. Microvascular invasion (MVI) was associated with HCC recurrence (χ2 = 13.253, P < 0.001). Despite postoperative antiviral therapy and chemotherapy, 22 of 44 patients with MVI experienced recurrence after surgical resection. The presence of MVI was 57.9% sensitive, 75.6% specific and 70.3% accurate in predicting postoperative recurrence. Of 84 tumors without MVI, univariate analysis confirmed that tumor margins, tumor margin grade, and tumor capsule detection on multiphasic CT were associated with HCC recurrence (P < 0.05). Univariate analyses showed no difference between groups with respect to hepatic capsular invasion, Ki-67 proliferation marker value, Edmondson-Steiner grade, largest tumor diameter, necrosis, arterial phase enhanced ratio, portovenous phase enhanced ratio, peritumoral enhancement, or serum α-fetoprotein level.
Non-smooth tumor margins, incomplete tumor capsules and missing tumor capsules correlated with postoperative HCC recurrence. HCC recurrence following curative resection may be predicted using CT.
Core tip: We discuss risk estimation for recurrence following curative resection of operable hepatocellular carcinoma that meets Barcelona Clinic Liver Cancer imaging criteria. Preoperative multiphasic computed tomography findings, including non-smooth tumor margins, incomplete tumor capsule and missing tumor capsule, can predict hepatocellular carcinoma recurrence following curative resection. Treatment should include wide margins during curative resection, followed by antiviral therapy and chemotherapy.
- Citation: Zhang W, Lai SL, Chen J, Xie D, Wu FX, Jin GQ, Su DK. Validated preoperative computed tomography risk estimation for postoperative hepatocellular carcinoma recurrence. World J Gastroenterol 2017; 23(35): 6467-6473
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6467
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third most common cause of cancer-related death worldwide[1-3]. Surgical resection and liver transplantation are potentially curative treatment options for patients with HCC[4]. However, the 5-year HCC recurrence rate may be as high as 70%[5]. Recent studies have shown that tumor size and number[6], microvascular invasion (MVI)[5,7-9] and α-fetoprotein (AFP) levels[10,11] are associated with recurrence following surgical resection. Although many potential risk factors for HCC postoperative recurrence have been described, a reliable preoperative method to estimate this risk has not been established.
Kanai et al[12] first described nodular HCC sub-classification based on microscopic, clinical and prognostic features; these being single nodular type, single nodular type with extra-nodular growth, and contiguous multinodular type. Studies have shown that non-smooth tumor margins are associated with increased MVI[13-16]. Although computed tomography (CT) examination is part of the standard of care for HCC evaluation, no study has demonstrated the validity of preoperative CT assessment to predict postoperative recurrence of solitary HCC.
Thymidine kinase 1 (TK1), a DNA synthesis enzyme, is an important serum proliferation marker that correlates with clinical stage and is an independent prognostic factor for recurrence-free survival in HCC[17-20]. However, to our knowledge, there are no reports about serum TK1 levels and postoperative HCC recurrence.
In this study, we retrospectively assessed whether preoperative CT findings could predict postoperative HCC recurrence. We specifically evaluated largest tumor diameter, tumor margins, tumor capsule, necrosis, peritumoral enhancement, tumor enhanced ratio and hepatic capsular invasion. Furthermore, we assessed whether MVI, serum AFP, and serum TK1 levels were correlated with postoperative recurrence of solitary HCC.
This retrospective study included 128 patients (105 males and 23 females), aged 24 to 79 years (mean, 47.7 years), with operable HCC (according to the Barcelona Clinic Liver Cancer imaging criteria) who underwent preoperative CT evaluation at our hospital from May 1, 2013 through May 30, 2014. Follow-up data were obtained from hospital medical records. HCC recurrence was defined as a pathologic or radiologic diagnosis of recurrent HCC during the follow-up period that ended on May 30, 2016. All patients in this study: (1) had no cancer-related treatment or liver biopsy prior to CT imaging; (2) underwent partial hepatectomy and had HCC diagnosis confirmed by histopathology; (3) had no macrovascular invasion or metastasis on CT imaging; and (4) had livers with a Child-Pugh classification of A or B. This study was approved by our hospital institutional review board and all patients provided written informed consent.
Hepatic CT images were obtained using a 64-MDCT scanner (SOMATOM Sensation 64; Siemens, Forchheim, Germany) with Z-axis modulation, a spiral pitch of 1, a 5 mm section thickness, a 2 mm reconstruction gap, a field of view of 311 mm, 120 kVp, 230 mA, and a standard reconstruction algorithm. Nonionic contrast medium (300 mg I/mL iopromide) was administered at an injection rate of 3 mL/s for a total dose of 100 mL. For the hepatic arterial and portovenous phases, scanning was begun approximately 25 and 60 s after contrast media injection, respectively. Equilibrium phase images were acquired approximately 180 s after contrast media injection. The scanning range was the whole-liver zone while patients held their breath. Coronary and sagittal images were reconstructed with 5 mm section thickness.
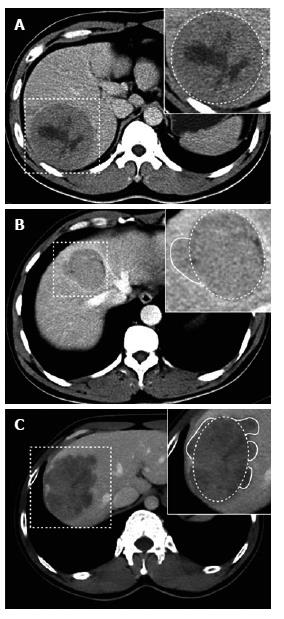
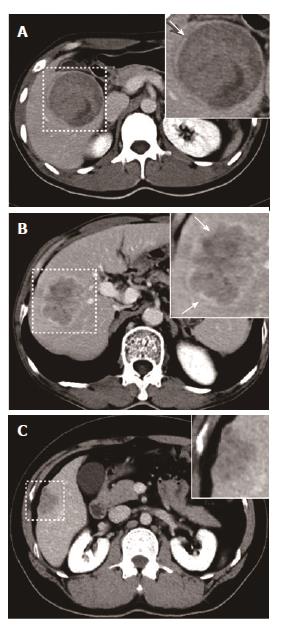
Tumor margins on the liver map (i.e. transverse, coronary and sagittal planes) were defined as three subtypes (smooth margin, non-smooth margin focal extranodular and non-smooth margin multinodular), based on previous reports[12-16,21,22]. The tumor margins were categorized into one of the following three grades: Grade 0: smooth margin; Grade 1: focal extranodular type; and Grade 2: multinodular type. The tumor capsule was defined as a linear and enhanced structure surrounding the tumor detected by equilibrium phase CT imaging. Tumor capsules were categorized into the following three groups: Grade 0: complete tumor encapsulation; Grade 1: incomplete tumor encapsulation; and Grade 2: no tumor encapsulation. Negative or positive peritumoral enhancement was determined by multi-phase CT imaging. Positive peritumoral enhancement was defined in comparison to liver parenchyma as a hyperdense area proximal to the tumor border during arterial phase imaging that changed to an isodense area during equilibrium phase imaging. The tumor enhanced ratio was calculated as (CTenhanced- CTunenhanced)/CTunenhanced.
All CT findings were reviewed by two radiologists experienced in liver CT evaluation: Lai SL (22 years of experience) and Xie D (21 years of experience). In the case of disagreements over CT findings, a third radiologist helped resolve discrepancies to achieve consensus.
The serum TK1 assay was performed using a commercial kit, based on an enhanced chemiluminescence dot blot assay. Serum AFP detection was carried out using enzyme immunoassay. Ki67 expression, hepatic capsular invasion and MVI were confirmed by immunohistochemistry. The Edmondson-Steiner (E-S) grade was determined using the WHO Liver Cancer Study Group guidelines[23].
Statistical analysis was performed using SPSS version 16.0 statistical software (SPSS, Chicago, IL, United States). An independent t-test was used to compare recurrence and non-recurrence groups with respect to largest tumor diameter, arterial phase enhanced ratio, portovenous phase enhanced ratio, serum AFP, serum TK1 and Ki67. Receiver operating characteristic curve analyses were used to determine the optimal cut-off value and diagnostic performance for P < 0.05. The chi-square test was used to compare tumor margins, necrosis, peritumoral enhancement, MVI and hepatic capsular invasion between groups. The tumor margin grade, E-S grade and tumor capsule were analyzed using the Mann-Whitney U test. A P-value of less than 0.05 was considered statistically significant.
Quantitative and qualitative findings are summarized in Table 1. MVI was higher in the recurrence group (22 of 38 patients) compared to the non-recurrence group (22 of 90 patients) (χ2 = 13.253, P < 0.001). Despite antiviral therapy and chemotherapy during follow-up, 22 of 44 patients with MVI experienced recurrence after surgical resection. The presence of MVI was 57.9% sensitive, 75.6% specific and 70.3% accurate in predicting postoperative recurrence.
| Risk factor | Follow-up recurrence | P value | ||||
| Yes | No | |||||
| MVI | Positive | 22 | 22 | 0.000b | ||
| Negative | 16 | 68 | ||||
| MVI-negative cases | ||||||
| Hepatic capsular invasion | Positive | 4 | 29 | 0.193 | ||
| Negative | 12 | 39 | ||||
| Ki67 | 30.3 ± 20.4 | 28.0 ± 21.0 | 0.686 | |||
| E-S grade1 | I | 1 | 6 | 0.646 | ||
| II | 8 | 36 | ||||
| III | 7 | 26 | ||||
| Largest tumor diameter, cm | 5.5 ± 3.0 | 4.8 ± 2.6 | 0.366 | |||
| Arterial phase enhanced ratio | 0.62 ± 0.34 | 0.66 ± 0.40 | 0.725 | |||
| Portovenous phase enhanced ratio | 0.77 ± 0.23 | 0.80 ± 0.33 | 0.685 | |||
| Tumor capsule | Complete | 3 | 35 | 0.023a | ||
| Incomplete | 7 | 19 | ||||
| Negative | 6 | 14 | ||||
| Necrosis | Positive | 8 | 28 | 0.521 | ||
| Negative | 8 | 40 | ||||
| Tumor margins | Smooth | 9 | 56 | 0.019a | ||
| Focal extranodular | 5 | 11 | ||||
| Multinodular | 2 | 1 | ||||
| Peritumoral enhancement | Positive | 2 | 3 | 0.24 | ||
| Negative | 14 | 65 | ||||
| AFP, ng/mL | 336.8 ± 480.4 | 357.6 ± 505.6 | 0.882 | |||
| TK1, U/L | 5.7 ± 7.8 | 2.6 ± 2.5 | 0.377 | |||
Of 84 recurrences without MVI, univariate analyses showed no difference between groups with respect to hepatic capsular invasion (χ2 = 1.691, P = 0.193), Ki67 value (P = 0.686), E-S grade (Z = -0.460, P = 0.646), largest tumor diameter (P = 0.366), necrosis (χ2 = 0.412, P = 0.521), arterial phase enhanced ratio (P = 0.725), portovenous phase enhanced ratio (P = 0.685), peritumoral enhancement (P = 0.240) or serum AFP level (P = 0.882). In 27 of 84 recurrences without MVI, serum TK1 did not differ between groups (P = 0.377). Univariate analyses showed that non-smooth tumor margins (χ2 = 5.042, P = 0.025), tumor margin grade (Z = -2.355, P = 0.019) and absence of tumor capsule on CT (Z = -2.279, P = 0.023) were correlated with tumor recurrence (Figure 1A-C and Figure 2A-C).
Surgical resection of HCC suffers from high rates of tumor recurrence[5]. A reliable and validated prognostic method to estimate individual HCC recurrence risk would help guide future treatment strategies. Factors commonly associated with high risk for postoperative HCC recurrence include tumor vascular invasion and poorly differentiated tumor grade[24]. In this study, however, E-S grade was not associated with tumor recurrence. We did confirm, however, that MVI may be a powerful independent prognostic factor for postoperative recurrence and metastasis[5,7,14,25-27]. Moreover, in this retrospective study, we further validated MVI as an independent prognostic factor correlated with HCC recurrence. A 50% HCC recurrence rate was found following surgical resection for tumors with MVI, despite antiviral therapy and chemotherapy. Thus, the possibility of recurrence for HCC with MVI is substantial. However, MVI can rarely be confirmed preoperatively and, consequently, it is difficult to choose an appropriate procedure (e.g., wide resection margins) to prevent HCC recurrence[28]. Thus, it is important to identify preoperative imaging markers that could predict HCC recurrence.
It has been reported that conventional CT could be used to reconstruct global HCC gene expression patterns[29]. When Zhou et al[30] investigated the correlation between CT-based radiomics signature and early HCC recurrence, they identified internal arteries and necrosis as independent risk factors. Their results, which were similar to those of previous studies[5,29], led to their hypothesis that internal arteries may be correlated with MVI. The finding that necrosis is a marker of early HCC recurrence[30], however, is inconsistent with our study findings. We suspect that different follow-up times (1 year in their study vs 2 years in ours), was responsible for this difference.
In this study, we further investigated whether preoperative CT findings correlate with HCC recurrence after surgical resection. Our finding that non-smooth tumor margins on CT could predict tumor recurrence has not been evaluated in previous studies. However, a previous study did show that non-smooth tumor margins on CT were predictive of MVI, and that MVI is an independent predictor of poor outcomes following surgical resection[13]. In our stratified analysis of tumors without MVI, tumor margins were significantly associated with postoperative recurrence during the follow-up period. This finding may be related to HCC multi-centricity being an important independent prognostic factor[31], and tumor aggressiveness should be considered[32]. We believe that non-smooth tumor margins can be considered an independent predictor of HCC recurrence.
Adachi et al[33] reported that vessels of the fibrous liver capsule were often invaded by tumor cells and that complete liver capsule fibrosis was a predictor of portal venous invasion. However, complete liver capsule fibrosis has been reported as a favorable prognostic marker because it can prevent HCC invasion into adjacent liver parenchyma[13]. Our results also showed that CT evidence of complete tumor capsules were associated with a low risk of postoperative recurrence. Peritumoral enhancement on magnetic resonance imaging (MRI) has been associated with a high risk of MVI[34]. In our study, peritumoral enhancement on CT was not related to HCC recurrence. The discrepancy in findings may be due to differences in imaging modalities used in various studies. Lu et al[35] reported that, when HCC was larger than 3 cm in diameter, the tumor tended to be more aggressive and was associated with a poor prognosis[36]. However, large tumor diameter was not a significant risk factor for postoperative recurrence in our study. At present, it is clear that HCC nodule size is not the only prognostic factor for recurrence[32]. Additionally, the relationship between postoperative HCC recurrence and AFP level remains controversial[5,10,11].
This study has two limitations. One potential limitation is the use of CT instead of MRI. Although CT is commonly used for HCC evaluation, MRI may provide additional information. A second limitation is the relatively small number of postoperative recurrences, except among those with preoperative evidence of MVI on CT. However, we further analyzed all cases and found similar results for the MVI-positive and MVI-negative groups. Thus, the study sample may reflect true population postoperative recurrences.
In conclusion, evaluation of patients with HCC should include preoperative CT findings of non-smooth tumor margins, incomplete tumor capsules and missing tumor capsules, which indicate possible postoperative recurrence. The treatment plan should include wide resection margins during curative resection, followed by antiviral therapy and chemotherapy.
Surgical resection of hepatocellular carcinoma (HCC) suffers from high rates of tumor recurrence. Although many potential risk factors for HCC postoperative recurrence have been described, a reliable preoperative method to estimate this risk has not been established. It has been reported that conventional computed tomography (CT) could be used to reconstruct global HCC gene expression patterns. Therefore, preoperative CT risk estimation for postoperative HCC recurrence should be clarified.
A reliable and validated prognostic method to estimate individual HCC recurrence risk would help guide future treatment strategies. The results of this study contribute to clarifying the correlation of CT signature with postoperative HCC recurrence.
In the study, preoperative CT provides evidence of non-smooth tumor margins, incomplete tumor capsules and missing tumor capsules, which had been clarified as independent risk factors of postoperative HCC recurrence.
This study suggests that preoperative CT is useful for predicting postoperative HCC recurrence. If the preoperative CT findings of a patient are non-smooth tumor margins, incomplete tumor capsules and missing tumor capsules, the treatment plan should include wide resection margins during curative resection, followed by antiviral therapy and chemotherapy.
The manuscript from Zhang W et al reported the correlation of preoperative CT findings with postoperative HCC recurrence. HCC recurrence following curative resection may be predicted using CT. The entire sets of data are nicely presented, and highly supportive of the conclusion.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Wan YL S- Editor: Qi Y L- Editor: Filipodia
E- Editor: Ma YJ
| 1. | Wang T, Liu M, Zheng SJ, Bian DD, Zhang JY, Yao J, Zheng QF, Shi AM, Li WH, Li L. Tumor-associated autoantibodies are useful biomarkers in immunodiagnosis of α-fetoprotein-negative hepatocellular carcinoma. World J Gastroenterol. 2017;23:3496-3504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 2. | McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 648] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 3. | Hao Y, Numata K, Ishii T, Fukuda H, Maeda S, Nakano M, Tanaka K. Rate of local tumor progression following radiofrequency ablation of pathologically early hepatocellular carcinoma. World J Gastroenterol. 2017;23:3111-3121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3587] [Article Influence: 275.9] [Reference Citation Analysis (4)] |
| 5. | Banerjee S, Wang DS, Kim HJ, Sirlin CB, Chan MG, Korn RL, Rutman AM, Siripongsakun S, Lu D, Imanbayev G. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology. 2015;62:792-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 6. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1566] [Article Influence: 92.1] [Reference Citation Analysis (1)] |
| 7. | Yu YQ, Wang L, Jin Y, Zhou JL, Geng YH, Jin X, Zhang XX, Yang JJ, Qian CM, Zhou DE. Identification of serologic biomarkers for predicting microvascular invasion in hepatocellular carcinoma. Oncotarget. 2016;7:16362-16371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, Chen J, Committee G. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279-9287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 185] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 10. | Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level &gt; 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 11. | Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013;19:634-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Kanai T, Hirohashi S, Upton MP, Noguchi M, Kishi K, Makuuchi M, Yamasaki S, Hasegawa H, Takayasu K, Moriyama N. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer. 1987;60:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol. 2014;203:W253-W259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 14. | Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 15. | Nagano Y, Shimada H, Takeda K, Ueda M, Matsuo K, Tanaka K, Endo I, Kunisaki C, Togo S. Predictive factors of microvascular invasion in patients with hepatocellular carcinoma larger than 5 cm. World J Surg. 2008;32:2218-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Hui AM, Takayama T, Sano K, Kubota K, Akahane M, Ohtomo K, Makuuchi M. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol. 2000;33:975-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Nisman B, Appelbaum L, Yutkin V, Nechushtan H, Hubert A, Uziely B, Pode D, Peretz T. Serum Thymidine Kinase 1 Activity Following Nephrectomy for Renal Cell Carcinoma and Radiofrequency Ablation of Metastases to Lung and Liver. Anticancer Res. 2016;36:1791-1797. [PubMed] |
| 18. | Liu Y, Ling Y, Qi Q, Tang Y, Xu J, Tong Z, Sheng G, Yang Q, Pan Y. Changes in serum thymidine kinase 1 levels during chemotherapy correlate with objective response in patients with advanced gastric cancer. Exp Ther Med. 2011;2:1177-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Nisman B, Allweis T, Kaduri L, Maly B, Gronowitz S, Hamburger T, Peretz T. Serum thymidine kinase 1 activity in breast cancer. Cancer Biomark. 2010;7:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Nisman B, Yutkin V, Nechushtan H, Gofrit ON, Peretz T, Gronowitz S, Pode D. Circulating tumor M2 pyruvate kinase and thymidine kinase 1 are potential predictors for disease recurrence in renal cell carcinoma after nephrectomy. Urology. 2010;76:513.e1-513.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Eguchi S, Takatsuki M, Hidaka M, Soyama A, Tomonaga T, Muraoka I, Kanematsu T. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34:1034-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Edmondson HA, STEINER PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 24. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 286] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 25. | Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, Chung AY, Ooi LL, Tan SB. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 412] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 26. | Kaibori M, Ishizaki M, Matsui K, Kwon AH. Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol. 2010;102:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1227] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 28. | Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, Inoue Y, Uchiyama K. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res. 2014;44:846-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Segal E, Sirlin CB, Ooi C, Adler AS, Gollub J, Chen X, Chan BK, Matcuk GR, Barry CT, Chang HY. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol. 2007;25:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 442] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 30. | Zhou Y, He L, Huang Y, Chen S, Wu P, Ye W, Liu Z, Liang C. CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol (NY). 2017;42:1695-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 31. | Shimada M, Hamatsu T, Yamashita Y, Rikimaru T, Taguchi K, Utsunomiya T, Shirabe K, Sugimachi K. Characteristics of multicentric hepatocellular carcinomas: comparison with intrahepatic metastasis. World J Surg. 2001;25:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Detry O, Govaerts L, Deroover A, Vandermeulen M, Meurisse N, Malenga S, Bletard N, Mbendi C, Lamproye A, Honoré P. Prognostic value of (18)F-FDG PET/CT in liver transplantation for hepatocarcinoma. World J Gastroenterol. 2015;21:3049-3054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, Tsuneyoshi M. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer. 1996;77:2022-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 345] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 35. | Lu XY, Xi T, Lau WY, Dong H, Xian ZH, Yu H, Zhu Z, Shen F, Wu MC, Cong WM. Pathobiological features of small hepatocellular carcinoma: correlation between tumor size and biological behavior. J Cancer Res Clin Oncol. 2011;137:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Moribe T, Iizuka N, Miura T, Kimura N, Tamatsukuri S, Ishitsuka H, Hamamoto Y, Sakamoto K, Tamesa T, Oka M. Methylation of multiple genes as molecular markers for diagnosis of a small, well-differentiated hepatocellular carcinoma. Int J Cancer. 2009;125:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |