Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6437
Peer-review started: June 7, 2017
First decision: July 13, 2017
Revised: July 25, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: September 21, 2017
Processing time: 108 Days and 2.1 Hours
To evaluate the relationship between the location of hepatocellular carcinoma (HCC) and the efficacy of transarterial chemoembolization (TACE).
We evaluated 115 patients (127 nodules), excluding recurrent nodules, treated with TACE between January 2011 and June 2014. TACE efficacy was evaluated according to mRECIST. The HCC location coefficient was calculated as the distance from the central portal portion to the HCC center (mm)/liver diameter (mm) on multiplanar reconstruction images rendered (MPR) to visualize bifurcation of the right and left branches of the portal vein and HCC center. The HCC location coefficient was compared between complete response (CR) and non-CR groups in Child-Pugh grade A and B patients.
The median location coefficient of HCC among all nodules, the right lobe, and the medial segment was significantly higher in the CR group than in the non-CR group in the Child-Pugh grade A patients (0.82 vs 0.62, P < 0.001; 0.71 vs 0.59, P < 0.01; 0.81 vs 0.49, P < 0.05, respectively). However, there was no significant difference in the median location coefficient of the HCC in the lateral segment between in the CR and in the non-CR groups (0.67 vs 0.65, P > 0.05). On the other hand, in the Child-Pugh grade B patients, the HCC median location coefficient in each lobe and segment was not significantly different between in the CR and in the non-CR groups.
Improved TACE efficacy may be obtained for HCC in the peripheral zone of the right lobe and the medial segment in Child-Pugh grade A patients.
Core tip: The relationship between hepatocellular carcinoma (HCC) location and transcatheter arterial chemoembolization (TACE) efficacy was evaluated. In Child-Pugh A, the median location coefficient of HCC among all nodules, right lobe, and medial segment was significantly higher in the complete response (CR) group than in the non-CR group, with no significant differences in the lateral segment. In Child-Pugh B, the median location coefficient of HCC in each lobe and segment was not significantly different between the two groups. Therefore, improved TACE efficacy may be obtained for HCC in the peripheral zone of the right lobe and medial segment in Child-Pugh A patients.
- Citation: Miki I, Murata S, Uchiyama F, Yasui D, Ueda T, Sugihara F, Saito H, Yamaguchi H, Murakami R, Kawamoto C, Uchida E, Kumita SI. Evaluation of the relationship between hepatocellular carcinoma location and transarterial chemoembolization efficacy. World J Gastroenterol 2017; 23(35): 6437-6447
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6437.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6437
Hepatocellular carcinoma (HCC) is one of the most common malignant diseases worldwide, and it is a lethal tumor whose prognosis largely depends on tumor stage at diagnosis and patient access to radical treatment[1,2]. Management of HCC has been standardized according to clinical staging systems, such as the Barcelona Clinical Liver Cancer (BCLC) classification, in which patients are stratified by tumor stage and underlying liver disease. According to the BCLC staging system, transarterial chemoembolization (TACE) is the current standard of care for BCLC stage B patients. Stage B is defined as an intermediate-stage disease[3] that involves highly heterogeneous patients who have Child-Pugh grade A or B liver function with four or more tumors irrespective of size or two to three tumors larger than 3 cm in diameter, in the absence of cancer-related symptoms, macrovascular invasion, or extrahepatic spread[4]. Several studies and well-designed randomized trials have shown that TACE has a positive effect on patient outcome and survival[5-7]. Although many studies have reported on several prognostic factors for stage-B HCC patients, tumor number, maximal tumor diameter, Child-Pugh score, and tumor response to TACE are fundamentally strong predictors of survival in patients with initially unresectable HCC[8,9]. Complete necrosis following repeated TACE was a significant independent predictor of favorable survival after the first TACE[10].
Most HCC patients have some degree of cirrhosis. Regardless of etiology, compared with noncirrhotic livers, cirrhotic livers show right-lobe and medial-segment atrophy and lateral-segment and caudate-lobe hypertrophy[11]. This pattern suggests that microcirculatory changes play a role in the development of cirrhosis within segments. Indeed, an in vivo mi-croscopic study[12] demonstrated differences in the blood flow in central and peripheral regions, indicating a direct and indirect reduction in the blood flow to the periphery of the main lobe.
Histopathological investigations of HCCs that were resected after TACE have shown that the most viable tissue is located at the periphery of the tumor[13]. The efficacy of TACE is limited by the dual blood supply (artery and portal vein) of liver tumors and collateral arterial supply after TACE, which make it difficult to achieve complete necrosis of tumor tissue because of insufficient tumor ischemia. However, complete necrosis is sometimes obtained in the long-term. Therefore, before performing this procedure, it is very important for the physician to predict whether TACE will be effective, particularly while considering other therapeutic modalities.
Thus, the aim of this retrospective study was to evaluate the relationship between the location of the HCC, in particular, the location close to the peripheral or hepatic portal portion of the liver, and the efficacy of TACE.
This retrospective study was conducted with the approval of our institutional review board. The requirement for informed consent was waived.
Between January 2011 and June 2014, 777 patients with HCC were treated with TACE in our institution. The eligibility criteria for the study were as follows: (1) diagnosis of HCC based on histologic findings, findings on dynamic contrast-enhanced multi-detector computed tomography (MDCT), or magnetic resonance imaging (MRI) performed in our institution in accordance with the American Association for the Study of Liver Disease[14] and/or findings of cirrhosis; (2) no indications for hepatic resection, percutaneous ethanol injection therapy, liver transplantation, or thermal ablation therapy; (3) presence of a bidimensionally measurable hepatic lesion; (4) adequate liver function (serum total bilirubin level < 2.0 mg/dL and Child-Pugh grade A or B); (5) adequate bone marrow function (leukocyte count > 3000 cells/mm3 and platelet count > 50000 cells/mm3); (6) adequate renal function (serum creatinine concentration ≤ 1.2 mg/dL); (7) no ascites; (8) absence of vascular invasion; (9) technical success of TACE; and (10) MDCT or MRI performed before TACE and at least 6 mo after TACE.
The exclusion criteria were as follows: (1) recurrent HCC; (2) tumor size > 7 cm; (3) inadequate iodized oil accumulation in the target lesion because of large tumor size or anastomosis with vital vessels; (4) balloon-occluded TACE; and (5) drug-eluting bead TACE. Overall, 115 patients with 127 HCC nodules were included in our study.
Experienced interventional radiologists performed all TACE procedures. After administering local anesthesia in all patients, we punctured the femoral artery using the Seldinger technique. A 4-French sheath (Super Sheath; Medikit, Miyazaki, Japan) was inserted via the femoral artery. After performing arterial portography and hepatic arteriography to confirm feeding arteries to HCC, we inserted a 2.0-French microcatheter (Gold Crest-MRT; Koshin Medical, Tokyo, Japan) into the feeding arteries. Then, an emulsion of anticancer drugs [cisplatin (50 mg/m2, up to 100 mg) or epirubicin (30 mg/m2)] and iodized oil [Lipiodol (the sum of the major axis of the tumor up to 15 mL) Ultra Fluid, Laboratoires Guerber, Aulnay-sous-Bois, France] was injected slowly under fluoroscopic guidance until the vascular bed of the target nodule was filled with the emulsion. We performed embolization with 2 mm pieces of gelatin sponge (Gelpart; Nippon Kayaku, Tokyo, Japan) following TACE.
Dynamic contrast-enhanced CT or MRI was used to evaluate the effect of TACE 6 mo after and on a per nodule basis, according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST)[15]: complete response (CR) = disappearance of any intratumoral arterial enhancement in all target lesions; partial response (PR) = at least a 30% decrease in the sum of the diameter of viable target lesions; stable disease (SD) = any cases that did not qualify as either PR or progressive disease; progressive disease (PD) = an increase by at least 20% of the sum of the diameters of the viable target lesions. The evaluation was performed by two independent and blinded observers. After individual evaluation, the findings were disclosed, and any discrepancy in the findings was discussed by both the observers.
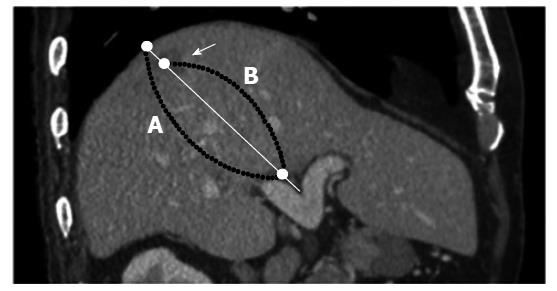
The location of the HCC was determined using the most recent dynamic contrast-enhanced MDCT (within 1 mo) before TACE. The MDCT row data of the arterial or portal phase of pre-TACE were transferred to a workstation (OsiriX 7.0; Pixmeo, Bernex, Switzerland). The location coefficient of HCC was defined as follows: multi-planar reconstructed (MPR) images were rendered to visualize the bifurcation of the right and left branches of the portal vein and the center of the HCC, which was defined as the intersection of the long axes with the short axes in the maximum cross-section of the target nodule. Next, we traced the straight line through the bifurcation of the right and left branches of the portal vein to the center of the target nodule and the peripheral surface of the liver using MPR images (Figure 1). The location coefficient of the HCC was determined as a ratio of the distance from an internal surface of the liver to the center of the HCC/diameter of the liver on the traced line. An increased location coefficient indicates a more peripheral nodule. The bifurcation was the baseline point of measurement of the HCC location because the bifurcation had few portal venous variations.
The location coefficient of the HCC was compared between the CR group and non-CR group, including the PR, SD, and PD subgroups, among all nodules and each lobe or segment in Child Pugh A and B patients. The relationship between the efficacy of TACE and the location of the HCC was assessed.
All statistical analysis was performed using R 2.15.1 (CRAN: the Comprehensive R Archive Network at http://cran. R-project.org/). We compared the location coefficient of the HCC between the CR group and non-CR group using the Mann-Whitney U test. Moreover, receiver operating characteristic (ROC) curve analyses were used to determine the appropriate cut-off points of the location coefficient to predict the efficacy of TACE. In all comparisons, the results of the statistical analyses were considered significant at a P-value < 0.05.
The median patient age was 73 year (range, 49-92 year; 88 men and 27 women). The tumor size ranged from 5.0 mm to 56.7 mm (mean ± standard deviation: 14.00 ± 10.30) (Table 1). Other patient characteristics are presented in Table 1. Among the 127 HCC nodules, 81 achieved CR 6 mo after TACE according to the mRECIST criteria. Local recurrences were classified into the PR, SD, or PD subgroup in 46 nodules. Twenty-six nodules were classified as PR, 13 as SD, and 7 as PD (Table 2). The median recurrence time was 2 mo. Table 2 summarizes the efficacy of TACE for each segment of the liver where the HCC nodule presented in Child-Pugh grade A and B patients.
| Characteristics | Values |
| Age | |
| Median (range) | 73 (49-92) |
| Sex | |
| Male/Female | 88 (76.5)/27 (23.5) |
| Etiology | |
| Hepatitis B/C/B + C | 13 (11.3)/85 (73.9)/2 (1.7) |
| Alcohol | 10 (8.7) |
| NASH | 2 (1.7) |
| PBC | 3 (2.6) |
| Child-Pugh class | |
| A/B | 99 (86.1)/16 (13.9) |
| Location of target nodules | |
| Anterior segment | 39 (30.7) |
| Posterior segment | 37 (29.1) |
| Medial segment | 27 (21.3) |
| Lateral segment | 24 (18.9) |
| Size of target nodules (mm)1 | 14.00 ± 10.30 (5.0-56.7) |
| Chemotherapeutic drug | |
| Cisplatin/Epirubicin | 87 (68.5)/40 (31.5) |
| CR | PR | SD | PD | Total | |
| All nodules | 81 | 26 | 13 | 7 | 127 |
| (63.8) | (20.5) | (10.2) | (5.5) | ||
| Anterior segment | 26 | 7 | 5 | 1 | 39 |
| Posterior segment | 22 | 8 | 3 | 4 | 37 |
| Medial segment | 14 | 8 | 4 | 1 | 27 |
| Lateral segment | 19 | 3 | 1 | 1 | 24 |
| Patient with Child-Pugh grade A | 56 | 17 | 10 | 3 | 86 |
| (65.1) | (19.8) | (11.6) | (3.5) | ||
| Anterior segment | 19 | 5 | 5 | 1 | 30 |
| Posterior segment | 14 | 5 | 1 | 1 | 21 |
| Medial segment | 9 | 6 | 3 | 0 | 18 |
| Lateral segment | 14 | 1 | 1 | 1 | 17 |
| Patients with Child-Pugh grade B | 25 | 9 | 3 | 4 | 41 |
| (61.0) | (22.0) | (7.3) | (9.7) | ||
| Anterior segment | 7 | 2 | 0 | 0 | 9 |
| Posterior segment | 8 | 3 | 2 | 3 | 16 |
| Medial segment | 5 | 2 | 1 | 1 | 9 |
| Lateral segment | 5 | 2 | 0 | 0 | 7 |
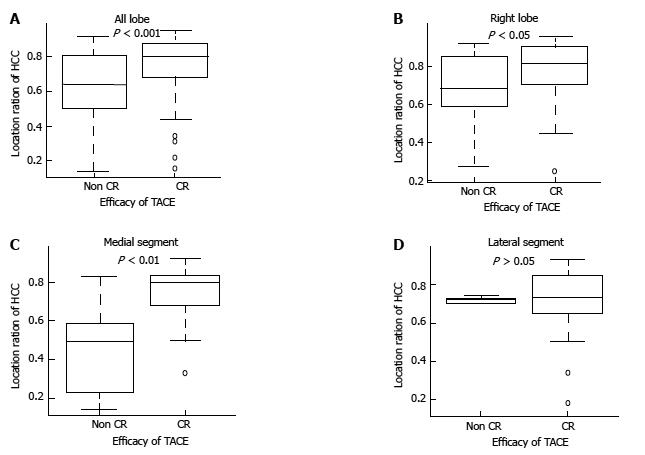
The median location coefficient of HCC was significantly higher in the CR group than the non-CR group among all 127 nodules (0.80 vs 0.63, P < 0.001). Among HCCs that presented in the right lobe and medial segment, the median location coefficient of HCC was also significantly higher in the CR group than in the non-CR group (0.81 vs 0.68, P < 0.05, 0.80 vs 0.49, P < 0.01, respectively). However, among HCCs in the lateral segment, there was no significant difference in the location coefficient of HCC between the CR group and non-CR group (0.74 vs 0.73, P > 0.05) (Figure 2 and Table 3).
| Non-CR group | CR group | P value | |
| All lobes | 0.63 | 0.8 | < 0.001 |
| Right lobe | 0.68 | 0.81 | < 0.05 |
| Medial segment | 0.49 | 0.8 | < 0.01 |
| Lateral segment | 0.73 | 0.74 | > 0.05 |
| Patients with Child-Pugh grade A | |||
| All lobes | 0.62 | 0.82 | < 0.001 |
| Right lobe | 0.59 | 0.71 | < 0.01 |
| Medial segment | 0.49 | 0.81 | < 0.05 |
| Lateral segment | 0.65 | 0.67 | > 0.05 |
| Patients with Child-Pugh grade B | |||
| All lobes | 0.73 | 0.75 | > 0.05 |
| Right lobe | 0.84 | 0.79 | > 0.05 |
| Medial segment | 0.43 | 0.69 | > 0.05 |
| Lateral segment | 0.72 | 0.69 | > 0.05 |
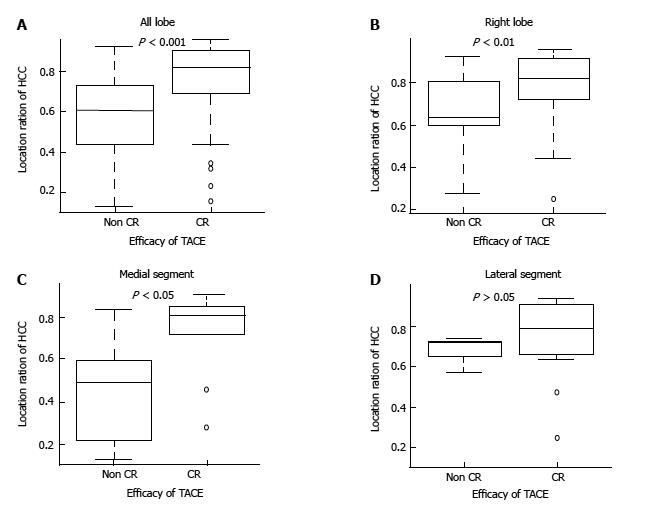
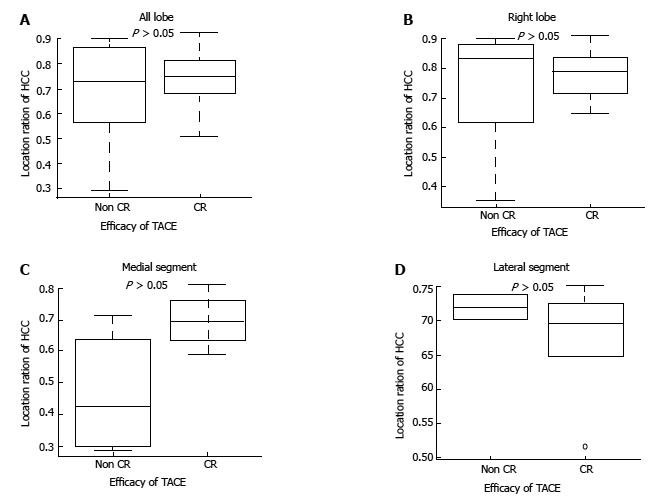
In the group classified as Child-Pugh grade A, the median location coefficient of HCC among all nodules in the right lobe and of those in the medial segment was also significantly higher in the CR group than in the non-CR group (0.82 vs 0.62, P < 0.001; 0.71 vs 0.59, P < 0.01; 0.81 vs 0.49, P < 0.05, respectively). However, there was no significant difference in the median location coefficient of HCC in the lateral segment between the CR group and non-CR group (0.67 vs 0.65, P > 0.05) (Table 3 and Figure 3). On the other hand, in the group classified as Child-Pugh grade B, the median location coefficient of HCC was not significantly different between the CR and non-CR groups for nodules in any lobe or segment (Figure 4).
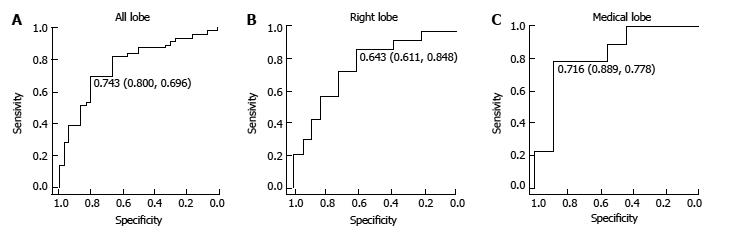
When we performed a ROC curve analysis for the location coefficient of HCC in all nodules, we found that the area under the curve (AUC) was 0.713 and that the cut-off value was 0.643, with a sensitivity of 56.5% and specificity of 85.2%. In the right lobe and medial segment, the ROC curve AUC was 0.678 and 0.852, respectively, and the cut-off value was 0.643 with a sensitivity of 50.0% and specificity of 89.6% and 0.674 with a sensitivity of 84.6% and specificity of 78.6%, respectively. In the group classified as Child-Pugh grade A, similar results were also obtained in all nodules, the right lobe, and medial segment; the AUC was 0.767, 0.753, and 0.827, respectively, and the cut-off value was 0.743, with a sensitivity of 80.0% and specificity of 69.6%, 0.643 with a sensitivity of 61.1% and specificity of 84.8%, and 0.716 with sensitivity of 88.9% and specificity of 77.8%, respectively (Figure 5).
In this retrospective study, we evaluated the relationship between the location of the HCC (in particular, the location close to the peripheral or hepatic portal portion of the liver) and the efficacy of TACE. Among all 127 nodules, the median location coefficient of the HCC was significantly higher in the CR group than in the non-CR group. Although the median location coefficient of HCC in the right lobe and medial segment was also significantly higher in the CR group than in the non-CR group, there was no significant difference in the location coefficient of HCC in the lateral segment. These results suggested that the location of HCC may influence the efficacy of TACE for the treatment of HCC in the right lobe and medial segment. Moreover, considering the liver function represented by the Child-Pugh grade, a significant difference was observed between the location of HCC and the efficacy of TACE only in patients classified as Child-Pugh grade A. In the patients classified as Child-Pugh grade B, the median location coefficient of HCC was not significantly different between the CR group and the non-CR group in each lobe and segment. These results may represent conditions that have hepatic circulatory, anatomical, or biological significance.
TACE is a useful palliative treatment for HCC because the hepatic artery delivers > 99% of the blood supply to hepatic tumors. Compact lipiodol uptake results in complete necrosis in the resected tissues[16-19], and complete deposition of iodized oil in HCC lesions correlates with near-total necrosis[20-22]. However, HCCs showing a high uptake of iodized oil may continue to be partially fed by the collateral arteries or the portal vein[23]. Chan et al[24] reported that TACE induces a posttreatment surge of angiogenic factors, such as vascular endothelial growth factor (VEGF), which can occur as early as a few hours after TACE. This process may contribute to revascularization of the tumor, thus leading to the recurrence of HCC and reduced efficacy of TACE[25,26].
Generally, residual HCC tumor cells are observed in the peripheral region of the HCC. The reasons for this are complex and may be partially explained by the following: (1) incomplete embolization or partial recanalization of the hepatic artery after embolization or (2) formation of collateral circulation or the opening of communicating vessels as a microcirculatory reaction. Notably, residual viable tumor cells after TACE are exposed to an extremely hypoxic or even anoxic environment. Ischemic hypoxia is considered a physiological stimulus for angiogenesis, including endothelial cell proliferation[22,23]. Tumor cells induce neovascularization by producing VEGF, which in turn can nourish more tumor cells. Thus, neovascularization and VEGF expression may be important reasons for the survival of residual tumor cells after TACE.
There are several reasons why the median location coefficient of HCC in the right lobe and medial segment was significantly higher in the CR group than in the non-CR group. An in vivo microscopic study[12] indicated a direct and indirect reduction in the blood flow to the periphery of the main lobe. Moreover, several reports have described functional and hemodynamic differences between the right and left lobes or between the peripheral (near the subcapsular area) and the central areas (near the porta hepatic) in the liver[27-30]. Okubo et al[31], in a study with gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MRI, reported that the right lobe or central zone showed significantly more enhancement than the left lobe or peripheral zone. The hemodynamic differences between the peripheral and central zones and between the right and left lobes could cause the differences in hepatic enhancement. These reports suggested that vascularity around the HCC in the peripheral area is less than that in the central area, and TACE may induce much more ischemia in the HCC tissue in the peripheral area than in the central area because of protecting the formation of collateral circulation or the opening of communicating vessels.
We also evaluated why a significant difference was found only in the right lobe and medial segment and not in the lateral segment. The abovementioned reports suggested that there were differences between the right and left lobes. However, the medial segment is in the left lobe. We do not have a definitive answer to this question, but several phenomena may provide clues. First, thin anastomosis branches often present between the right hepatic and the middle hepatic arteries[32]. Second, cirrhotic livers associated with all etiologies demonstrate atrophy of the right lobe and medial segment as well as hypertrophy of the caudate lobe and lateral segment[11]. These phenomena suggest that differences in the frequency of revascularization between the central and peripheral areas also exist in the medial segment.
Our study demonstrated a significant difference between the location of HCC and the efficacy of TACE only in the Child-Pugh grade A patients. In Child-Pugh grade B patients, there was no relationship between any nodules, even in the right lobe and medial segment. These results suggested that hepatic hemodynamics may change depending on cirrhotic liver status. Indeed, qualitative analysis indicated that the parenchymal enhancement in the cirrhotic liver was significantly heterogeneous compared with that in the normal liver[33]. Moreover, the quantitative liver-spleen contrast ratio was significantly decreased with an elevation of the Child-Pugh score[31]. These reports suggest that hemodynamic features are different depending on cirrhotic liver status. In our study, considering a cut-off value of the location coefficient of HCC on the ROC curve, a boundary line that demonstrated a significant, effective difference in patients with Child-Pugh grade A was expected in the range of 0.6-0.7 in the right lobe and median segment. Considering these results and the changing hepatic hemodynamics that depend on cirrhotic liver status, we concluded that livers with advanced cirrhosis had atrophy of the peripheral area in the right lobe and medial segment. Consequently, there was no significant difference between the location of the HCC and the efficacy of TACE in Child-Pugh grade B patients.
A novel finding in our study was that the median location coefficient of HCC in the right lobe and medial segment was significantly higher in the CR group than in the non-CR group. Therefore, the coefficient could be used as an index when choosing an HCC treatment strategy. When HCC is located in the peripheral area in the right lobe or medial segment, TACE is much more suitable for treatment. However, when HCC is located in the central area in the right lobe or medial segment, an early combination of local ablation therapy (such as radiofrequency ablation and percutaneous ethanol injection) with TACE may be efficacious. Indeed, the synergistic effect of such an approach has already been demonstrated in both nonsurgical and recurrent HCC patients[34-36]. Therefore, the combination of local ablation therapy with TACE may be considered as a treatment of HCC in the central area.
There are several limitations in our study. First, there were very few HCC nodules in the caudate lobe in our study. If there were more HCCs in the caudate lobe, further studies could evaluate the difference in the efficacy of TACE in each lobe or segment in detail. Second, the baseline point of the center when measuring the HCC location was the bifurcation of the portal vein because of few portal vein variations in this study. The HCC location coefficient and the boundary line, demonstrating the difference in efficacy of TACE between the peripheral area and central area, may change when other baseline points, such as the bifurcation of the right and left branches of the hepatic artery, are defined. Therefore, further studies are necessary to confirm our findings.
In conclusion, our study suggested that improved efficacy of TACE may be obtained for treatment of HCC in the peripheral area of the right lobe and medial segment in Child-Pugh grade A patients.
We appreciate the contributions of Dr. Nobuhiko Taniai (Gastrointestinal and Hepato-Biliary-Pancreatic Surgery), Dr. Keiko Kaneko, and Dr. Takeshi Fukuda (Gastroenterology and Hepatology Medicine, Nippon Medical School) to this work.
Transarterial chemoembolization (TACE) is the current standard of care for patients with Barcelona clinical liver cancer stage B, which includes a highly heterogeneous group of patients. TACE has a positive effect on patient outcome and survival. Studies have shown that tumor number, maximal tumor diameter, Child-Pugh score, and tumor response to TACE are fundamentally strong predictors of survival in patients with initially unresectable hepatocellular carcinoma (HCC). Most HCC patients have some degree of cirrhosis. Microcirculatory changes play a role in the development of cirrhosis within segments. Histopathological investigations of HCCs that were resected after TACE have shown that the most viable tissue is located at the tumor periphery. Considering all these factors, it is very important for the physician to distinguish effective and ineffective TACE before performing this procedure. In this study, we evaluated the relationship between HCC location-in particular, a location close to the peripheral or hepatic portal portion of the liver-and TACE efficacy.
Predicting whether TACE will be effective before performing this procedure is very important for the physician, especially while considering other therapeutic modalities. The results of this study evaluating the relationship between HCC location and TACE efficacy may prove to be an index when determining a HCC treatment strategy.
Several studies and well-designed randomized trials have shown that TACE has a positive effect on patient outcome and survival. However, no previous study has evaluated the relationship between the efficacy of this procedure and HCC. This retrospective study evaluated the relationship between the location of HCC and the efficacy of TACE and suggested that an improved efficacy of TACE may be obtained for treatment of HCC in the peripheral area of the right lobe and medial segment in patients with Child-Pugh grade A tumors. For patients with Child-Pugh grade B or HCC located in the central area or in the lateral segment, it might be difficult to obtain complete necrosis with TACE only.
This study suggests that improved TACE efficacy may be obtained for HCC in the peripheral zone of the right lobe and the medial segment in Child-Pugh grade A patients. When HCC is located in the central area in the right lobe or the medial segment, an early combination of local ablation therapy with TACE may be efficacious.
TACE: A minimally invasive procedure performed in in-terventional radiology to reduce a tumor’s blood supply. Child-Pugh score: A score to assess the prognosis of liver cirrhosis.
In this retrospective study, the authors evaluated the relationship between the location of the HCC (in particular, the location close to the peripheral or hepatic portal portion of the liver) and the efficacy of TACE. It is an interesting study on TACE for HCC. The results demonstrated a significant difference between the location of HCC and the efficacy of TACE only in the Child-Pugh grade A patients. When HCC is located in the peripheral area in the right lobe or medial segment, TACE is much more suitable for treatment. When HCC is located in the central area in the right lobe or medial segment, an early combination of local ablation therapy (such as radiofrequency ablation and percutaneous ethanol injection) with TACE may be efficacious. These findings will provide very important clinical guidance for the selection of TACE for HCC.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: El-Bendary MM, Qin JM, Rodriguez-Peralvarez ML S- Editor: Ma YJ L- Editor: A
E- Editor: Ma YJ
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13555] [Article Influence: 677.8] [Reference Citation Analysis (1)] |
| 2. | Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Tremosini S, Reig M, de Lope CR, Forner A, Bruix J. Treatment of early hepatocellular carcinoma: Towards personalized therapy. Dig Liver Dis. 2010;42 Suppl 3:S242-S248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 301] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2605] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 6. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1985] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 7. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 618] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2267] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 9. | Rou WS, Lee BS, Moon HS, Lee ES, Kim SH, Lee HY. Risk factors and therapeutic results of early local recurrence after transcatheter arterial chemoembolization. World J Gastroenterol. 2014;20:6995-7004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Shim JH, Kim KM, Lee YJ, Ko GY, Yoon HK, Sung KB, Park KM, Lee SG, Lim YS, Lee HC. Complete necrosis after transarterial chemoembolization could predict prolonged survival in patients with recurrent intrahepatic hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2010;17:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Niggemann P, Murata S, Naito Z, Kumazaki T. A comparative study of the microcirculatory changes in the developing liver cirrhosis between the central and peripheral parts of the main lobe in mice. Hepatol Res. 2004;28:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Ozaki K, Matsui O, Kobayashi S, Minami T, Kitao A, Gabata T. Morphometric changes in liver cirrhosis: aetiological differences correlated with progression. Br J Radiol. 2016;89:20150896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Nakamura H, Tanaka T, Hori S, Yoshioka H, Kuroda C, Okamura J, Sakurai M. Transcatheter embolization of hepatocellular carcinoma: assessment of efficacy in cases of resection following embolization. Radiology. 1983;147:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 142] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4500] [Article Influence: 225.0] [Reference Citation Analysis (0)] |
| 15. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2583] [Cited by in RCA: 3277] [Article Influence: 218.5] [Reference Citation Analysis (36)] |
| 16. | Matsuo N, Uchida H, Soda S, Ohshima M, Nakano H, Ohishi H, Nagano N, Kitamura I, Nidhimurs Y, Nidhimine K. Histopathological study of the resected specimens after segmental Lp-TACE using lipiodol mixed with anticancer agent for hepatocellular carcinoma. Anti-tumor effect and influence on non-tumor area. Acta Hepatol Jpn. 1990;31:1084-1093. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Raby N, Karani J, Michell M, Gimson A, Nunnerley H, Williams R. Lipiodol enhanced CT scanning in assessment of hepatocellular carcinoma. Clin Radiol. 1989;40:480-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Matsuo N, Uchida H, Sakaguchi H, Nishimine K, Nishimura Y, Hirohashi S, Ohishi H. Optimal lipiodol volume in transcatheter arterial chemoembolotherapy for hepatocellular carcinoma: study based on lipiodol accumulation patterns and histopathologic findings. Semin Oncol. 1997;24:S6-61-S6-70. [PubMed] |
| 19. | Kwan SW, Fidelman N, Ma E, Kerlan RK Jr, Yao FY. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl. 2012;18:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Choi BI, Kim HC, Han JK, Park JH, Kim YI, Kim ST, Lee HS, Kim CY, Han MC. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992;182:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Jinno K, Moriwaki S, Tanada M, Wada T, Mandai K, Okada Y. Clinicopathological study on combination therapy consisting of arterial infusion of lipiodol-dissolved SMANCS and transcatheter arterial embolization for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1992;31 Suppl:S7-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3236] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 23. | Ernst O, Sergent G, Mizrahi D, Delemazure O, Paris JC, L’Herminé C. Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization: comparison of planned periodic chemoembolization and chemoembolization based on tumor response. AJR Am J Roentgenol. 1999;172:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Chan SL, Mok T, Ma BB. Management of hepatocellular carcinoma: beyond sorafenib. Curr Oncol Rep. 2012;14:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 26. | Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. In-creased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 27. | Jacobsson H, Jonas E, Hellström PM, Larsson SA. Different concentrations of various radiopharmaceuticals in the two main liver lobes: a preliminary study in clinical patients. J Gastroenterol. 2005;40:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Imaeda T, Kanematsu M, Asada S, Seki M, Doi H, Saji S. Utility of Tc-99m GSA SPECT imaging in estimation of functional volume of liver segments in health and liver diseases. Clin Nucl Med. 1995;20:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Feld R, Wechsler RJ, Dumsha JZ, Westerberg S, Munoz S, Boiskin I, Rubin R. Significance of the computed tomography finding of subcapsular hepatic necrosis in liver transplantation. Abdom Imaging. 1996;21:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Jang HJ, Khalili K, Yu H, Kim TK. Perfusion and parenchymal changes related to vascular alterations of the liver. Abdom Imaging. 2012;37:404-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Okubo H, Mogami M, Ozaki Y, Igusa Y, Aoyama T, Amano M, Kokubu S, Miyazaki A, Watanabe S. Liver function test by gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging with consideration of intrahepatic regional differences. Hepatogastroenterology. 2013;60:1547-1551. [PubMed] |
| 32. | Hiramatsu K, Koda E, Mori M, Isobe Y. X-ray Anatomy of the Abdominal Vascular System. Tokyo: Igaku-Shoin; 1982; 63-81. [PubMed] |
| 33. | Tamada T, Ito K, Higaki A, Yoshida K, Kanki A, Sato T, Higashi H, Sone T. Gd-EOB-DTPA-enhanced MR imaging: evaluation of hepatic enhancement effects in normal and cirrhotic livers. Eur J Radiol. 2011;80:e311-e316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Cheng BQ, Jia CQ, Liu CT, Fan W, Wang QL, Zhang ZL, Yi CH. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA. 2008;299:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Ishii H, Okada S, Sato T, Nose H, Okusaka T, Yoshimori M, Takayasu K, Takayama T, Kosuge T, Yamasaki S. Effect of percutaneous ethanol injection for postoperative recurrence of hepatocellular carcinoma in combination with transcatheter arterial embolization. Hepatogastroenterology. 1996;43:644-650. [PubMed] |
| 36. | Sato M, Watanabe Y, Iseki N, Ueda S, Kawach K, Kimura S, Itoh Y, Ohkubo K, Onji M. Chemoembolization and percutaneous ethanol injection for intrahepatic recurrence of hepatocellular carcinoma after hepatic resection. Hepatogastroenterology. 1996;43:1421-1426. [PubMed] |