Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6403
Peer-review started: April 21, 2017
First decision: July 28, 2017
Revised: July 31, 2017
Accepted: August 25, 2017
Article in press: August 25, 2017
Published online: September 21, 2017
Processing time: 154 Days and 9.8 Hours
To determine whether Nucb2/nesfatin1 production is regulated by the cannabinoid system through the intracellular mTOR pathway in the stomach.
Sprague Dawley rats were treated with vehicle, rimonabant, rapamycin or rapamycin+rimonabant. Gastric tissue obtained from the animals was used for biochemical assays: Nucb2 mRNA measurement by real time PCR, gastric Nucb2/nesfatin protein content by western blot, and gastric explants to obtain gastric secretomes. Nucb2/nesfatin levels were measured in gastric secretomes and plasma using enzyme-linked immunosorbent assay.
The inhibition of cannabinoid receptor 1 (CB1) by the peripheral injection of an inverse agonist, namely rimonabant, decreases food intake and increases the gastric secretion and circulating levels of Nucb2/nesfatin-1. In addition, rimonabant treatment activates mTOR pathway in the stomach as showed by the increase in pmTOR/mTOR expression in gastric tissue obtained from rimonabant treated animals. These effects were confirmed by the use of a CB1 antagonist, AM281. When the intracellular pathway mTOR/S6k was inactivated by chronic treatment with rapamycin, rimonabant treatment was no longer able to stimulate the gastric secretion of Nucb2/nesfatin-1.
The peripheral cannabinoid system regulates food intake through a mechanism that implies gastric production and release of Nucb2/Nesfatin-1, which is mediated by the mTOR/S6k pathway.
Core tip: The peripheral pharmacological blockade of the cannabinoid receptor 1 (CB1) induces a decrease in food intake in rats by stimulating the gastric secretion, and consequently the circulating levels, of the anorexigenic peptide Nucb2/nesfatin-1. The present data show that gastric CB1 receptors modulate Nucb2/Nesfatin-1 production in the stomach at the intracellular level, which is mediated by the mTOR pathway.
- Citation: Folgueira C, Barja-Fernandez S, Prado L, Al-Massadi O, Castelao C, Pena-Leon V, Gonzalez-Saenz P, Baltar J, Baamonde I, Leis R, Dieguez C, Pagotto U, Casanueva FF, Tovar SA, Nogueiras R, Seoane LM. Pharmacological inhibition of cannabinoid receptor 1 stimulates gastric release of nesfatin-1 via the mTOR pathway. World J Gastroenterol 2017; 23(35): 6403-6411
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6403
Nucb2/nesfatin-1 is a 42 kDa peptide, first discovered as an anorexigenic factor that regulates energy balance. The product of the Nucb2 gene is a 396 amino acid secreted peptide that, after being processed by the enzyme prohormone convertase (PC-1/2), becomes the N-terminal fragment known as nesfatin-1[1]. Nucb2 is mainly expressed in the gastric mucosa and adipose tissue, although its expression has also been detected elsewhere, such as hypothalamus, brain stem, pancreas and testis[1-5]. In the gastrointestinal tract, Nucb2 is most highly expressed in the X/A cells from the oxyntic mucosa of the stomach, which also synthesizes ghrelin, the main peptide hormone in the gastrointestinal tract[4,6,7].
Despite being secreted by the same cells, Nucb2 and ghrelin have opposing biological functions and regulation. Central and peripheral administration of Nucb2 suppresses feeding behavior[1,8], while ghrelin stimulates appetite[9,10]. Interestingly, the fact that Nucb2 and ghrelin are produced in the same gastric cells but in different vesicles suggests that both proteins might experience differential regulation at the gastric level to maintain energy balance. Supporting this theory, Nucb2/nesfatin-1 production and regulation by nutritional status decreases during fasting[1], while ghrelin levels are up-regulated[11]. Lastly, this decrease in Nucb2 production during fasting conditions is concomitant with inhibition of the mTOR/pS6k1 pathway, a sensor of the metabolic status of organisms[12]. Ghrelin secretion, meanwhile, is negatively associated with mTOR/pS6k1 pathway activity under fasting conditions[13].
The cannabinoid system plays an important role as an endogenous regulator of energy balance, acting at multiple levels. One of those mechanisms includes its interaction with gastric ghrelin production to regulate appetite and body weight[14]. More specifically, the blockade of the cannabinoid receptor CB1 decreases ghrelin expression in the stomach, and this effect was mediated by activation of the mTOR/pS6k1 pathway[14]. Taking into account the opposing functions and regulation of ghrelin and Nucb2/nesfatin at the gastric level, as well as their opposite relationship with the mTOR/S6K1 pathway, we sought to investigate whether the cannabinoid system might also regulate Nucb2/nesfatin-1 production in the stomach and whether the mTOR/pS6k1 intracellular pathway mediates this effect.
The authors of this manuscript declare that the animal work in this study was approved by the Animal Care Committee of Santiago de Compostela University (Santiago de Compostela, Spain) in accordance with our institutional guidelines and the European Union standards for the care and use of experimental animals. The approaches in the present manuscript were performed under the procedure 15005/2015/003 reviewed and approved by the Faculty Animal Committee at the University of Santiago de Compostela.
Male Sprague-Dawley rats were housed in air-conditioned rooms (22-24 °C) under a controlled light/dark cycle (12 h light, 12 h darkness) with free access to food and water. The animals were euthanatized, trunk blood was collected and immediately centrifuged, and the plasma was stored at -80 °C for biochemical measurements.
A group of overnight fasted adult male rats was treated with Rimonabant intraperitoneal (i.p.) at a dose of 3 mg/kg (drug or vehicle, a solution of saline-DMSO 70%). To corroborate the data, a second set of animals was treated with AM-281 (Tocris Cookson Inc., Ellisville, MO), a CB1 antagonist. Animals were assigned to one of two experimental groups: the first group of rats was fasted for 36 h before receiving either an i.p. injection of Rimonabant or AM281 (3 mg/kg) 1 h prior to euthanasia (Rimonabant or AM281 group). The second group was fasted for 36 h prior to receiving an injection of vehicle (DMSO) 1 h before euthanasia (Control group). After sacrifice, the stomach was surgically excised and the plasma collected.
An additional set of animals was utilized to perform the food intake study. A group of overnight fasted adult male rats was treated with Rimonabant (i.p.) at a dose of 3 mg/kg or vehicle (a solution of saline-DMSO 70%) and immediately after treatment allowed free access to chow diet. One hour after the injection, the amount of food eaten was recorded for each rat.
Effects of pharmacological blockade of CB1 on gastric Nucb2 production, gastric Nucb2 secretion and plasma Nucb2/Nesfatin-1 in animals with a pharmacological inhibition of the gastric mTOR signaling by rapamycin treatment
Adult male rats were assigned to one of two experimental groups: 36-hour fasted animals treated with rapamycin (1 mg/Kg i.p. for 6 d) (Rapa group); 36-h fasted animals treated with rapamycin (1 mg/Kg i.p. for 6 d) and rimonabant (3 mg/Kg i.p.) 1 h before euthanasia (rapa+rimo group). Rapamycin was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, United States).
Tissue explant culture: Tissue explants were obtained from adult Sprague-Dawley rats. To obtain ex vivo tissue explants, after euthanasia the stomachs were rapidly excised and transported to the lab in Krebs-Ringer-HEPES buffer (NaCl, 125 mmol/L; KCl, 5 mmol/L; MgSO4, 1.2 mmol/L; KH2PO4, 1.3 mmol/L; CaCl2, 2 mmol/L; glucose, 6 mmol/L; HEPES, 25 mmol/L; pH 7.4). After removing the blood vessels and connective tissue, the stomach was washed with sterile Krebs-Ringer-HEPES buffer. The tissue explants were placed in six-well dishes containing 2.5 mL of Dulbecco’s modified Eagle’s medium supplemented with L-glutamine (200 mmol/L), penicillin (1000 U/mL) and streptomycin (100 μg/mL). After a pre-incubation period of 1 h at 37 °C under a humidified atmosphere of 95% air-5% CO2, the media was aspirated, and 2.5 mL of fresh medium was added into each well. The culture medium was collected after 2 h and tissue was weighed[11]. The samples were stored at -80 °C until used for biochemical analysis or processed to sample concentration by ultracentrifugation units (Amicon Ultra 3-kDa cut off, Millipore, Billerica, United States) for Western blot analyses.
The fundus of the stomach was obtained after euthanasia from animal models and homogenizated using a TissueLyser II (Qiagen, Tokyo, Japan) in cold RIPA buffer [containing 200 mmol/L Tris/HCl (pH 7.4), 130 mmol/L NaCl, 10% (v/v) glycerol, 0.1%(v/v) SDS, 1%(v/v) Triton X-100, 10 mmol/L MgCl2] with anti-proteases and anti-phosphatases (Sigma-Aldrich; St. Louis, MO, United States). The tissue lysates were centrifuged for 10 min at 18000 g in a microfuge at 4 °C. Then, 30 μg of stomach mucosa were separated in 10% sodium-dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and transferred onto nitrocellulose membranes. Equal loading was confirmed by measuring the amount of β-actin in whole tissue protein extracts. The membranes were probed successively with primary antibodies and peroxide-conjugated secondary antibodies (Jackson Immunoresearch, Baltimore, United States). Specific antigen-antibody binding was visualized using a chemiluminescence method according to the manufacturer’s instructions (Pierce ECL Western Blotting Sustrate, Thermo Scientific). Primary anti-nesfatin-1 (1-82) was purchased from Phoenix Pharmaceuticals, Inc. (Burlingame, United States) and anti- β-actin (A-5316) was purchased from Sigma Chemical Co., (St Louis, MO, United States).
Western blots were performed using independent samples from different rats belonging to each group. β-actin detection was performed for all western blots as a loading control. The Western blot data are expressed as the mean ± SEM of percentages normalized to β-actin levels (arbitrary units).
Total RNA was isolated from the stomach mucosa of animals using TRIzol (Invitrogen, CA, United States), according to the manufacturer’s recommendations. The extracted total RNA was purified with DNase treatment using a DNA-free kit as a template (Ambion, United States) to generate first-strand cDNAs using a High-capacity cDNA Reverse Transcription kit (Applied Biosystems, United States). Quantitative real-time PCR was performed using a StepOne Plus Instrument (Applied Biosystems) with specific Taqman qRT-PCR primers and probes. For analyses, the NUCB2 gene expression levels (Assay ID: Rn01510621_m1) were normalized to the mRNA expression levels of the housekeeping gene hypoxanthine phosphoribosyltransferase 1 (HPRT1) (Assay ID: Rn01527840_m1, TaqMan: Applied Biosystems) and are expressed relative to the average value of the control group.
Variations in the Nucb2 mRNA expression were measured in the rat gastric mucosa by real-time PCR and immunoblotting was performed to test the Nucb2 protein content in the mucosa.
To measure plasma Nucb2/Nesfatin-1 levels, blood samples were collected in tubes containing EDTA (1 mg/mL blood) and immediately centrifuged. Gastric tissues explants obtained from the experimental animals were incubated as described below[11]. The secretome collected from the gastric explants were immediately processed for sample concentration (corrected per gram of tissue) by ultra-centrifugation units (Amicon Ultra UFC800324 and UFC500324 off, Millipore, Billerica, United States). Nucb2/Nesfatin-1 secretion was measured by ELISA in the secretome and plasma using reagents kits and methods provided by Phoenix Pharmaceuticals, Inc. (Burlingame, United States). The assay sensitivity limit was 0.1 ng/mL. The results are expressed as ng/mL of NUCB2/nesfatin-1.
The data are presented as the mean ± SEM. Statistical analyses were performed with GraphPad Prism 5 software. Analyses comparing different groups were performed using the non-parametric Mann-Whitney U test. The statistical significance is indicated as follows: aP < 0.05 and bP < 0.01.
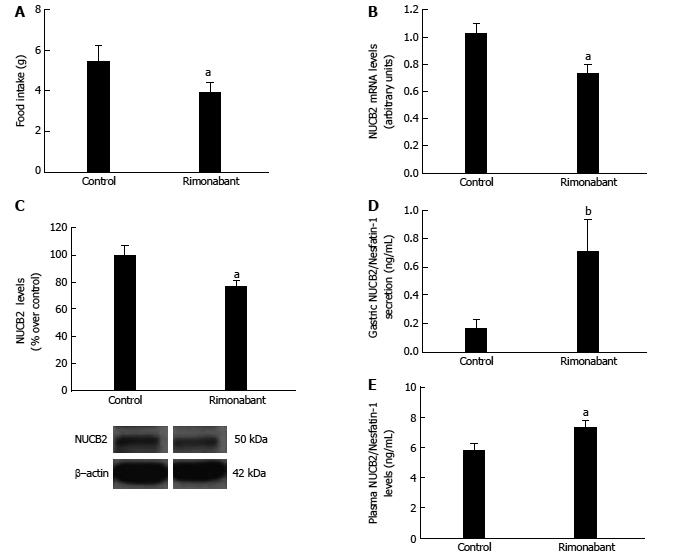
Animals administered rimonabant (i.p.) showed a decrease in food intake after 1 h compared to control group (Figure 1A). Gastric mRNA levels of Nucb2 (Control: 1.02 ± 0.06 vs Rimonabant: 0.74 ± 0.06 arbitrary units, aP < 0.05) (Figure 1B) and Nucb2 protein content in gastric mucosa (Control: 100 ± 6.81 vs Rimonabant: 76.23 ± 4.76, aP < 0.05) (Figure 1C) were significantly reduced after the blockade of the peripheral CB1 receptors with rimonabant. The decreased content in Nucb2 in the gastric mucosa in the group of animals treated with rimonabant was associated with an increased secretion of Nucb2/Nesfatin-1 from the stomach, as found by Nucb2/Nesfatin-1 values measured in the secretome obtained from gastric explants (control 0.16 ± 0.06 vs rimonabant 0.71 ± 0.22, bP < 0.01 ) (Figure 1D). The circulating levels of Nucb2/Nesfatin-1 measured in plasma were accordingly increased after rimonabant treatment (control 5.83 ± 0.39 ng/mL vs rimonabant 7.37 ± 0.4 ng/ml, aP < 0.05) (Figure 1E).
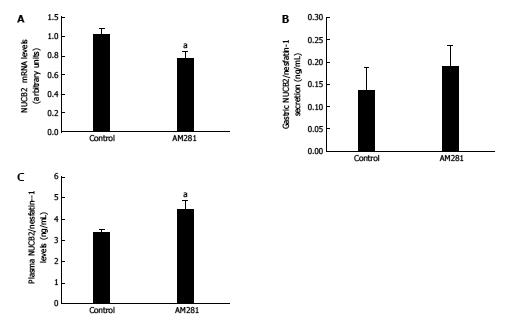
In keeping with data from the rimonabant treatment, the i.p. injection of a CB1 antagonist (AM281) caused a decrease in mRNA levels of Nucb2 (Control: 1.02 ± 0.06 vs AM-281: 0.78 ± 0.06 arbitrary units, aP < 0.05) (Figure 2A). Moreover, the measurement of Nucb2 secretion from gastric explants from animals treated with AM-281 showed a tendency towards an increase in gastric Nucb2 secretion, although it was not statistically significant (Figure 2B). In addition, circulating Nucb2/nesfatin-1 levels analyzed by ELISA showed a significant increase after AM281 treatment (Control: 3.39 ± 0.13 ng/mL vs AM281: 4.46 ± 0.38 ng/mL, aP < 0.05) (Figure 2C).
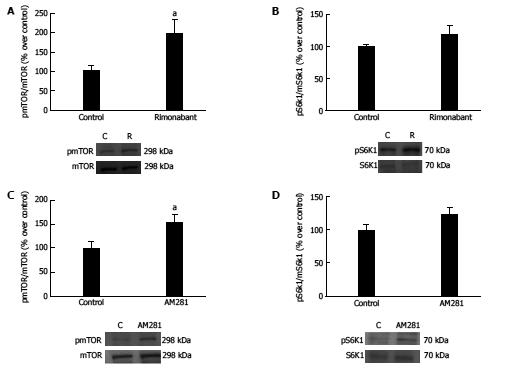
We found that gastric CB1 blockade with rimonabant treatment in the fasting state induces activation of the mTOR/S6K1 pathway (vehicle: 100 ± 13.99 vs rimonabant: 198.11 ± 34.5; P < 0.05) (Figure 3A). Accordingly, in the present work we observed that AM281 administration to fasted animals induces activation of the mTOR/pS6K1 pathway, as observed by increased expression of pmTOR/mTOR in the gastric mucosa of AM281 treated animals compared to controls (vehicle, 100 ± 13.41 vs AM281, 154.13 ± 15.31, P < 0.05) (Figure 3C).
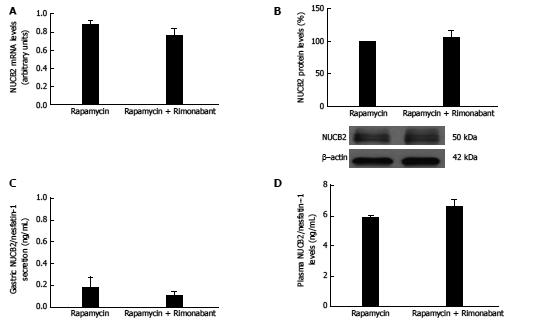
In light of mTOR pathway activation in response to gastric CB1 blockade with rimonabant, the next step was to determine whether this pathway might be mediating the effect of cannabinoids on Nucb2/nesfatin-1 production. To this end, we administered rimonabant to a group of animals subjected to chronic rapamycin treatment to establish mTOR pathway inactivation. We find that, contrary to the effects found in control rats, i.p. administration of rimonabant to animals previously treated with rapamycin did not affect gastric Nucb2 mRNA levels (Figure 4A), gastric Nucb2/Nesfatin-1 protein levels (Figure 4B), gastric secretion of Nucb2/Nesfatin-1 (Figure 4C) or circulating levels of Nucb2/Nesfatin-1 (Figure 4D).
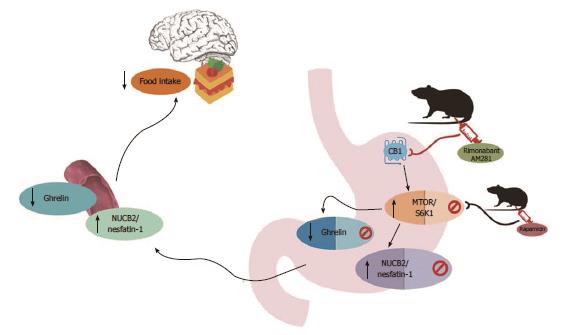
Ghrelin and Nucb2/Nesfatin-1 are co-expressed in the same endocrine cells of the stomach, together with the cannabinoid receptor CB1[4]. In the present study we show, for the first time to our knowledge, that Nucb2/Nesfatin-1 regulation in the stomach is governed by the cannabinoid system in an inverse relation to the described in the bibliography for ghrelin[14]. First, we found that blockade of the endogenous CB1 receptor at peripheral level with two different antagonists, rimonabant and AM281, increases gastric Nucb2/Nesfatin-1 secretion, which is reflected by an increase in the circulating levels of this peptide. An anorexigenic effect of Nucb2/nesfatin-1 has been extensively described[4] animals treated with rimonabant showed a significant decrease in food intake. The anorexigenic effect after CB1 blockade might be attributed to the increased production of Nucb2/nesfatin-1 in the stomach. Moreover, it was previously shown that the peripheral blockade of CB1 with rimonabant resulted in decreased food intake that was mediated by a decrease in the production of the orexigenic ghrelin[14]. Altogether, the data in the present study shows that Nucb2/nesfatin-1 is regulated in the stomach in a way that is diametrically opposed to those previously described for ghrelin, suggesting an equilibrium at the gastric level between the anorexigenic Nucb2/nesfatin-1 and the orexigenic ghrelin that regulates food intake to maintain body weight.
The next step of the present work was to un-derstand the intracellular mechanism mediating the effect of the cannabinoid system on gastric Nucb2/nesfatin-1. It was previously demonstrated that the regulation of ghrelin production in the stomach by the cannabinoid system is driven by the mTOR/S6K1 pathway[14]. In this sense, it was also reported that Nucb2/nesfatin-1 production in the stomach is modulated by mTOR signaling during nutritional changes[12]. Taking into account that ghrelin and Nucb2/nesfatin-1 are produced in the same cell type in the stomach, we wanted to determine whether the intracellular mTOR/S6K1 pathway may also be involved in the gastric regulation of Nucb2/nesfatin-1 by the cannabinoid system. As expected, pharmacological blockade of CB1 with rimonabant, or AM281, activated the mTOR/S6K1 pathway, which is a sensor of nutritional status.
In light of the present findings the blockade of the cannabinoid system by CB1 antagonism with rimonabant or AM281 exerts the activation of the mTOR pathway and the increase of the anorexigenic signal Nucb2/nesfatin-1 that induces satiation.
To further confirm our results and to more com-pletely explore the mechanism mediating the effects of cannabinoid on Nucb2/nesfatin-1 production and release, we investigated whether injection of rimonabant would be effective in animals with mTOR/S6K1 pathway inactivation. In agreement with our hypothesis, peripheral injection of rimonabant did not affect gastric Nucb2/nesfatin-1 production or circulating levels in rats with an inactivated mTOR/S6K1 pathway by rapamycin treatment.
In summary, the main findings of this present research are as follows: (1) peripheral CB1 receptors modulate food intake and the data might suggest that these regulation is drive through a mechanism that implies gastric Nucb2/nesfatin-1 production; (2) gastric CB1 receptors modulate Nucb2/nesfatin-1 production in the stomach at the intracellular level mediated by the mTOR pathway; and (3) taking into account previous data with reference to cannabinoid regulation of ghrelin[14] and the present, we can conclude that Nucb2/nesfatin-1 and ghrelin are regulated at the gastric level by the cannabinoid system in a diametrically oppositional manner to regulate the food intake and maintain energy balance (Figure 5).
Altogether, the data in the present paper show for the first time that the cannabinoid system, and more precisely CB1, regulates the nutrient sensor mTOR, consequently controlling the production of the anorexigenic hormone Nucb2/Nesfatin-1. Moreover, this newly described mechanism also provides clues to the balance of anorexigenic and orexigenic signals in the stomach that maintain energy homeostasis. Further work should focus on testing whether this gastric mechanism is affected under pathological conditions such as obesity or anorexia, which lead to an unhealthy body weight. Moreover, the possibility of modulating a peripheral target, such as Nucb2/Nesfatin-1, may represent a novel pharmacological therapy for obesity, thereby potentially avoiding the non-desirable off-target effects of many drugs.
Nowadays the more effective treatment against obesity is gastric surgery which suggests that gastrointestinal tract signals are crucial factors in the regulation of energy homeostasis. Although several works in the last years has been focused in the role of different gastric signals with a relevant role in body weight regulation the full mechanisms governing this actions has not been elucidated.
The discovery of new mechanisms at gastric level represents the possibility of new therapeutic targets to treat obesity avoiding the collateral effects at central level that suppose a serious problem in the pharmacological design of drugs.
The gastric mechanism described in the present regulating the production by the stomach of one of the newest gastrointestinal hormone related with a decreasing effect in appetite was has been previously reported with an inhibitory effect in the release of the only known gastric hormone with orexigenic effects. This data highlight the existence of a gastric mechanism controlling appetite through the differential regulation of anorexigenic and orexigenic gastric signals.
This is an interesting manuscript on the regulation of food intake through a mechanism that implies gastric production and release of Nucb2/Nesfatin-1.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Desiderio J, Schuller HM, Temraz S, Ulasoglu C
S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 752] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 2. | Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088-5094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | García-Galiano D, Pineda R, Ilhan T, Castellano JM, Ruiz-Pino F, Sánchez-Garrido MA, Vazquez MJ, Sangiao-Alvarellos S, Romero-Ruiz A, Pinilla L. Cellular distribution, regulated expression, and functional role of the anorexigenic peptide, NUCB2/nesfatin-1, in the testis. Endocrinology. 2012;153:1959-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Taché Y, Sachs G, Lambrecht NW. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 5. | Kim J, Chung Y, Kim H, Im E, Lee H, Yang H. The Tissue Distribution of Nesfatin-1/NUCB2 in Mouse. Dev Reprod. 2014;18:301-309. [PubMed] |
| 6. | Dornonville de la Cour C, Björkqvist M, Sandvik AK, Bakke I, Zhao CM, Chen D, Håkanson R. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept. 2001;99:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 258] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2834] [Cited by in RCA: 2768] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 8. | Shimizu H, Oh-I S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2466] [Cited by in RCA: 2450] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 10. | Al Massadi O, López M, Tschöp M, Diéguez C, Nogueiras R. Current Understanding of the Hypothalamic Ghrelin Pathways Inducing Appetite and Adiposity. Trends Neurosci. 2017;40:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Seoane LM, Al-Massadi O, Caminos JE, Tovar SA, Dieguez C, Casanueva FF. Sensory stimuli directly acting at the central nervous system regulate gastric ghrelin secretion. an ex vivo organ culture study. Endocrinology. 2007;148:3998-4006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Li Z, Xu G, Li Y, Zhao J, Mulholland MW, Zhang W. mTOR-dependent modulation of gastric nesfatin-1/NUCB2. Cell Physiol Biochem. 2012;29:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Xu G, Li Y, An W, Li S, Guan Y, Wang N, Tang C, Wang X, Zhu Y, Li X. Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake. Endocrinology. 2009;150:3637-3644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Senin LL, Al-Massadi O, Folgueira C, Castelao C, Pardo M, Barja-Fernandez S, Roca-Rivada A, Amil M, Crujeiras AB, Garcia-Caballero T. The gastric CB1 receptor modulates ghrelin production through the mTOR pathway to regulate food intake. PLoS One. 2013;8:e80339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |