Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6385
Peer-review started: June 6, 2017
First decision: June 22, 2017
Revised: July 3, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: September 21, 2017
Processing time: 110 Days and 22.7 Hours
An awareness of the expected time for therapies to induce symptomatic improvement and remission is necessary for determining the timing of follow-up, disease (re)assessment, and the duration to persist with therapies, yet this is seldom reported as an outcome in clinical trials. In this review, we explore the time to clinical response and remission of current therapies for inflammatory bowel disease (IBD) as well as medication, patient and disease related factors that may influence the time to clinical response. It appears that the time to therapeutic response varies depending on the indication for therapy (Crohn’s disease or ulcerative colitis). Agents with the most rapid time to clinical response included corticosteroids, calcineurin inhibitors, exclusive enteral nutrition, aminosalicylates and anti-tumor necrosis factor therapy which will work in most patients within the first 2 mo. Vedolizumab, methotrexate and thiopurines had a longer time to clinical response and can take several months to achieve maximal efficacy. Factors affecting the time to clinical response of therapies included use of concomitant therapy, disease duration, smoking status, disease phenotype and advanced age. There appears to be marked variation in time to clinical response for therapies used in IBD which is further influenced by disease and patient related factors. Understanding the expected time to therapeutic response is integral to inform further decision making, maintain a patient-centered approach and ensure treatment is given an appropriate timeframe to achieve maximal benefit prior to cessation.
Core tip: There appears to be marked variation in time to clinical response for therapies used in inflammatory bowel disease which is further influenced by disease and patient related factors. The most rapid response can be expected with corticosteroids, calcineurin inhibitors, exclusive enteral nutrition, aminosalicylates and anti-tumor necrosis factor therapy (within 2 mo), while methotrexate, thiopurines and vedolizumab can take several months to achieve maximal response. There is a lack of reporting of the time to response of therapies in clinical trials for inflammatory bowel disease and this remains an area that should be addressed in future studies.
- Citation: Vasudevan A, Gibson PR, Langenberg DRV. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: What should the clinician expect, what should patients be told? World J Gastroenterol 2017; 23(35): 6385-6402
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6385.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6385
The therapeutic armamentarium for inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), continues to expand, providing valuable additional opportunities to achieve optimal long term outcomes for patients. Equally, however, there is added complexity, commensurate with the number of options, an enhanced understanding of the risks and benefits, plus the differential effects of treatments on objective disease outcomes (e.g., mucosal healing), clinical remission and/or patient-reported outcomes.
Yet when better outcomes are potentially achievable, there are higher expectations. It is increasingly important with current therapeutics that the physician plans ahead, given delays in escalating treatment are likely just as common and detrimental as delays in diagnosis are in IBD[1]. Hence an awareness of the approximate time expected to achieve a treatment goal is fundamental to making decisions such as whether to persist with a therapy or switch to an alternative. Equally, one does their patient a disservice by prematurely switching therapies before an agent is given an appropriate length of time to achieve efficacy.
Achieving an optimal time to therapeutic response has further benefits in the doctor-patient relationship, as it allows the clinician to provide the patient a cogent framework of the expected period to see response to a new drug and hopefully empower the patient to persevere with, and maintain adherence to therapy. This is particularly relevant for therapies that have a longer time to therapeutic response, such as the immunomodulators, where it might take several months to reach maximal therapeutic efficacy without the patient necessarily experiencing any symptom benefit for a significant part of this.
Hence, time-to-therapeutic response is an important yet underestimated factor in the day-to-day management of IBD and has not been a major focus of attention in the literature to date. This review attempts to address this unmet need by analyzing the available literature relating to the expected time-to-clinical-response for currently available therapies in IBD and measures that can assist the clinician in determining whether a medication has reached its therapeutic potential. We will also analyze disease and patient related factors that may impact on the time-to-clinical-response of therapies. Therapies discussed in the review will include corticosteroids, aminosalicylates (5-ASA), thiopurines, methotrexate, anti-tumor necrosis factor (anti-TNF) therapies, vedolizumab, calcineurin inhibitors and exclusive enteral nutrition.
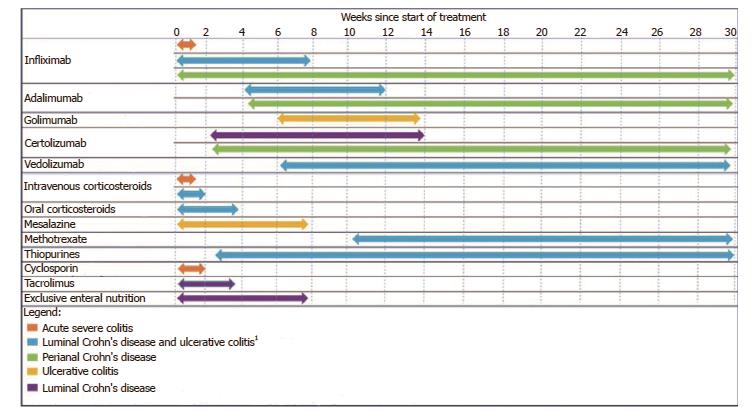
The concept of time-to-clinical-response is schematically represented in Figure 1. Given the lack of focus on time to response in previous literature, there is no broadly accepted definition. Table 1 provides a summary of some of the important components of time to response. These include the earliest time at which a patient can expect a response, the time at which most patients (i.e., greater than 50%) expecting to benefit from therapy will achieve a response and the time point where therapeutic benefit remains improbable, the so-called time to futility. For this review, time-to-clinical-response refers to the time from the initiation of therapy until the patient achieves a clinical response. It only pertains to patients who attain a clinical response and can thereby aid the clinician in judging the likelihood of a response being achieved based upon the elapsed time on therapy. Where available, estimates of timeframes in which the majority of patients who ultimately respond to therapy will be expected to respond to therapy will be reported. The time to futility of therapy is reported in Table 1, however, this will not be a primary focus of this review.
| Agent | Earliest published clinical response | Earliest published objective response | Time to response in most patients1 | Time to futility | Use of therapeutic drug monitoring | Comments |
| Mesalazine (oral) | 1 wk[21] | 3 wk[148] | 4 wk | > 12 wk | N | A higher dose may lead to a more rapid response |
| Prednisolone (oral) | 2 wk[28] | 2 wk[28] | 3 to 7 wk | 8 wk | N | May take longer for CD |
| Corticosteroids (IV) | 3 d[31] | 1 wk[127] | 3-5 d | 7-10 d | N | |
| Infliximab (IV) | 1 wk[48] | 8 wk[149] | 2-8 wk | > 6 mo | Y | |
| Adalimumab (SC) | 4 wk[59] | 8 wk[59] | 4-8 wk | > 6 mo | Y | Response time better with 160/80 mg vs 40/40 mg induction dosing |
| Certolizumab (SC) | 2wk[61] | 10 wk[64] | 10 wk | > 16 wk | N | |
| Golimumab (SC) | 6 wk[65] | 6 wk[65] | 6 wk | > 14 wk | Y | |
| Certolizumab (SC) | 2 wk[61] | 10 wk[64] | 10 wk | > 16 wk | N | |
| Vedolizumab (IV) | 6 wk[78] | 6 wk[78] | 19 wk | 12 mo | N2 | Response time may be better for UC vs CD |
| Thiopurines (oral) | 2 wk[80] | 3 mo[88] | > 6-9 mo | Y | Endoscopic response may take much longer than clinical response | |
| 10 to 12 wk | ||||||
| Methotrexate (oral or SC) | 9 wk[111] | 12 wk[110] | 9 wk | > 6 mo | N | Response time and efficacy may be better in 1) CD vs UC, 2) SC vs oral |
| Cyclosporin (IV then oral) | 1 wk[127] | 1 wk[127] | 4 to 5 d | > 14 d | Y | |
| Tacrolimus (oral) | 2 wk[122] | 2 wk[122] | 2 wk | 4 wk | Y | |
| EEN (oral) | 10 d[131] | 4 wk[139] | 3 to 4 wk | 8 wk | N |
The methods of determining when a “response” has occurred are heterogeneous and include both clinical symptoms and endoscopic (or objectively-assessed) findings. The correlation between symptomatic improvement and achievement of endoscopic remission differs between UC and CD, with improvement in symptoms correlating better with mucosal healing in UC than CD[2-5]. There are data to support early clinical remission, albeit not response, to be predictive of endoscopic improvement and healing at 12 mo[6]. The value of symptomatic improvement, however, cannot be discounted from a patient’s perspective given the correlation with long-term steroid-free remission and the inherent part that alleviation of symptoms plays in improving quality of life[5,7]. Moreover, the complex interplay of patient symptoms and structural damage in IBD is being increasingly recognized with both symptoms and endoscopic findings important factors in determining overall disease severity and burden[8].
Thus, response is a multi-faceted concept and this review will primarily address the time-to-clinical-response and time-to-clinical-remission, given that the focus here is to engender a patient-centered approach when clinicians discuss therapeutic options with their patients. Time to endoscopic response and remission will also be reported where data are available, although this is a secondary focus.
Given the heterogeneity across studies in defining clinical response, clinical remission and endoscopic remission, we have used broad outcome measures of “clinical or endoscopic improvement” or “clinical or endoscopic remission or mucosal healing” as defined by the authors of each study. For the purpose of this review, clinical response will consider symptom improvement only rather than an improvement in symptoms and laboratory indices.
A summary of the relative time-to-therapeutic-response of different therapies for IBD is presented in Table 1 and Figure 1. Medication related factors that influence the time to response of different therapies are discussed within each of the therapeutic classes.
Aminosalicylates are more effective at inducing response and remission in patients with mild-moderate ulcerative colitis than placebo[9], but their evidence for efficacy in patients with Crohn’s disease is poor[10,11]. Therefore, with regards to ulcerative colitis, available data indicate that it generally takes two to four weeks to achieve clinical response with oral and/or topical aminosalicylates. Mesalazine induces endoscopic remission in 67% of patients at 4 wk for active colitis for both 2 and 4 g preparations, while another study found higher endoscopic remission rates of 78% and 69% after 8 wk with multimatrix mesalazine 4.8 g and 2.4 g, respectively, suggesting some patients who will achieve endoscopic remission may take up to 8 wk[12,13].
Key issues for aminosalicylates include whether the formulation, the dose and/or the route(s) of delivery influence the speed of onset of action.
Formulation: Trials of sulfasalazine, olsalazine and balsalazide in mild to moderate UC demonstrated an improvement in clinical symptoms and endoscopic response with 2 to 3 wk of therapy in most patients[14-17]. In contrast according to published data, coated mesalazine preparations demonstrated a somewhat slower clinical and endoscopic improvement within 4 to 8 wk[18,19]. Two head-to-head studies suggested that an equimolar dose of balsalazide resulted in a more rapid clinical and endoscopic response than delayed-release mesalazine (using Eudragit S-coated tablets) therapy for patients with left-sided disease[17,20].
Dose: Time to therapeutic response for aminosalicylates may also be dose-dependent as demonstrated by Orchard et al[21] who found that 4.8 g daily of mesalazine (delayed release, Asacol MR®) improved and resolved symptoms more rapidly than 2.4 g daily (median 7 d vs 9 d, 19 d vs 29 d respectively). Another study found a numerically faster time-to-clinical-response with mesalazine 4.5 g than 3 g or 1.5 g daily[22]. Kamm et al[13] described numerically higher endoscopic remission rates of 78% after 8 wk with mesalazine MMX 4.8 g vs 2.4 g daily (69%), but whether this equates to faster response in those who achieve remission was not reported[12,13].
Route of delivery: Combined oral and topical mesalazine was associated with more rapid resolution of rectal bleeding (mean 11.9 d) than with oral mesalazine only (25.5 d) for left-sided colitis in the only randomized study reporting this end-point[23]. Another randomized trial of sixty patients with distal UC comparing oral mesalazine with mesalazine enemas or combination topical and oral treatment found a median time to resolution of rectal bleeding of 8 d on combination therapy and bleeding were significantly lower after ten days with either topical or combination therapy compared to oral mesalazine only. The findings indicate that topical therapy alone or in combination with oral therapy achieves symptom resolution more rapidly than oral therapy[23,24]. The efficacy of topical 5-ASA does not appear dose-dependent in the single study where this was specifically examined, but rapidity of response was not addressed[25].
A clinical response to steroids should be expected within 1 to 4 wk of commencing therapy for both CD and UC (not applicable to acute severe colitis) with response occurring more rapidly with intravenous than oral therapy[26,27]. There are several types of corticosteroids available for the treatment of IBD and the influence of route of administration and type of corticosteroid are relevant to determining the time to response, as discussed below.
CD: Although most patients with CD can expect a response to high dose oral corticosteroids within 4 wk, some data suggest a more prolonged course may be necessary to capture response. For example, clinical response after three to seven weeks of 1 mg/kg per day oral prednisolone in a prospective cohort study of 146 patients with active ileocolonic or colonic CD increased from 63% to 92% between weeks 4 and 7 respectively, although only 29% achieved endoscopic remission[2]. These data suggest that a clinician should wait up to 7 wk before deciding that a response to high doses of prednisolone is unlikely if that approach is clinically acceptable.
UC: Response to oral prednisolone is rapid in UC, with 17%-76% achieving clinical remission and 65%-78% endoscopic improvement after 2 wk of oral prednisone in two randomized studies, with the higher response rates noted by Truelove et al[28] who used both oral and rectal prednisolone in combination[28,29]. Other studies have also suggested a response within the first two weeks of a tapering dose of oral prednisolone beginning at 40 mg/d in the majority of patients with moderate UC[30].
Route of administration: (1) CD: While direct comparisons between intravenous and oral corticosteroids are not available in CD, response appears rapid with intravenous corticosteroids, with 78% of patients having symptom resolution after five days of intravenous hydrocortisone (300 mg daily), which increased to 93% after 10 d in one randomized study comparing intravenous hydrocortisone to corticotrophin, to which response rates were also high (71% and 82% at days 5 and 10, respectively)[27,31]. (2) UC: Moderate UC has been shown to typically improve within five days of intravenous corticosteroids, including patients who failed to respond to high-dose oral prednisolone. Time frames for expected response are well described for acute severe colitis, where most patients appear to respond to therapy within 3 or 5 d of intravenous steroids (methylprednisolone 60 mg/d or hydrocortisone 300-400 mg/d), although these are observational data only[32-35]. A lack of response within 5 d is associated with a higher rate of subsequent colectomies again in observational studies, and therapy beyond 7 d is unlikely to be beneficial[36].
Type of glucocorticoid: (1) CD: Several randomized studies have suggested the mean times to clinical response and remission with budesonide in CD were comparable to systemic corticosteroids, ranging from 22 to 27 d[37-40].
(2) UC: Induction of remission when using budesonide MMX 9 mg daily in mild to moderate UC should occur within 4 to 8 wk of commencing therapy, with 42%-47% of patients achieving an endoscopic or clinical improvement in randomized controlled trials (RCT)[41,42].
Dose: The effect of corticosteroid dose on time to response has not been evaluated. One randomized study assessed response rates with 20 mg, 40 mg and 60 mg daily of oral prednisolone for outpatient management of ulcerative colitis and suggested a higher response rate at both 2 wk and 3-5 wk of follow-up with 40 and 60 mg/d of therapy (both 50% at 2 wk, then 65% at 3-5 wk respectively) compared to 20 mg daily (20% then 30%), but did not specifically assess time to response[30]. Determining the appropriate dose of steroid has traditionally been either empiric or weight-based. Accordingly, corticosteroid dosing evaluated in clinical trials has varied; for instance, studies have used 1 mg/kg/d or 40-60 mg/d of prednisolone, 9 mg of budesonide orally, while for intravenous therapy includes 300-400 mg/d of hydrocortisone (divided doses) or 60 mg/d of methylprednisolone[26,43,44].
Pertinent issues relating to time to therapeutic response of anti-TNF therapy include the associations with serum drug levels and antibodies, plus concomitant therapy.
Infliximab: (1) CD: Clinical response and remission after administration of infliximab appear to be rapid in luminal CD, taking 8 and 9 d respectively in one observational study of 129 patients[45]. Clinical response rates in RCTs of infliximab in CD were 61% and 81% for weeks 2 and 4 respectively after a single infusion of infliximab[46,47]. Clinical remission rates were reportedly 88% one week after a single infliximab dose for colonic CD although the data were observational only[48]. Rates of mucosal healing in Crohn’s disease have ranged from 30%-67% after 6 mo of infliximab, with higher rates typically observed in ’real-world’ clinical cohorts than trials[49,50].
(2) UC: Clinical and endoscopic response to infliximab in patients with moderate to severe chronic active ulcerative colitis appears to take several weeks, although this may be due to a lack of reporting of early outcomes after initiation of therapy in the outpatient setting, given that response rates reported for acute severe colitis are generally more rapid than this. Nevertheless, about half of patients previously not responding to either intravenous or oral corticosteroids experienced a clinical response two weeks after the first infusion of infliximab in one prospective uncontrolled study[51]. Such early response rates have not been reported in RCTs, but data from such studies have shown a significantly higher rate of clinical response (69% vs 37%), remission (39% vs 15%) and mucosal healing (62% vs 33%) by week 8 with 5 mg/kg induction dosing versus placebo[52]. For infliximab use in ulcerative colitis, two large randomized studies of moderately severe ulcerative colitis showed a significantly higher rate of mucosal healing by week 8 with after both 5 mg/kg and 10 mg/kg induction therapy (62% vs 33% with placebo)[52].
For acute severe colitis, a clinical response to infliximab therapy should be expected within the first 7 d after therapy[53]. Achieving a higher serum infliximab level during induction has been associated with a higher rate of short term mucosal healing and an accelerated induction regimen of infliximab in acute severe colitis has been associated with a more prolonged time to colectomy than standard induction, although the rapidity of response has not been directly assessed[54,55]. Indeed, recent data suggest that a rebound of higher C-reactive protein, lower albumin and/or symptoms within a few days after the first dose of infliximab should prompt concerns of infliximab non-response and a potentially higher risk of colectomy[55]. A trial is currently underway to assess the utility of an accelerated induction regimen of infliximab in acute severe colitis and this may provide further information on the effect of dose and drug levels on time to response[56].
Adalimumab: (1) CD: An initial response to adalimumab typically occurs within the first few weeks of therapy, as inferred from a phase 2 RCT showing clinical remission rates of 36% at week 4 following induction treatment with 160/80 mg at week 0 and 2 for CD, compared to 12% with placebo[57]. While clinical remission rates were higher from week 1 than placebo in this study (16% vs 7% respectively), this only reached statistical significance at week 4. Moreover, the rate of mucosal healing for moderately severe ileocolonic CD with induction 160/80 mg adalimumab followed by 40 mg fortnightly was significantly higher than placebo at 12 wk (27% vs 13%) and sustained until 52 wk (24% vs 0%) in another RCT[58]. Endoscopic remission rates were 52% and 28% at weeks 12 and 52 respectively in this study, the latter likely reflecting secondary loss of response during maintenance therapy.
(2) UC: Clinical remission and mucosal healing rates with adalimumab induction with 160/80 mg regimen in patients with moderate to severe UC after 8 wk was achieved in 19 and 47% respectively, with separation in clinical remission rates as early as week 4 compared to placebo in a RCT[59]. The lower remission rates in this study may relate to the high proportion (75%) of patients who had failed other therapies prior study enrolment[59]. In another RCT assessing long term remission rates with adalimumab in moderate to severe UC, mucosal healing rates were 41% at week 8 and 25% at week 52 with fortnightly adalimumab 40 mg, compared to 32% and 15% for placebo, respectively[60]. Mucosal healing rates following adalimumab induction for UC have varied between 32% and 47% in RCTs[59,60].
Certolizumab pegol: CD: Certolizumab pegol at a dose of 400 mg given subcutaneously at weeks 0, 2 and 4 wk, about a third of patients with CD will have a clinical response to therapy within 2 wk, increasing to 41% by week 6 based on RCT data[61]. One study found response rates peak at 10 wk of 400mg 4-weekly therapy[62], while another study found response rates, as per a reduction in CDAI of 100, peaked by week 16 and declined thereafter[63]. Endoscopic activity was assessed at week 10 in one prospective, open label clinical trial of patients on 400 mg certolizumab 4-weekly and showed endoscopic remission occurred in 37%, reducing to 27% by week 54 in CD[64].
Golimumab: UC: Golimumab is administered subcutaneously and has been approved for use in ulcerative colitis in many countries. Approximately half of patients will achieve a clinical response by 6 wk with regimens of 100/200 mg and 400/200 mg as induction at weeks 0 and 2 for moderate to severe ulcerative colitis from one large RCT[65]. Observational data suggest that response rates may continue to increase up until week 14, when reported to be between 69% and 86%[66,67]. Mucosal healing appears to be rapid, with 42 and 45% of patients achieving mucosal healing 6 wk after induction therapy with 100/200 mg and 400/200 mg, respectively[65]. In-travenous induction therapy for golimumab does not appear to confer any additional benefit in terms of response rate compared to subcutaneous induction, although time to response of this strategy has not been evaluated[68]. Since there are no data evaluating clinical response rates beyond week 14, the benefits of continuing therapy beyond this time point in patients who have not achieved a response remains uncertain.
Demographic factors: One study assessed baseline factors that were predictive of a more rapid attainment of clinical remission with induction certolizumab therapy for CD and found that younger age, non-smokers, the absence of previous IBD surgery and a lower disease activity score were associated with a more rapid attainment of clinical remission[69].
Anti-TNF drug levels: Currently, data supporting a correlation between anti-TNF drug levels and time to therapeutic response are limited. For golimumab, drug levels at weeks 2 and 4 were shown to correlate with week 6 clinical response rates, but the effect on time to therapeutic response was not further assessed as response was only evaluated at a single time point in this study[70]. Higher certolizumab plasma concentrations at week 8 are associated with higher rates of clinical remission and endoscopic remission at week 10, but not a higher clinical response rate[71]. There is a lack of data concerning the relationship between time-to-response for infliximab and adalimumab in relation to drug levels.
Concurrent immunomodulator therapy: While time to response has not been directly compared between anti-TNF monotherapy and in combination with immunomodulator therapy, there was a trend toward a higher rate of clinical remission at week 10 with combination infliximab and azathioprine compared to infliximab alone in the Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease (SONIC) Study, and a significantly higher rate of endoscopic remission at 26 wk with adalimumab and azathioprine, suggesting combination therapy may work faster than either therapy alone[50,72]. Similar findings of more rapid clinical remission have also been found for certolizumab therapy when used with concomitant immunomodulator therapy in CD[69].
Vedolizumab appears to have a generally slower time to response compared to other biologic agents. This may relate to the mechanism of anti-integrin therapy, with inhibition of lymphocyte gut migration taking more time to achieve therapeutic efficacy[73].
CD: Clinical remission with vedolizumab appears to take at least 10 to 14 wk in CD. This slower onset of action of vedolizumab, compared to anti-TNF therapies for instance, was evident in the RCTs GEMINI 2 and 3 registration trials of vedolizumab comparing therapy to placebo induction and maintenance therapy in CD[74]. Clinical remission rates increased from 15% to 27% between weeks 6 and 10 while remained stable in the placebo group (12%) at these time points in GEMINI 3 (i.e., those with prior anti-TNF failure), and in GEMINI 2 there was a significant increase in clinical remission after 6 wk compared to placebo[73]. Subsequent real-world observational data have also demonstrated that clinical remission rates tend to increase from week 6 to week 14 and in one study the median time to clinical response was 19 wk[75]. Mucosal healing rates of 30% were attained after a median of 22 wk in CD in one observational study[76]. Furthermore, GEMINI 2 found that almost 40% of initial vedolizumab responders remained in clinical remission to 52 wk although clinical remission rates only became superior to placebo after 30 wk[77].
UC: Response and remission rates appear more rapid in UC than CD. At week 6 after a 2-dose induction, clinical response, remission rates and mucosal healing were significantly higher with vedolizumab than placebo (47%, 17% and 41% vs 26%, 5% and 25% respectively) in one RCT[78]. Moreover, maintenance vedolizumab resulted in higher rates of clinical and endoscopic remission at week 52 than week 6 and mean partial Mayo scores continued to decline until week 52, suggesting that maximal response often takes several months.
While immunomodulators appear to reduce the formation of antidrug antibodies to vedolizumab[78], the low proportion of patients who actually formed antidrug antibodies in trials might imply that combination therapy may be unnecessary and hence may not provide additional benefit to time to therapeutic response, in contrast to anti-TNF therapies[78].
Thiopurines, including azathioprine and 6-mercaptopurine, appear to exert their effect via the metabolites, 6-thioguanine nucleotides (6-TGNs). Thiopurines inhibit the synthesis of DNA, RNA and proteins leading to inhibition of lymphocyte proliferation and apoptosis, thereby immunosuppression[79].
CD: Azathioprine or mercaptopurine take at least 4 to 8 wk to achieve clinical remission[80]. A small RCT suggested that a median of 10.7 wk was required to achieve clinical remission with oral azathioprine 2 mg/kg/d[81]. Time to clinical response with 1.5 mg/kg/d of mercaptopurine was assessed in an RCT by Present et al[82] in patients with CD failing corticosteroids or sulfasalazine. They demonstrated a wide-ranging time to therapeutic response from two weeks to nine months with a median of 3.1 mo; 19% of cases took more than 4 mo to achieve a sustained clinical response[82]. Similarly, in another RCT, Ardizzone et al[83] found a clinical remission rate gain from 33% to 63% from 3 to 6 mo after initiating azathioprine 2 mg/kg/d in patients with corticosteroid-dependent CD. Endoscopic remission rates also appear to be slow to achieve with only 17% of patients on azathioprine in the SONIC study achieving mucosal healing after 26 wk of therapy[50]. Yet another RCT comparing budesonide to azathioprine with 1 year follow up found mucosal healing occurred with thiopurines in 83% of steroid-dependent CD by 12 mo, implying that endoscopic improvement continues to slowly accumulate over extended periods[84].
UC: Both clinical and endoscopic response to thiopurines in UC appear to take a minimum of one month but more typically 3-6 mo for steroid-dependent UC[85,86]. Ardizzone et al[87] found that more than half of patients on azathioprine were in steroid-free endoscopic and clinical remission after 6 mo for patients with steroid-dependent UC, which was significantly higher than that with mesalazine (53% vs 21%) in an RCT. Another RCT showed a significant decrease in a composite disease activity score (including endoscopic, clinical and biochemical findings) at 3 and 6 mo after commencing azathioprine 2.5 mg/kg/d for steroid-dependent UC, implying that time to therapeutic response is likely between 3 and 6 mo[88].
Metabolite levels: Azathioprine undergoes rapid non-enzymatic conversion to 6-mercaptopurine which is then further metabolized to 6-TGNs in erythrocytes and leukocytes[89]. The therapeutic effect of thiopurines appears commensurate with 6-TGN concentrations, with steady state achieved at two to four weeks, so response can only be expected after this period[90]. Intravenous loading with azathioprine has been associated with a more rapid time to therapeutic response initially (1 wk for clinical response and 4 wk for endoscopic improvement). However, when compared to oral azathioprine at 8 wk, no difference in remission rates or 6-TGN levels had persisted[80,91]. Using dose titration guided by therapeutic drug monitoring, the mean time to therapeutic response decreased from 22 to 19 wk[92]. While prospective studies have not demonstrated a difference in outcomes between patients treated with weight-based and individualized, metabolite-guided dosing of thiopurines[93,94], robust retrospective data elucidated higher rates of clinical remission with 6-TGN levels above 230-260 pmol/8 × 108 RBCs[95-97]. Indeed, 78%-90% of patients had improved clinical outcomes from dose optimization after having a sub-therapeutic 6-TGN level[98-100].
Addition of allopurinol: A combination of allopurinol 100 mg and 25%-50% of the standard thiopurine dose has been utilized to overcome a number of thiopurine related side effects and correct an unfavorable metabolite profile (so called hypermethylators), with high clinical efficacy[101-105]. This has piqued interest as to whether combination allopurinol-thiopurine therapy might be able to achieve not only higher rates of, but quicker time to therapeutic response, especially compared to slow-titrating introductory dosing protocols (as advocated by treatment guidelines[26,106] and widely used to mitigate early side effects[107]). The result of controlled trials addressing this issue are awaited[108]. Additional strategies that may affect time to response of therapies are summarized in Table 2.
| Clinical scenario | Method | Improves time to response | Improves response rate | Improves tolerability | Published data? | Comments | Ref. | |
| Corticosteroids | CD and UC | Intravenous administration | - | - | Yes | [27,32,150] | ||
| Anti-tumour necrosis factor-α | Initial or for flare to recapture response (CD and UC) | Addition of azathioprine | - | Yes | [50] | |||
| Thiopurine | CD and UC | Addition of allopurinol | - | - | Yes | [105,108] | ||
| Split dosing of thiopurine | - | - | Yes | [151] | ||||
| Methotrexate | CD | High dose parenteral with corticosteroids if relapse on lower dose | - | - | Yes | Can recapture response | [152] | |
| Tacrolimus | UC | Target levels of 10-15 ng/mL | - | - | Yes | [123] | ||
| Aminosalicylates | UC | Maximize dose | - | Yes | [21] | |||
| Distal UC | Choice of formulation (balsalazide) | - | - | Yes | [17,20] |
Methotrexate is a folic acid analogue used in the treatment of multiple autoimmune conditions, including IBD. Methotrexate has been shown effective as an induction and maintenance therapy in CD particularly when administered parenterally. The role of methotrexate in UC is less clear, although a placebo-controlled trial of subcutaneous methotrexate did suggest clinical efficacy in the induction of steroid-free remission[109].
CD: A clinical response should be expected within 12 wk on parenterally-administered methotrexate according to an open label, non-randomized trial by Kozarek et al[110]. An observational study suggested that the median clinical response time was 9 wk for both oral and parenteral therapy (although 86% were on parenteral therapy in this study) and clinical remission took 22 wk[111]. Despite most patients clinically responding within the first several weeks of therapy, a subgroup may take up to 6 mo to respond[83].
UC: While a placebo-controlled trial of oral methotrexate 12.5 mg in patients with steroid-refractory UC showed no significant difference in clinical remission between the groups, those who reached clinical remission with methotrexate took a mean of 4.1 mo to do so[112]. Subsequent uncontrolled observations by Kozarek et al[110] showed a rapid clinical response (71% within 12 wk) with high-dose intramuscular dosing in UC, albeit in only 7 patients. The METEOR RCT, assessed the efficacy of parenteral methotrexate 25 mg weekly in steroid-dependent UC and found almost a third of patients had corticosteroid-free clinical remission by week 16, which increased to 40% by week 24[109]. Endoscopic remission was achieved in a numerically greater proportion then did the placebo group, but the difference did not reach statistical significance, perhaps due to lack of statistical power.
Route of administration: It is likely that parenteral methotrexate generally achieves a faster time to therapeutic response in a higher proportion of patients due to greater bioavailability, though supportive evidence is found only in the rheumatological literature[113].
Drug levels: Currently there is no reliable method to apply therapeutic drug monitoring of methotrexate in routine IBD care and this is therefore not useful in predicting time to therapeutic response. Serum methotrexate levels are only detectable for about 24 h post-dose and appear not to correlate with clinical response in IBD[114], adenosine levels do not correlate with efficacy[115], and methotrexate polyglutamates [active metabolite(s) of methotrexate] have displayed inconsistent results[116-118]. Finally, although folic acid is an important adjunct to methotrexate use and may reduce gastrointestinal upset and hepatic dysfunction, it has no apparent impact on time to therapeutic response[119].
Tacrolimus and cyclosporin are calcineurin inhibitors with evidence in IBD primarily for steroid-refractory UC. Small case series have suggested efficacy of tacrolimus for induction of remission in luminal CD[120]. Cyclosporin is an effective rescue therapy in acute severe colitis and has been used in moderate to severe chronic UC. Oral cyclosporine does not appear to be an effective induction therapy in CD[121].
Tacrolimus: (1) UC: When targeting a trough level of 10-15 ng/mL, tacrolimus induces clinical response and mucosal healing rates of 50%-68% and 44%-79% respectively, in patients with UC within 2 wk based on results from two RCTs, with lower rates noted in the larger of these studies[122,123]. In contrast, clinical remission was reported in only 9%-20% after 2 wk in the aforementioned studies, but amongst those who continued tacrolimus, remission rates increased to 29% by week 12, with mucosal healing rates increasing from 67% to 86% over the same time period[122,123]. (2) CD: Small case series suggest a similarly rapid clinical response with tacrolimus in CD refractory to other therapies, occurring within 30-40 d[124,125]. However, time to therapeutic response appears longer than UC, with one study finding that 36% achieved clinical remission by 20 d which increased to 64% at 120 d[125].
Cyclosporin: UC: Clinical response to cyclosporine in acute severe colitis failing to respond to intravenous corticosteroids is usually rapid, with a median response time reported to be 4-5 d, with the vast majority responding within 7 d in two randomized studies[53,126]. Clinical remission rates were approximately 65% with cyclosporine monotherapy and 93% in combination with steroids after 7 d, according to the results of a randomized study by D’Haens et al[127] In the above studies, endoscopic response was described within 7 d of initiation, with a continued improvement in endoscopic activity noted between weeks 1 and 4[126,127].
Dose and drug levels: Targeting high levels (10-15 ng/mL) of tacrolimus for induction appears more effective than low levels (5-10 ng/mL) with possibly a more rapid time to therapeutic response[123]. For ongoing maintenance thereafter, targeting trough levels of 5-10 ng/mL appears sufficient[122-125].
For cyclosporin, no additional benefit in clinical response was achieved with 4 mg/kg compared to 2 mg/kg intravenously in an RCT for acute severe colitis with a median time to response of 4 d in both groups, suggesting that doses above 2 mg/kg do not produce a more rapid time to response[126].
Route of administration: One observational study suggested that oral and intravenous tacrolimus achieved similar rates of clinical response by 14 d in steroid-refractory colitis, with comparable serum tacrolimus levels achieved with both strategies[128]. A retrospective study of oral and intravenous cyclosporin in ulcerative colitis actually found a higher early clinical response rate (exact timing not specified) with oral compared to intravenous cyclosporin (100% vs 65%) despite comparable serum drug levels, predominantly due to higher rates of side effects necessitating treatment cessation with intravenous cyclosporin[129]. Time to response was not directly assessed in this study and groups significantly differed with higher proportions of inpatients and intravenous corticosteroid failures in the intravenous cyclosporin group.
Exclusive enteral nutrition (EEN) involves the administration of a liquid nutrition formula to meet all nutritional requirements, replacing normal diet, either orally or via nasoenteric tube. It is mostly used for induction of remission in CD typically over a duration of 6-8 wk. While most data emanates from pediatric studies, efficacy is also likely in adults[130].
Time to response with EEN seems rapid, with 75% of adult patients with active CD achieving clinical remission after 10 d of an elemental feeding in one small RCT[131]. Other small RCTs have demonstrated clinical remission rates of 25% to 80% within 3-4 wk of commencement[132-138]. In two non-randomized cohort studies utilizing objective disease activity endpoints, 44% of patients achieved mucosal healing after 4 wk of EEN and, in a pediatric cohort, 36% had mucosal healing after 8 wk of EEN[139,140].
There are several patient and disease-related factors that appear important when predicting the likelihood of response and time to therapeutic response in individual patients (see Table 3). Agents tend to achieve a more rapid time to therapeutic response in UC than CD. This may relate to the transmural nature of CD thus treatment takes longer to achieve resolution of inflammatory changes. This difference in time to therapeutic response is exemplified by the vedolizumab registration studies, where induction therapy appeared to have higher response rates in UC than CD and the benefits in CD were predominantly observed later during the maintenance phase[73,78].
| Variable | Parameter | Effect on time to response | Effect on response rate | Medications implicated | Level of evidence1 | Ref. |
| Age | > 65 yr | ↑ | ? | Anti-tumour necrosis factor-α (anti-TNF) | 2b | Lobatón et al[143] |
| Increased body mass index | BMI > 25 | - | ↓ | Azathioprine | 2b | Holtmann et al[153] |
| Weight > 82 kg | - | ↓ | Anti-TNF | 1b | Reinisch et al[59] | |
| Concomitant therapies | ↓ | ↑ | Immunomodulators with anti-TNF | 1b | Colombel et al[50] | |
| Sandborn et al[69] | ||||||
| Smoking status | Current smoker | ↑ | ↓ | Anti-TNF | 1b, 2b | Arnott et al[154] |
| Sandborn et al[69] | ||||||
| Disease duration | > 2 yr | - | ↓ | Anti-TNF | 1b | Colombel et al[155] |
| Schreiber et al[156] | ||||||
| D'Haens et al[157] |
Patients who have not achieved an adequate response to prior therapies may have a slower response to subsequent therapies. This was noted with vedolizumab induction, where previous anti-TNF failures achieved clinical remission rates that only became significantly different to placebo after 10 wk, in contrast to the overall cohort where clinical remission rates were higher at week 6 compared to placebo[74]. Similar findings of a longer time to clinical remission were found in patients who had received infliximab previously and were subsequently treated with induction certolizumab for CD[69].
While several additional factors intuitively could affect time to therapeutic response such as nutritional status, age and the intestinal microbiome, published data are lacking. Advancing age is typically associated with a reduced glomerular filtration rate, greater oxidative stress, increased volume of distribution, co-morbid conditions, decreased hepatic metabolism and frailty-any of which may affect treatment choice and a therapy’s time to clinical response[141,142]. For instance, one retrospective study found that rates of clinical response to anti-TNF therapy for IBD in patients > 65 years was significantly less at week 10 but not significantly different at 6 mo than matched controls with similar co-morbidities aged < 65 years, suggesting a slower onset of response overall[143]. The limited available evidence also suggests that obesity can affect the rate of response, although the effect on time to response has not been studied. For instance, higher baseline weight was associated with a lower rate of clinical remission following induction therapy with adalimumab in the ULTRA-1 study and has been associated with an earlier loss of response to infliximab and adalimumab in IBD[59,144,145].
Although many of these factors are not modifiable, intervening early in the disease course and/or simple complementary measures such as improving nutrition may allow for a more rapid time to therapeutic response.
This review has elucidated multiple important principles of time to therapeutic response with direct applicability to clinical practice. First, patience is critical (for patients and clinicians alike) to reap the maximal benefits of some agents, particularly thiopurines, vedolizumab and methotrexate, with cumulative gains in response to these agents up to 12 mo after commencement. The clinical benefits indeed might lag significantly beyond the drug reaching therapeutic levels. For instance, this was reflected in a study assessing whether initial intravenous loading of azathioprine might hasten time to response. Indeed, therapeutic levels of 6-TGN were achieved rapidly. However, this did not improve the time to therapeutic response compared to standard oral administration[80]. Thus assessment of response should be delayed until sufficient time has lapsed to reach clinical efficacy. Whilst symptomatic improvement may occur relatively early following the initiation of many therapies, there often appears to be a lag in achieving mucosal healing, which should be considered when interpreting an early endoscopic assessment. This concept was demonstrated in a randomized controlled trial by Sutherland et al[146] comparing 4 g aminosalicylate enemas to placebo enemas for the management of distal UC. Clinical response rates and endoscopy were measured at 3 and 6 wk, with response rates reaching 60% by week 3 in the aminosalicylate arm, then plateauing to reach 68% by week 6. The mucosal healing rates over the same period increased from 25% to 42%, suggesting a later rise[24]. Additionally, patients kept a symptom diary for the first two weeks of this trial and rectal bleeding had ceased in over half of patients by day 6, so the symptom improvement may have occurred more rapidly than the time of outcome measurement. Hence, it may be appropriate to delay assessment until after the expected time to therapeutic response has passed.
Secondly, it follows that a prolonged period of bridging therapy such as co-administration of EEN, corticosteroids or even perhaps tacrolimus is an important component of treatment planning, particularly in patients who are acutely unwell, so that relapse is avoided if possible, prior to the expected maximal response of a newly introduced therapy. Thirdly, in agents where a delayed time to therapeutic response is more likely, one must consider whether potential methods of hastening onset of action can be employed, such as thiopurine and allopurinol in combination or perhaps using EEN in combination with an anti-TNF agent in CD as induction therapy.
Significantly, the lack of reporting of time-to-response in clinical trials is a deficiency. Mostly, data must be extrapolated from studies rather than being directly reported. Given its utility in clinical practice and the relatively basic calculations required, this should be addressed in future studies. The expected time-to-response can potentially assist in trial design by providing an estimate of the necessary duration of a study to show maximal efficacy. For example, for vedolizumab for the management of CD, the CDAI-100 response rate was not significant at week 6 and only surpassed placebo around week 28[73]. Similarly, modest early response rates were also noted with another anti-integrin therapy natalizumab at week 10 with response rates increasing during maintenance therapy[147]. The use of week 6 rather than week 10 or later as the response times for vedolizumab has been a criticism of the large randomized studies[77]. Hence, such information can potentially be reported in early studies and thus provide further insight into the rapidity of action of drugs or more widely, drug classes, which can be incorporated into future studies of that therapy, plus into clinical practice.
Finally, the concept of time-to-response is a broad term and it is likely that it can be further divided based on the proportion of patients who have respond to therapy, as shown in Table 1. There is a time point at which most patients will respond and then following this there is progressively diminishing returns in the likelihood of further response, culminating in a point where response is unlikely; the time to futility. This concept remains clinically important, as the yield from persisting with therapy beyond this point is low and thus place patients at higher risk, for minimal or no benefit. Again, such information be ideally included in published data to better aid clinicians with therapeutic decisions.
In a chronic disease like IBD, where many therapies are effective yet of relatively slow onset of action, time-to-therapeutic response is of central importance both quantitatively to frame the patient’s expectations and the clinician’s decision making but also conceptually-encouraging the mindset of forward treatment planning, overlapping bridging therapies and accuracy in determining therapeutic failure. Furthermore, in contrast to the homogenized, group-based reporting of results from the seminal RCTs as the reference standard for each new agent, time to therapeutic response is inherently patient-centric and individualized. This is not only because it has the potential to vary by indication, dosage, demographic, clinical factors and is dependent on which endpoint is chosen (i.e., clinical response, remission, or endoscopic remission) but one can argue that the time to therapeutic response is of far greater relevance to patients and perhaps even to clinicians in day-to-day practice, thus future studies should examine and incorporate this paradigm further.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gazouli M, Jadallah KA, Mitsui K S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Markowitz J. Early inflammatory bowel disease: different treatment response to specific or all medications? Dig Dis. 2009;27:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, Rene E. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Groupe d’Etude Thérapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990;98:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 476] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 3. | Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF, Florent C, Bouvry M, Mary JY, Modigliani R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 326] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Peyrin-Biroulet L, Reinisch W, Colombel JF, Mantzaris GJ, Kornbluth A, Diamond R, Rutgeerts P, Tang LK, Cornillie FJ, Sandborn WJ. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 5. | Jharap B, Sandborn WJ, Reinisch W, D’Haens G, Robinson AM, Wang W, Huang B, Lazar A, Thakkar RB, Colombel JF. Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther. 2015;42:1082-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Kiss LS, Szamosi T, Molnar T, Miheller P, Lakatos L, Vincze A, Palatka K, Barta Z, Gasztonyi B, Salamon A. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn’s disease. Aliment Pharmacol Ther. 2011;34:911-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Turner D, Griffiths AM, Veerman G, Johanns J, Damaraju L, Blank M, Hyams J. Endoscopic and clinical variables that predict sustained remission in children with ulcerative colitis treated with infliximab. Clin Gastroenterol Hepatol. 2013;11:1460-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Peyrin-Biroulet L, Panés J, Sandborn WJ, Vermeire S, Danese S, Feagan BG, Colombel JF, Hanauer SB, Rycroft B. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clin Gastroenterol Hepatol. 2016;14:348-354.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Parker CE, Bhanji T, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;4:CD000543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Ford AC, Kane SV, Khan KJ, Achkar JP, Talley NJ, Marshall JK, Moayyedi P. Efficacy of 5-aminosalicylates in Crohn’s disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Lim WC, Wang Y, MacDonald JK, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev. 2016;7:CD008870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Miglioli MB, Brunetti G. Delayed release mesalazine in the treatment of mild ulcerative colitis: a dose ranging study. Eur J Gastroenterol Hepatol. 1990;2:229-234. |
| 13. | Kamm MA, Sandborn WJ, Gassull M, Schreiber S, Jackowski L, Butler T, Lyne A, Stephenson D, Palmen M, Joseph RE. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132:66-75; quiz 432-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 14. | Baron JH, Connell AM, Lennard-jones JE, Jones FA. Sulphasalazine and salicylazosulphadimidine in ulcerative colitis. Lancet. 1962;1:1094-1096. [PubMed] |
| 15. | Lennard-Jones JE, Longmore AJ, Newell AC, Wilson CW, Jones FA. An assessment of prednisone, salazopyrin, and topical hydrocortisone hemisuccinate used as out-patient treatment for ulcerative colitis. Gut. 1960;1:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 149] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Selby WS, Barr GD, Ireland A, Mason CH, Jewell DP. Olsalazine in active ulcerative colitis. Br Med J (Clin Res Ed). 1985;291:1373-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Green JR, Lobo AJ, Holdsworth CD, Leicester RJ, Gibson JA, Kerr GD, Hodgson HJ, Parkins KJ, Taylor MD. Balsalazide is more effective and better tolerated than mesalamine in the treatment of acute ulcerative colitis. The Abacus Investigator Group. Gastroenterology. 1998;114:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Oliva-Hemker M, Hutfless S, Al Kazzi ES, Lerer T, Mack D, LeLeiko N, Griffiths A, Cabrera J, Otley A, Rick J. Clinical Presentation and Five-Year Therapeutic Management of Very Early-Onset Inflammatory Bowel Disease in a Large North American Cohort. J Pediatr. 2015;167:527-532.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed-release 5-aminosalicylic acid (mesalazine) and sulfasalazine as maintenance treatment for patients with ulcerative colitis. Gastroenterology. 1988;94:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 126] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Levine DS, Riff DS, Pruitt R, Wruble L, Koval G, Sales D, Bell JK, Johnson LK. A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:1398-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Orchard TR, van der Geest SA, Travis SP. Randomised clinical trial: early assessment after 2 weeks of high-dose mesalazine for moderately active ulcerative colitis-new light on a familiar question. Aliment Pharmacol Ther. 2011;33:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Kruis W, Bar-Meir S, Feher J, Mickisch O, Mlitz H, Faszczyk M, Chowers Y, Lengyele G, Kovacs A, Lakatos L. The optimal dose of 5-aminosalicylic acid in active ulcerative colitis: a dose-finding study with newly developed mesalamine. Clin Gastroenterol Hepatol. 2003;1:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Safdi M, DeMicco M, Sninsky C, Banks P, Wruble L, Deren J, Koval G, Nichols T, Targan S, Fleishman C. A double-blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. Am J Gastroenterol. 1997;92:1867-1871. [PubMed] |
| 24. | Sandborn WJ, Hanauer S, Lichtenstein GR, Safdi M, Edeline M, Scott Harris M. Early symptomatic response and mucosal healing with mesalazine rectal suspension therapy in active distal ulcerative colitis--additional results from two controlled studies. Aliment Pharmacol Ther. 2011;34:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Hanauer SB. Dose-ranging study of mesalamine (PENTASA) enemas in the treatment of acute ulcerative proctosigmoiditis: results of a multicentered placebo-controlled trial. The U.S. PENTASA Enema Study Group. Inflamm Bowel Dis. 1998;4:79-83. [PubMed] |
| 26. | Lichtenstein GR, Abreu MT, Cohen R, Tremaine W; American Gastroenterological Association. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 344] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 27. | Shepherd HA, Barr GD, Jewell DP. Use of an intravenous steroid regimen in the treatment of acute Crohn’s disease. J Clin Gastroenterol. 1986;8:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Truelove SC, Watkinson G, Draper G. Comparison of corticosteroid and sulphasalazine therapy in ulcerative colitis. Br Med J. 1962;2:1708-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Hawthorne AB, Record CO, Holdsworth CD, Giaffer MH, Burke DA, Keech ML, Hawkey CJ. Double blind trial of oral fluticasone propionate v prednisolone in the treatment of active ulcerative colitis. Gut. 1993;34:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Baron JH, Connell AM, Kanaghinis TG, Lennard-Jones JE, Jones AF. Out-patient treatment of ulcerative colitis. Comparison between three doses of oral prednisone. Br Med J. 1962;2:441-443. [PubMed] |
| 31. | Chun A, Chadi RM, Korelitz BI, Colonna T, Felder JB, Jackson MH, Morgenstern EH, Rubin SD, Sacknoff AG, Gleim GM. Intravenous corticotrophin vs. hydrocortisone in the treatment of hospitalized patients with Crohn’s disease: a randomized double-blind study and follow-up. Inflamm Bowel Dis. 1998;4:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Truelove SC, Jewell DP. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet. 1974;1:1067-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 338] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Truelove SC, Willoughby CP, Lee EG, Kettlewell MG. Further experience in the treatment of severe attacks of ulcerative colitis. Lancet. 1978;2:1086-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 142] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Järnerot G, Rolny P, Sandberg-Gertzén H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology. 1985;89:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 226] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Travis SP, Farrant JM, Ricketts C, Nolan DJ, Mortensen NM, Kettlewell MG, Jewell DP. Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 518] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 36. | Molnár T, Farkas K, Nyári T, Szepes Z, Nagy F, Wittmann T. Response to first intravenous steroid therapy determines the subsequent risk of colectomy in ulcerative colitis patients. J Gastrointestin Liver Dis. 2011;20:359-363. [PubMed] |
| 37. | Tremaine WJ, Hanauer SB, Katz S, Winston BD, Levine JG, Persson T, Persson A; Budesonide CIR United States Study Group. Budesonide CIR capsules (once or twice daily divided-dose) in active Crohn’s disease: a randomized placebo-controlled study in the United States. Am J Gastroenterol. 2002;97:1748-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Gross V, Andus T, Caesar I, Bischoff SC, Lochs H, Tromm A, Schulz HJ, Bär U, Weber A, Gierend M. Oral pH-modified release budesonide versus 6-methylprednisolone in active Crohn’s disease. German/Austrian Budesonide Study Group. Eur J Gastroenterol Hepatol. 1996;8:905-909. [PubMed] |
| 39. | Tursi A, Giorgetti GM, Brandimarte G, Elisei W, Aiello F. Beclomethasone dipropionate for the treatment of mild-to-moderate Crohn’s disease: an open-label, budesonide-controlled, randomized study. Med Sci Monit. 2006;12:PI29-PI32. [PubMed] |
| 40. | Thomsen OO, Cortot A, Jewell D, Wright JP, Winter T, Veloso FT, Vatn M, Persson T, Pettersson E. A comparison of budesonide and mesalamine for active Crohn’s disease. International Budesonide-Mesalamine Study Group. N Engl J Med. 1998;339:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Sandborn WJ, Travis S, Moro L, Jones R, Gautille T, Bagin R, Huang M, Yeung P, Ballard ED 2nd. Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology. 2012;143:1218-1226.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 42. | D'Haens GR, Kovács A, Vergauwe P, Nagy F, Molnár T, Bouhnik Y, Weiss W, Brunner H, Lavergne-Slove A, Binelli D. Clinical trial: Preliminary efficacy and safety study of a new Budesonide-MMX® 9 mg extended-release tablets in patients with active left-sided ulcerative colitis. J Crohns Colitis. 2010;4:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Carter MJ, Lobo AJ, Travis SP; IBD Section, British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-V16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 772] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 44. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 701] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 45. | Cohen RD, Tsang JF, Hanauer SB. Infliximab in Crohn’s disease: first anniversary clinical experience. Am J Gastroenterol. 2000;95:3469-3477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2268] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 47. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3052] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 48. | Nikolaus S, Raedler A, Kühbacker T, Sfikas N, Fölsch UR, Schreiber S. Mechanisms in failure of infliximab for Crohn’s disease. Lancet. 2000;356:1475-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Tursi A, Elisei W, Giorgetti GM, Penna A, Picchio M, Brandimarte G. factors influencing mucosal healing in Crohn’s disease during infliximab treatment. Hepatogastroenterology. 2013;60:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2372] [Article Influence: 158.1] [Reference Citation Analysis (1)] |
| 51. | Sands BE, Tremaine WJ, Sandborn WJ, Rutgeerts PJ, Hanauer SB, Mayer L, Targan SR, Podolsky DK. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 292] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 52. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2880] [Article Influence: 144.0] [Reference Citation Analysis (2)] |
| 53. | Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, Zerbib F, Savoye G, Nachury M, Moreau J. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 438] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 54. | Papamichael K, Van Stappen T, Vande Casteele N, Gils A, Billiet T, Tops S, Claes K, Van Assche G, Rutgeerts P, Vermeire S. Infliximab Concentration Thresholds During Induction Therapy Are Associated With Short-term Mucosal Healing in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2016;14:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 55. | Gibson DJ, Heetun ZS, Redmond CE, Nanda KS, Keegan D, Byrne K, Mulcahy HE, Cullen G, Doherty GA. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:330-335.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 56. | Austin Health. Optimising Infliximab Induction Therapy for Acute Severe Ulcerative Colitis (PREDICT-UC). Bethesda (MD): National Library of Medicine (US). Cited 2016-12-20. Available from: https://clinicaltrials.gov/show/NCT02770040. |
| 57. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1193] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 58. | Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W; EXTEND Investigators, Kumar A, Lazar A, Camez A, Lomax KG, Pollack PF, D’Haens G. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102-1111.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 59. | Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 60. | Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257-265.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 941] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 61. | Sandborn WJ, Schreiber S, Feagan BG, Rutgeerts P, Younes ZH, Bloomfield R, Coteur G, Guzman JP, D’Haens GR. Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol. 2011;9:670-678.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 62. | Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, Bernstein CN, Staun M, Thomsen OØ, Innes A; CDP870 Crohn’s Disease Study Group. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 63. | Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 807] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 64. | Hébuterne X, Lémann M, Bouhnik Y, Dewit O, Dupas JL, Mross M, D’Haens G, Mitchev K, Ernault É, Vermeire S, Brixi-Benmansour H, Moreels TG, Mary JY, Marteau P, Colombel JF. Endoscopic improvement of mucosal lesions in patients with moderate to severe ileocolonic Crohn’s disease following treatment with certolizumab pegol. Gut. 2013;62:201-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 65. | Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85-95; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 681] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 66. | Bosca-Watts MM, Cortes X, Iborra M, Huguet JM, Sempere L, Garcia G, Gil R, Garcia M, Muñoz M, Almela P. Short-term effectiveness of golimumab for ulcerative colitis: Observational multicenter study. World J Gastroenterol. 2016;22:10432-10439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Castro-Laria L, Argüelles-Arias F, García-Sánchez V, Benítez JM, Fernández-Pérez R, Trapero-Fernández AM, Gallardo-Sánchez F, Pallarés-Manrique H, Gómez-García M, Cabello-Tapia MJ. Initial experience with golimumab in clinical practice for ulcerative colitis. Rev Esp Enferm Dig. 2016;108:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Rutgeerts P, Feagan BG, Marano CW, Padgett L, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Zhang H, Colombel JF. Randomised clinical trial: a placebo-controlled study of intravenous golimumab induction therapy for ulcerative colitis. Aliment Pharmacol Ther. 2015;42:504-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Sandborn WJ, Melmed GY, McGovern DP, Loftus EV Jr, Choi JM, Cho JH, Abraham B, Gutierrez A, Lichtenstein G, Lee SD, Randall CW, Schwartz DA, Regueiro M, Siegel CA, Spearman M, Kosutic G, Pierre-Louis B, Coarse J, Schreiber S. Clinical and demographic characteristics predictive of treatment outcomes for certolizumab pegol in moderate to severe Crohn’s disease: analyses from the 7-year PRECiSE 3 study. Aliment Pharmacol Ther. 2015;42:330-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Adedokun OJ, Xu Z, Marano CW, Strauss R, Zhang H, Johanns J, Zhou H, Davis HM, Reinisch W, Feagan BG. Pharmacokinetics and Exposure-response Relationship of Golimumab in Patients with Moderately-to-Severely Active Ulcerative Colitis: Results from Phase 2/3 PURSUIT Induction and Maintenance Studies. J Crohns Colitis. 2017;11:35-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 71. | Colombel JF, Sandborn WJ, Allez M, Dupas JL, Dewit O, D’Haens G, Bouhnik Y, Parker G, Pierre-Louis B, Hébuterne X. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:423-431.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 72. | Matsumoto T, Motoya S, Watanabe K, Hisamatsu T, Nakase H, Yoshimura N, Ishida T, Kato S, Nakagawa T, Esaki M. 854 Comparison of Adalimumab Monotherapy and a Combination With Azathioprine for Patients With Crohn’s Disease: A Prospective, Multicenter, Open-Labeled Clinical Trial (DIAMOND Study). Gastroenterology. 2016;150:S182. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 73. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1564] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 74. | Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, D’Haens G, Ben-Horin S, Xu J, Rosario M. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 535] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 75. | Dulai PS, Singh S, Jiang X, Peerani F, Narula N, Chaudrey K, Whitehead D, Hudesman D, Lukin D, Swaminath A. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am J Gastroenterol. 2016;111:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 76. | Vivio EE, Kanuri N, Gilbertsen JJ, Monroe K, Dey N, Chen CH, Gutierrez AM, Ciorba MA. Vedolizumab Effectiveness and Safety Over the First Year of Use in an IBD Clinical Practice. J Crohns Colitis. 2016;10:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 77. | Bryant RV, Sandborn WJ, Travis SP. Introducing vedolizumab to clinical practice: who, when, and how? J Crohns Colitis. 2015;9:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1862] [Article Influence: 155.2] [Reference Citation Analysis (1)] |
| 79. | Moon W, Loftus EV Jr. Review article: recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2016;43:863-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 80. | Sandborn WJ, Tremaine WJ, Wolf DC, Targan SR, Sninsky CA, Sutherland LR, Hanauer SB, McDonald JW, Feagan BG, Fedorak RN. Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn’s disease. North American Azathioprine Study Group. Gastroenterology. 1999;117:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 162] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Willoughby JM, Beckett J, Kumar PJ, Dawson AM. Controlled trial of azathioprine in Crohn’s disease. Lancet. 1971;2:944-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 202] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 706] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 83. | Ardizzone S, Bollani S, Manzionna G, Imbesi V, Colombo E, Bianchi Porro G. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn’s disease: a randomised, investigator-blind study. Dig Liver Dis. 2003;35:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Mantzaris GJ, Christidou A, Sfakianakis M, Roussos A, Koilakou S, Petraki K, Polyzou P. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis. 2009;15:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 85. | Kirk AP, Lennard-Jones JE. Controlled trial of azathioprine in chronic ulcerative colitis. Br Med J (Clin Res Ed). 1982;284:1291-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 86. | Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. Br Med J. 1974;4:627-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 228] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 87. | Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 362] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 88. | Rosenberg JL, Wall AJ, Levin B, Binder HJ, Kirsner JB. A controlled trial of azathioprine in the management of chronic ulcerative colitis. Gastroenterology. 1975;69:96-99. [PubMed] |
| 89. | Teml A, Schaeffeler E, Herrlinger KR, Klotz U, Schwab M. Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing. Clin Pharmacokinet. 2007;46:187-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Sandborn WJ. Rational dosing of azathioprine and 6-mercaptopurine. Gut. 2001;48:591-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 91. | Sandborn WJ, Van O EC, Zins BJ, Tremaine WJ, Mays DC, Lipsky JJ. An intravenous loading dose of azathioprine decreases the time to response in patients with Crohn’s disease. Gastroenterology. 1995;109:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 96] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 92. | Dubinsky MC, Reyes E, Ofman J, Chiou CF, Wade S, Sandborn WJ. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol. 2005;100:2239-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Reinshagen M, Schütz E, Armstrong VW, Behrens C, von Tirpitz C, Stallmach A, Herfarth H, Stein J, Bias P, Adler G. 6-thioguanine nucleotide-adapted azathioprine therapy does not lead to higher remission rates than standard therapy in chronic active crohn disease: results from a randomized, controlled, open trial. Clin Chem. 2007;53:1306-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 94. | Dassopoulos T, Dubinsky MC, Bentsen JL, Martin CF, Galanko JA, Seidman EG, Sandler RS, Hanauer SB. Randomised clinical trial: individualised vs. weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014;39:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 95. | Moreau AC, Paul S, Del Tedesco E, Rinaudo-Gaujous M, Boukhadra N, Genin C, Peyrin-Biroulet L, Roblin X. Association between 6-thioguanine nucleotides levels and clinical remission in inflammatory disease: a meta-analysis. Inflamm Bowel Dis. 2014;20:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 96. | Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 722] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 97. | Cuffari C, Hunt S, Bayless T. Utilisation of erythrocyte 6-thioguanine metabolite levels to optimise azathioprine therapy in patients with inflammatory bowel disease. Gut. 2001;48:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 98. | Haines ML, Ajlouni Y, Irving PM, Sparrow MP, Rose R, Gearry RB, Gibson PR. Clinical usefulness of therapeutic drug monitoring of thiopurines in patients with inadequately controlled inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1301-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 99. | Kennedy NA, Asser TL, Mountifield RE, Doogue MP, Andrews JM, Bampton PA. Thiopurine metabolite measurement leads to changes in management of inflammatory bowel disease. Intern Med J. 2013;43:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Smith M, Blaker P, Patel C, Marinaki A, Arenas M, Escuredo E, Anderson S, Irving P, Sanderson J. The impact of introducing thioguanine nucleotide monitoring into an inflammatory bowel disease clinic. Int J Clin Pract. 2013;67:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Sparrow MP, Hande SA, Friedman S, Lim WC, Reddy SI, Cao D, Hanauer SB. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther. 2005;22:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 102. | Rahhal RM, Bishop WP. Initial clinical experience with allopurinol-thiopurine combination therapy in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1678-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 103. | Leung Y, Sparrow MP, Schwartz M, Hanauer SB. Long term efficacy and safety of allopurinol and azathioprine or 6-mercaptopurine in patients with inflammatory bowel disease. J Crohns Colitis. 2009;3:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 104. | Ansari A, Patel N, Sanderson J, O’Donohue J, Duley JA, Florin TH. Low-dose azathioprine or mercaptopurine in combination with allopurinol can bypass many adverse drug reactions in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2010;31:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 105. | Pavlidis P, Stamoulos P, Abdulrehman A, Kerr P, Bull C, Duley J, Ansari A. Long-term Safety and Efficacy of Low-dose Azathioprine and Allopurinol Cotherapy in Inflammatory Bowel Disease: A Large Observational Study. Inflamm Bowel Dis. 2016;22:1639-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 106. | Lee KM, Kim YS, Seo GS, Kim TO, Yang SK; IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of Thiopurines in Inflammatory Bowel Disease: A Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intest Res. 2015;13:193-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 107. | Yip JS, Woodward M, Abreu MT, Sparrow MP. How are Azathioprine and 6-mercaptopurine dosed by gastroenterologists? Results of a survey of clinical practice. Inflamm Bowel Dis. 2008;14:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Kiszka-Kanowitz M, Theede K, Mertz-Nielsen A. Randomized clinical trial: a pilot study comparing efficacy of low-dose azathioprine and allopurinol to azathioprine on clinical outcomes in inflammatory bowel disease. Scand J Gastroenterol. 2016;51:1470-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Carbonnel F, Colombel JF, Filippi J, Katsanos KH, Peyrin-Biroulet L, Allez M, Nachury M, Novacek G, Danese S, Abitbol V. Methotrexate Is Not Superior to Placebo for Inducing Steroid-Free Remission, but Induces Steroid-Free Clinical Remission in a Larger Proportion of Patients With Ulcerative Colitis. Gastroenterology. 2016;150:380-388.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 110. | Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 351] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 111. | Chong RY, Hanauer SB, Cohen RD. Efficacy of parenteral methotrexate in refractory Crohn’s disease. Aliment Pharmacol Ther. 2001;15:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 112. | Oren R, Arber N, Odes S, Moshkowitz M, Keter D, Pomeranz I, Ron Y, Reisfeld I, Broide E, Lavy A. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology. 1996;110:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 188] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 113. | Islam MS, Haq SA, Islam MN, Azad AK, Islam MA, Barua R, Hasan MM, Mahmood M, Safiuddin M, Rahman MM. Comparative efficacy of subcutaneous versus oral methotrexate in active rheumatoid arthritis. Mymensingh Med J. 2013;22:483-488. [PubMed] |
| 114. | Egan LJ, Sandborn WJ, Tremaine WJ, Leighton JA, Mays DC, Pike MG, Zinsmeister AR, Lipsky JJ. A randomized dose-response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 115. | Egan LJ, Sandborn WJ, Mays DC, Tremaine WJ, Lipsky JJ. Plasma and rectal adenosine in inflammatory bowel disease: effect of methotrexate. Inflamm Bowel Dis. 1999;5:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 116. | Dervieux T, Furst D, Lein DO, Capps R, Smith K, Walsh M, Kremer J. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum. 2004;50:2766-2774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 263] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 117. | Fischer M, Siva S, Cook GK, Jones DR, Fadda HM. Methotrexate Polyglutamate Monitoring in Patients With Crohn’s Disease. Clin Pharmacol Drug Dev. 2017;6:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 118. | Brooks AJ, Begg EJ, Zhang M, Frampton CM, Barclay ML. Red blood cell methotrexate polyglutamate concentrations in inflammatory bowel disease. Ther Drug Monit. 2007;29:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Shea B, Swinden MV, Ghogomu ET, Ortiz Z, Katchamart W, Rader T, Bombardier C, Wells GA, Tugwell P. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. J Rheumatol. 2014;41:1049-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 120. | McSharry K, Dalzell AM, Leiper K, El-Matary W. Systematic review: the role of tacrolimus in the management of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:1282-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | McDonald JW, Feagan BG, Jewell D, Brynskov J, Stange EF, Macdonald JK. Cyclosporine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2005;CD000297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 122. | Ogata H, Kato J, Hirai F, Hida N, Matsui T, Matsumoto T, Koyanagi K, Hibi T. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2012;18:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 123. | Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, Suzuki Y, Hibi T. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006;55:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 328] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 124. | Baumgart DC, Pintoffl JP, Sturm A, Wiedenmann B, Dignass AU. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease--a long-term follow-up. Am J Gastroenterol. 2006;101:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 125. | Tamaki H, Nakase H, Matsuura M, Inoue S, Mikami S, Ueno S, Uza N, Kitamura H, Kasahara K, Chiba T. The effect of tacrolimus (FK-506) on Japanese patients with refractory Crohn’s disease. J Gastroenterol. 2008;43:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 126. | Van Assche G, D’Haens G, Noman M, Vermeire S, Hiele M, Asnong K, Arts J, D’Hoore A, Penninckx F, Rutgeerts P. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 272] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 127. | D'Haens G, Lemmens L, Geboes K, Vandeputte L, Van Acker F, Mortelmans L, Peeters M, Vermeire S, Penninckx F, Nevens F. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology. 2001;120:1323-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 269] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 128. | Fellermann K, Tanko Z, Herrlinger KR, Witthoeft T, Homann N, Bruening A, Ludwig D, Stange EF. Response of refractory colitis to intravenous or oral tacrolimus (FK506). Inflamm Bowel Dis. 2002;8:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 129. | Actis GC, Aimo G, Priolo G, Moscato D, Rizzetto M, Pagni R. Efficacy and efficiency of oral microemulsion cyclosporin versus intravenous and soft gelatin capsule cyclosporin in the treatment of severe steroid-refractory ulcerative colitis: an open-label retrospective trial. Inflamm Bowel Dis. 1998;4:276-279. [PubMed] |
| 130. | Wall CL, Day AS, Gearry RB. Use of exclusive enteral nutrition in adults with Crohn’s disease: a review. World J Gastroenterol. 2013;19:7652-7660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 131. | Giaffer MH, North G, Holdsworth CD. Controlled trial of polymeric versus elemental diet in treatment of active Crohn’s disease. Lancet. 1990;335:816-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 132. | Gassull MA, Fernández-Bañares F, Cabré E, Papo M, Giaffer MH, Sánchez-Lombraña JL, Richart C, Malchow H, González-Huix F, Esteve M; Eurpoean Group on Enteral Nutrition in Crohn’s Disease. Fat composition may be a clue to explain the primary therapeutic effect of enteral nutrition in Crohn’s disease: results of a double blind randomised multicentre European trial. Gut. 2002;51:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 133. | Bamba T, Shimoyama T, Sasaki M, Tsujikawa T, Fukuda Y, Koganei K, Hibi T, Iwao Y, Munakata A, Fukuda S. Dietary fat attenuates the benefits of an elemental diet in active Crohn’s disease: a randomized, controlled trial. Eur J Gastroenterol Hepatol. 2003;15:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 134. | Mansfield JC, Giaffer MH, Holdsworth CD. Controlled trial of oligopeptide versus amino acid diet in treatment of active Crohn’s disease. Gut. 1995;36:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 135. | Verma S, Brown S, Kirkwood B, Giaffer MH. Polymeric versus elemental diet as primary treatment in active Crohn’s disease: a randomized, double-blind trial. Am J Gastroenterol. 2000;95:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 136. | Rigaud D, Cosnes J, Le Quintrec Y, René E, Gendre JP, Mignon M. Controlled trial comparing two types of enteral nutrition in treatment of active Crohn’s disease: elemental versus polymeric diet. Gut. 1991;32:1492-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 129] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 137. | Middleton SJ, Rucker JT, Kirby GA, Riordan AM, Hunter JO. Long-chain triglycerides reduce the efficacy of enteral feeds in patients with active Crohn’s disease. Clin Nutr. 1995;14:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 138. | González-Huix F, de León R, Fernández-Bañares F, Esteve M, Cabré E, Acero D, Abad-Lacruz A, Figa M, Guilera M, Planas R. Polymeric enteral diets as primary treatment of active Crohn’s disease: a prospective steroid controlled trial. Gut. 1993;34:778-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 137] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 139. | Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: cytokine production and endoscopic and histological findings. Inflamm Bowel Dis. 2005;11:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 140. | Fell JM, Paintin M, Arnaud-Battandier F, Beattie RM, Hollis A, Kitching P, Donnet-Hughes A, MacDonald TT, Walker-Smith JA. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2000;14:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 282] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 141. | Juneja M, Schwartz M, El Mourabet M, Regueiro M, Harrison J, Barrie AM, Swoger JM, Baidoo L, Saul MI, Binion DG. W1276 W1276 Patterns of Medical Treatment and Polypharmacy in Geriatric Crohn’s Disease. Gastroenterology. 2010;138:S-689. [DOI] [Full Text] |
| 142. | Katz S, Pardi DS. Inflammatory bowel disease of the elderly: frequently asked questions (FAQs). Am J Gastroenterol. 2011;106:1889-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 143. | Lobatón T, Ferrante M, Rutgeerts P, Ballet V, Van Assche G, Vermeire S. Efficacy and safety of anti-TNF therapy in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 144. | Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2118-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 145. | Bultman E, de Haar C, van Liere-Baron A, Verhoog H, West RL, Kuipers EJ, Zelinkova Z, van der Woude CJ. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn’s disease patients. Aliment Pharmacol Ther. 2012;35:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 146. | Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E, Borgen L. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 491] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 147. | Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, van Deventer S, Goldblum R, Despain D, Hogge GS, Rutgeerts P; International Efficacy of Natalizumab as Active Crohn’s Therapy (ENACT-1) Trial Group; Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 664] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 148. | Sninsky CA, Cort DH, Shanahan F, Powers BJ, Sessions JT, Pruitt RE, Jacobs WH, Lo SK, Targan SR, Cerda JJ. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis. A multicenter study. Ann Intern Med. 1991;115:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 148] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 149. | Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 726] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 150. | Llaó J, Naves JE, Ruiz-Cerulla A, Marín L, Mañosa M, Rodríguez-Alonso L, Cabré E, Garcia-Planella E, Guardiola J, Domènech E. Intravenous corticosteroids in moderately active ulcerative colitis refractory to oral corticosteroids. J Crohns Colitis. 2014;8:1523-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 151. | Shih DQ, Nguyen M, Zheng L, Ibanez P, Mei L, Kwan LY, Bradford K, Ting C, Targan SR, Vasiliauskas EA. Split-dose administration of thiopurine drugs: a novel and effective strategy for managing preferential 6-MMP metabolism. Aliment Pharmacol Ther. 2012;36:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 152. | Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Koval J, Wong CJ, Hopkins M. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000;342:1627-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 512] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 153. | Holtmann MH, Krummenauer F, Claas C, Kremeyer K, Lorenz D, Rainer O, Vogel I, Böcker U, Böhm S, Büning C. Significant differences between Crohn’s disease and ulcerative colitis regarding the impact of body mass index and initial disease activity on responsiveness to azathioprine: results from a European multicenter study in 1,176 patients. Dig Dis Sci. 2010;55:1066-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 154. | Arnott ID, McNeill G, Satsangi J. An analysis of factors influencing short-term and sustained response to infliximab treatment for Crohn’s disease. Aliment Pharmacol Ther. 2003;17:1451-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 155. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1618] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 156. | Schreiber S, Colombel JF, Bloomfield R, Nikolaus S, Schölmerich J, Panés J, Sandborn WJ; PRECiSE 2 Study Investigators. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol. 2010;105:1574-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 157. | D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 939] [Article Influence: 55.2] [Reference Citation Analysis (0)] |