Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6350
Peer-review started: June 18, 2017
First decision: June 26, 2017
Revised: July 8, 2017
Accepted: August 8, 2017
Article in press: August 8, 2017
Published online: September 14, 2017
Processing time: 89 Days and 18.7 Hours
To identify which technique is better for avoiding biliary reflux and gastritis between uncut Roux-en-Y and Billroth II reconstruction.
A total of 158 patients who underwent laparoscopy-assisted distal gastrectomy for gastric cancer at the First Hospital of Jilin University (Changchun, China) between February 2015 and February 2016 were randomized into two groups: uncut Roux-en-Y (group U) and Billroth II group (group B). Postoperative complications and relevant clinical data were compared between the two groups.
According to the randomization table, each group included 79 patients. There was no significant difference in postoperative complications between groups U and B (7.6% vs 10.1%, P = 0.576). During the postoperative period, group U stomach pH values were lower than 7 and group B pH values were higher than 7. After 1 year of follow-up, group B presented a higher incidence of biliary reflux and alkaline gastritis. However, histopathology did not show a significant difference in gastritis diagnosis (P = 0.278), and the amount of residual food and gain of weight between the groups were also not significantly different. At 3 mo there was no evidence of partial recanalization of uncut staple line, but at 1 year the incidence was 13%.
Compared with Billroth II reconstruction, uncut Roux-en-Y reconstruction is secure and feasible, and can effectively reduce the incidence of alkaline reflux, residual gastritis, and heartburn. Despite the incidence of recanalization, uncut Roux-en-Y should be widely applied.
Core tip: Because of the challenge of recanalization, the uncut Roux-en-Y reconstruction is still controversial and needs further study. This study is the first randomized controlled trial concentrating on uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer. This study aimed to compare uncut Roux-en-Y and Billroth II reconstruction in terms of postoperative complications, including biliary reflux and gastritis. Despite the incidence of recanalization, uncut Roux-en-Y reconstruction is secure and feasible, and can effectively reduce the incidence of alkaline reflux, residual gastritis, and heartburn.
- Citation: Yang D, He L, Tong WH, Jia ZF, Su TR, Wang Q. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: Which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol 2017; 23(34): 6350-6356
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6350.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6350
There remains no clear consensus regarding the preferred reconstructive surgical procedure after laparoscopy-assisted distal gastrectomy (LADG) for gastric cancer[1,2]. Compared with Japan[3] and Korea[4], early gastric cancer only accounts for a small percentage in China, and most gastric cancer cases are found in advanced stages at the initial diagnosis. It is inappropriate for surgeons to perform Billroth I anastomosis after subtotal gastrectomy. In 2005, Uyama first combined LADG with uncut Roux-en-Y reconstruction; however, its use remains controversial[5,6]. In our department, we usually prefer Billroth II and uncut Roux-en-Y reconstruction.
The current study aimed to compare these two reconstruction techniques in terms of postoperative complications, including biliary reflux and gastritis.
This is a randomized controlled trial which was evaluated and approved by the ethics committee at our institution, and registered in clinicaltrials.gov with the number NCT02694081. Between February 2015 and February 2016, a total of 158 patients with gastric cancer treated at the First Hospital of Jilin University (Changchun, China), who met the inclusion criteria and provided informed consent, were randomized into one of two groups: uncut Roux-en-Y group (group U) or Billroth II group (group B). Randomization was done after laparoscopic exploration with the randomization table, which was produced using SPSS v18.0 for Windows software by the Division of Clinical Research at our hospital. Patients as well as investigators (assessing outcomes and analyzing data) were masked. The inclusion criteria were: (1) distal gastric cancer diagnosed by endoscopy, CT scan, and pathology study; (2) patients who underwent LADG; and (3) age between 18 and 75 years. The exclusion criteria were: (1) late-stage gastric carcinoma or pyloric obstruction; (2) preoperative esophageal reflux symptoms, esophagitis, or hiatal hernia; and (3) systemic disease including diabetes, severe chronic lung disease, cirrhosis, or esophageal varices.
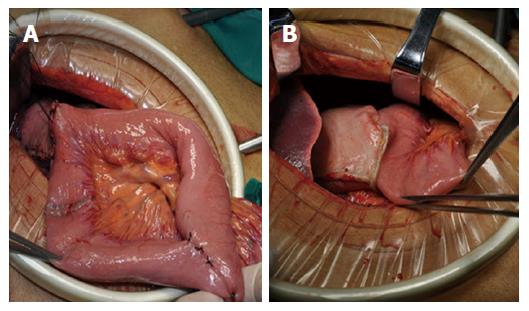
All included patients underwent LADG with D2 lymphadenectomy, which was performed by the same surgical team. For reconstruction, a 5-cm mini-laparotomy was made to complete a delta-shaped Billroth II anastomosis using a 80 mm linear stapler[7]. In the uncut Roux-en-Y group, gastrojejunostomy was performed at 25 cm distal to the Treitz ligament, and jejunum-jejunum anastomosis at 40 cm from the afferent limb. The blade of the linear stapler (Covidien GIA8038S, Medtronic, Minneapolis, MN, the United States of America) was removed to perform the uncut procedure of the afferent jejunal limb, 5 cm proximal to the gastrojejunostomy in the jejunum (Figure 1A and B).
During the postoperative period, omeprazole 40 mg was given to all patients twice a day. Ambulation was encouraged from the first day after operation, and the nasogastric tube was kept in place for 5 d. All patients received uniform diet guidance after discharge.
Demographic data, clinical outcomes, and follow-up data were collected. Change of potential of hydrogen (pH) in the remnant stomach was recorded at 8:00 am on the day before surgery as well as 1-5 d after surgery.
Three months later, an upper esophagogastroduodenal series was performed in each patient with 100 mL of meglumine diatrizoate. The full emptying rate at 30 min and the ratio of partial recanalization were collected.
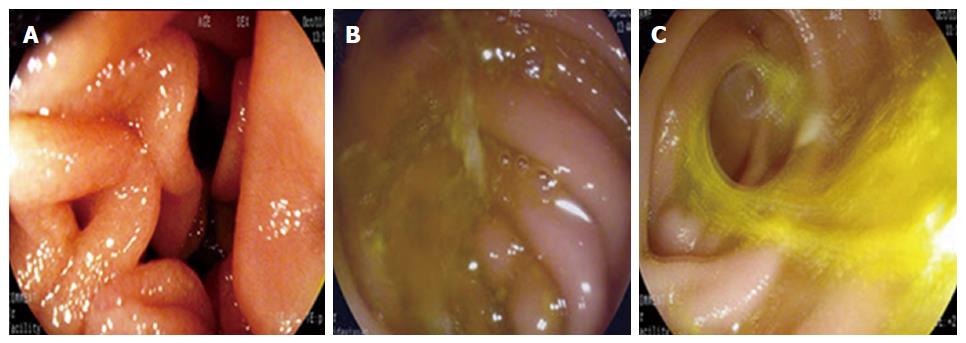
Twelve months later, items were monitored as follows: (1) number of patients with heartburn symptoms; (2) changes in body weight within 1 year; and (3) gastric residue, residual gastritis, and biliary reflux (RGB; monitored by endoscopy). Combined with standard RGB[8], a modified biliary reflux classification in three grades was applied-grade 0: absence of bile (Figure 2A); grade 1: small amount of bile located in the bottom of residual stomach without overflow (Figure 2B); and grade 2: bile spilled into the jejunum with tidal rhythm (Figure 2C).
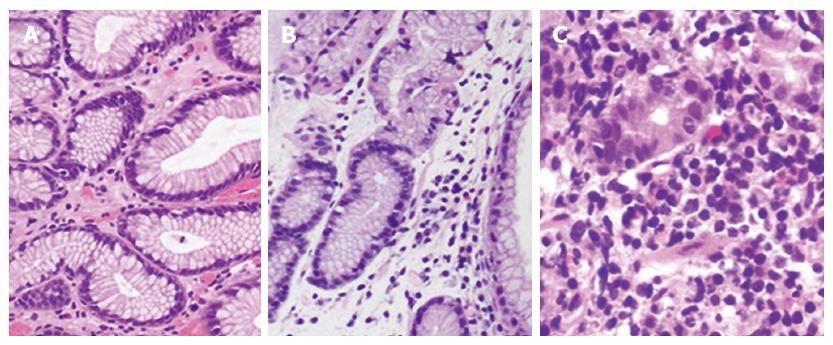
Gastric tissue biopsies were taken to compare the degree of gastritis at 2 cm from anastomosis and then evaluated by two pathologists. Classification included three grades: grade 0, normal mucosa with a small amount of lymphocytes and transparent microscopic field (Figure 3A); grade 1, intermediate between grades 0 and 2 (Figure 3B); and grade 2, acute inflammation with fully infiltrated tissue by lymphocytes or inflammatory cells (Figure 3C).
All statistical analyses were performed using SPSS v18.0 for Windows software. Continuous variables are expressed as mean and SD and compared by Student’s t-test. Categorical variables were analyzed by Pearson χ2 test. The pH variables were compared by repeated measures analysis of variance. A two-tailed P value < 0.05 was considered statistically significant.
According to the randomization table, each group included 79 patients. Baseline data are shown in Table 1. There were no significant difference in gender or pathological data (P > 0.05), but the average age of group U patients was older than group B patients (58.0 ± 11.4 vs 61.8 ± 11.4 years, P = 0.030). The surgical time was slightly longer in group U (154.8 ± 17.8 vs 145.5 ± 15.1 mins, P = 0.001), but there was no difference in blood loss (74.1 ± 26.7 vs 74.0 ± 36.6 mL, P > 0.05).
| Variable | Group U | Group B | P value |
| Age (yr) | 58.0 ± 11.4 | 61.8 ± 11.4 | 0.030 |
| Gender | 0.287 | ||
| Male | 60 (75.9) | 54 (68.4) | |
| Female | 19 (24.1) | 25 (31.6) | |
| Pathological tumor stage | 0.822 | ||
| IB | 3 (3.8) | 2 (2.5) | |
| IIA | 31 (39.2) | 27 (34.2) | |
| IIIB | 28 (35.4) | 29 (36.7) | |
| IIIA | 17 (21.5) | 21 (26.6) | |
| Operative time (min) | 154.8 ± 17.8 | 145.5 ± 15.1 | 0.001 |
| Blood loss (mL) | 74.1 ± 26.7 | 74.0 ± 36.6 | 0.980 |
There was no significant difference in postoperative complications between the two groups (7.6% vs 10.1%, P = 0.576). One patient in each group underwent reoperation because of intra-abdominal bleeding. In group U, a patient with ileus required reoperation after 1 mo of conservative treatment. In group B, a patient received emergency endoscopy to insert a stomach tube into the afferent loop to release pressure due to A-loop syndrome. For both groups, no gastroparesis syndrome was found during the postoperative period (Table 2). According to the Clavien-Dindo classification for surgical complications[9], in group U grade I complications were recorded in 3.8%, grade II in 1.3%, and grade IIIb in 2.5% of the cases. In group B grade I complications were recorded in 3.8%, grade II in 2.5%, grade IIIa in 1.3%, and grade IIIb in 2.5% of the cases. There was still no significant difference between the two groups (P = 0.954).
| Item | Group U | Group B | P value |
| Duodenal stump leakage | 1 (1.3) | 3 (3.8) | 0.620 |
| Chylous fistula | 1 (1.3) | 0 | 1.000 |
| Ileus | 1 (1.3) | 1 (1.3) | 1.000 |
| Anastomotic bleeding | 0 | 1 (1.3) | 1.000 |
| Intra-abdominal bleeding | 2 (2.5) | 2 (2.5) | 1.000 |
| Incision infection | 1 (1.3) | 0 | 1.000 |
| Gastroparesis syndrome | 0 | 0 | - |
| A-loop syndrome | 0 | 1 (1.3) | 1.000 |
| Total | 6 (7.6) | 8 (10.1) | 0.576 |
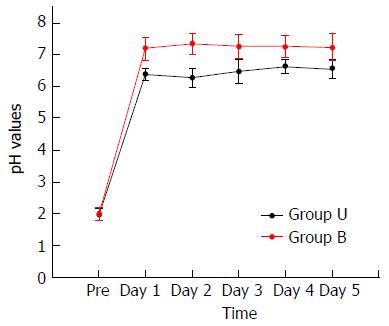
The stomach pH was lower in group U patients, with a significant statistical difference (P < 0.05) (Table 3).
| Time | Group U | Group B | P value |
| Preoperative | 1.97 ± 0.19 | 1.99 ± 0.21 | |
| Day 1 | 6.38 ± 0.18 | 7.21 ± 0.36 | |
| Day 2 | 6.28 ± 0.29 | 7.35 ± 0.32 | |
| Day 3 | 6.48 ± 0.38 | 7.27 ± 0.38 | |
| Day 4 | 6.64 ± 0.22 | 7.27 ± 0.35 | |
| Day 5 | 6.56 ± 0.29 | 7.24 ± 0.42 | |
| 0.000 |
During the postoperative period, all stomach pH values in group U patients were below 7.00. Conversely, all stomach pH values in group B patients were higher than 7.00 (Figure 4).
Three months later, regarding the postoperative esophagogastroduodenal series after 30 min, a higher ratio of full emptying was seen in group B patients (88.2% vs 76.6%, P = 0.061), but with no statistical significance.
At the end of the 1-year follow-up period, three patients in group U and one patient in group B were lost to follow-up. Besides, seven patients in group U and six patients in group B had died. Therefore, the survival rates at 1 year for group U and B patients were 90.79% and 92.31%, respectively, and showed no significant difference (P = 0.735). The biliary reflux incidence in group B was higher than that in group U patients with a significant difference (60.9% vs 90.3%, P = 0.000). The ratio of gastritis in group B (72.2%) was significantly higher than that in group U (55.1%). However, the result of biopsy showed no significant difference (63.8% vs 70.8%, P = 0.278), but the linear-by-linear association was significant (Ptrend = 0.015). Besides, there was no significant difference for the incidence of diarrhea, residual food, or gain of weight between the two groups (Table 4).
| Item | Group U | Group B | P value |
| 3 mo later | n = 77 | n = 76 | |
| Esophagogastroduodenal series | |||
| Full emptying at 30 min | 59 (76.6) | 67 (88.2) | 0.061 |
| Partial recanalization | 0 | - | - |
| 1 yr later | n = 69 | n = 72 | |
| Heartburn | 7 (10.1) | 17 (23.6) | 0.033 |
| Weight gain (kg) | -0.04 ± 3.6 | -0.18 ± 3.8 | 0.723 |
| Endoscopic finding | |||
| Residual food | 8 (11.6) | 3 (4.2) | 0.178 |
| Grade 0 | 61 | 69 | |
| Grade 1 | 7 | 3 | |
| Grade 2 | 1 | 0 | |
| Grade 3-4 | 0 | 0 | |
| Gastritis | 38 (55.1) | 52 (72.2) | 0.044 |
| Grade 0 | 31 | 20 | |
| Grade 1 | 30 | 35 | |
| Grade 2 | 8 | 17 | |
| Grade 3-4 | 0 | 0 | |
| Bile reflux | 42 (60.9) | 65 (90.3) | 0.000 |
| Grade 0 | 27 | 7 | |
| Grade 1 | 34 | 23 | |
| Grade 2 | 8 | 42 | |
| Partial recanalization | 9 (13.0) | - | - |
| Biopsy of gastritis | 44 (63.8) | 51 (70.8) | 0.278 |
| Grade 0 | 25 | 21 | |
| Grade 1 | 39 | 45 | |
| Grade 2 | 5 | 6 |
In 1988, uncut Roux-en-Y reconstruction was first reported by Stiegman and Goff[10]. Some studies over the years have confirmed that this reconstruction can preserve myoneural continuity to eliminate Roux stasis syndrome[11-13]. Because uncut Roux-en-Y is a modification of Billroth II reconstruction, it makes sense to compare Billroth II and uncut Roux-en-Y to determine the better procedure after LADG.
Our study showed there was no significant difference for the incidence of postoperative complications between the two groups (7.6% vs 10.1%, P > 0.05). Moreover, for the severity of postoperative complications, according to the Clavien-Dindo classification of surgical complications, there was no significant difference (P = 0.954). A-loop syndrome does not occurred in the uncut Roux-en-Y group, and the incidence of duodenal stump leakage was lower than that of the Billroth II group. The reason for this may be that the Braun anastomosis effectively relieves the pressure of the afferent loop[14], but more cases should be included to confirm this difference.
For biliary reflux, during the postoperative period, all group U pH values were lower than 7.00, thus representing an acidic stomach environment. In group B patients, all pH values were higher than 7.00, representing an alkaline stomach environment with alkaline reflux, which can be considered an important risk factor for gastric stump cancer[15] (Figure 2). In addition, according to patient outcomes at the 1-year follow-up, the bile reflux incidence in group B patients was significantly higher than that in group U (P = 0.000). However, the incidence of bile reflux in group U patients was 60.9%, which is higher than the equivalent statistic in Park and Kim’s report (less than 30%)[4]. There are two main reasons that can explain these data. On one hand, the incidence of partial recanalization reached 13.0%. In this study, for partial recanalization, all those cases were first observed by endoscopy, and the result would be confirmed by esophagogastroduodenal series if there was doubt. Recanalization eventually allowed bile access to the gastric remnant. On the other hand, for some other cases, the bile reflux happened through the efferent loop, and a small amount of bile was usually found in the bottom of residual stomach with no overflow (grade 1).
Regarding gastritis, the incidence in group B patients (72.2%) was significantly higher than that in group U patients (55.1%), with a higher incidence of heartburn in group B patients as well (23.6%). These results allow us to conclude that uncut Roux-en-Y can reduce the occurrence of residual gastritis and heartburn proportion in Billroth II reconstruction. However, the biopsy results showed no significant difference between the two groups (P = 0.278).
We draw two conclusions from these results. First, the pattern of bile reflux is different. The majority of group U patients were classified into grade 1 (49.3%), characterized by a small bile amount usually located in the bottom of the stomach as gastric residue, whereas most group B patients belonged to grade 2 (58.3%), characterized by considerable tidal rhythm. Hence, the stomach bile was fresh and temporary, which may be less corrosive to the gastric mucous membranes. Park showed a correlation between bile reflux and the degree of gastritis[4], but perhaps it depends not only on the amount but also on the pattern of biliary reflux. Second, the follow-up time was too short to show differences in the percentage of residual gastritis on biopsy, so the linear-by-linear association P value was also calculated. The result showed a significant difference (Ptrend = 0.015), meaning that the severity of residual gastritis for group B was worse than that for group U on biopsy.
For residual food, there was no significant difference between the two groups (11.6% vs 4.2%, P > 0.05). For gastrointestinal anastomosis of both procedures, the stoma was extensive along the greater curvature. No gastroparesis syndrome was found during follow-up, which is perhaps related to myoneural continuity. At 3-mo follow-up, the incidence of full emptying at 30 min reached 76.6% and 88.2% in group U and B patients, respectively (Table 4). As a result, no retention of afferent loop stump was found during the follow-up, and the incidence of residual food was lower than that in other studies[4,16].
This study adopted body weight change to evaluate postoperative nutritional status of patients, and 1 year later, the weight change values of group U and group B patients were -0.04 ± 3.6 kg and -0.18 ± 3.8 kg, respectively, with no significant difference. Moreover, there was no significant difference in survival rates of group U and group B patients after 1 year (90.79% vs 92.31%, respectively; P > 0.05).
In conclusion, the uncut Roux-en-Y digestive reconstruction procedure is secure and feasible. Moreover, it can effectively reduce the incidence of alkaline reflux, residual gastritis, and heartburn seen in classical Billroth II procedure. Besides, the uncut technique still needs improvement so that the risk of staple line dehiscence is minimized, with a longer follow-up period to reevaluate the exact risk. Despite the incidence of recanalization[17-21], uncut Roux-en-Y should be widely applied.
In 1988, uncut Roux-en-Y reconstruction was first reported by Stiegman and Goff. Some studies over the years have confirmed that this reconstruction can preserve myoneural continuity to eliminate Roux stasis syndrome. However, because of the challenge of recanalization, the uncut Roux-en-Y is still controversial and really needs further study. Since uncut Roux-en-Y is a modification of Billroth II reconstruction, it makes sense to compare Billroth II and uncut Roux-en-Y to determine the better procedure after laparoscopy-assisted distal gastrectomy (LADG).
Compared with Japan and Korea, early gastric cancer only accounts for a small percentage in China, and most gastric cancer cases are found in advanced stages at the initial diagnosis. It is inappropriate for surgeons to perform Billroth I anastomosis after subtotal gastrectomy. In 2005, Uyama first combined LADG with uncut Roux-en-Y reconstruction, and since then it has been the hotspot for many years. However, its use remains controversial. Some surgeons believe that it is better than Billroth II and Roux-en-Y reconstructions, while others do not.
This study is the first randomized controlled trial concentrating on uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer. It aimed to compare uncut Roux-en-Y and Billroth II reconstruction in terms of postoperative complications, including biliary reflux and gastritis. Despite the incidence of recanalization, uncut Roux-en-Y reconstruction is secure and feasible, and can effectively reduce the incidence of alkaline reflux, residual gastritis, and heartburn.
There remains no clear consensus regarding the preferred reconstructive surgical procedure after LADG for gastric cancer. In this study, uncut Roux-en-Y reconstruction is secure and feasible, and can effectively reduce the incidence of alkaline reflux, residual gastritis, and heartburn. Therefore, uncut Roux-en-Y should be widely applied in the future. Besides, the uncut technique still needs improvement so that the risk of staple line dehiscence is minimized, with a longer follow-up period to reevaluate the exact risk.
Interesting comparison of two techniques of postgastrectomy reconstruction.
Manuscript source: Unsolicited manuscript
Specialty type: World Journal of Gastroenterology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bang YJ, Kirshtein B, Tarantino G S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Tran TB, Worhunsky DJ, Squires MH, Jin LX, Spolverato G, Votanopoulos KI, Cho CS, Weber SM, Schmidt C, Levine EA. To Roux or not to Roux: a comparison between Roux-en-Y and Billroth II reconstruction following partial gastrectomy for gastric cancer. Gastric Cancer. 2016;19:994-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakamura K, Hirano M, Esaki M. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017;52:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Park JY, Kim YJ. Uncut Roux-en-Y Reconstruction after Laparoscopic Distal Gastrectomy Can Be a Favorable Method in Terms of Gastritis, Bile Reflux, and Gastric Residue. J Gastric Cancer. 2014;14:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Uyama I, Sakurai Y, Komori Y, Nakamura Y, Syoji M, Tonomura S, Yoshida I, Masui T, Inaba K, Ochiai M. Laparoscopy-assisted uncut Roux-en-Y operation after distal gastrectomy for gastric cancer. Gastric Cancer. 2005;8:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 6. | Huang Y, Wang S, Shi Y, Tang D, Wang W, Chong Y, Zhou H, Xiong Q, Wang J, Wang D. Uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Expert Rev Gastroenterol Hepatol. 2016;10:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 7. | Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, Wada Y, Ohtoshi M. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002;195:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Kubo M, Sasako M, Gotoda T, Ono H, Fujishiro M, Saito D, Sano T, Katai H. Endoscopic evaluation of the remnant stomach after gastrectomy: proposal for a new classification. Gastric Cancer. 2002;5:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24843] [Article Influence: 1183.0] [Reference Citation Analysis (0)] |
| 10. | Van Stiegmann G, Goff JS. An alternative to Roux-en-Y for treatment of bile reflux gastritis. Surg Gynecol Obstet. 1988;166:69-70. [PubMed] |
| 11. | Frankel LA. Roux stasis syndrome: treatment by pacing and prevention by use of an ‘uncut’ Roux limb. Arch Surg. 1992;127:1135-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Tu BN, Kelly KA. Elimination of the Roux stasis syndrome using a new type of “uncut Roux” limb. Am J Surg. 1995;170:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Zhang YM, Liu XL, Xue DB, Wei YW, Yun XG. Myoelectric activity and motility of the Roux limb after cut or uncut Roux-en-Y gastrojejunostomy. World J Gastroenterol. 2006;12:7699-7704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (3)] |
| 14. | Wang F, Zu HL, Jiang H, Kang Y, Dong PD, Xue YW. Clinical investigation of combined Billroth II with Braun anastomosis for patients with gastric cancer. Hepatogastroenterology. 2014;61:1812-1816. [PubMed] |
| 15. | Lorusso D, Linsalata M, Pezzolla F, Berloco P, Osella AR, Guerra V, Di Leo A, Demma I. Duodenogastric reflux and gastric mucosal polyamines in the non-operated stomach and in the gastric remnant after Billroth II gastric resection. A role in gastric carcinogenesis? Anticancer Res. 2000;20:2197-2201. [PubMed] |
| 16. | Csendes A, Burgos AM, Smok G, Burdiles P, Braghetto I, Díaz JC. Latest results (12-21 years) of a prospective randomized study comparing Billroth II and Roux-en-Y anastomosis after a partial gastrectomy plus vagotomy in patients with duodenal ulcers. Ann Surg. 2009;249:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Tu BN, Sarr MG, Kelly KA. Early clinical results with the uncut Roux reconstruction after gastrectomy: limitations of the stapling technique. Am J Surg. 1995;170:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Sardiñas C, Gattorno F. Evaluation of gastric emptying with the “uncut” Roux en Y technique. Ann Ital Chir. 1998;69:41-46; discussion 46-47. [PubMed] |
| 19. | Richardson WS, Spivak H, Hudson JE, Budacz MA, Hunter JG. Teflon buttress inhibits recanalization of uncut stapled bowel. J Gastrointest Surg. 2000;4:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Morton JM, Lucktong TA, Trasti S, Farrell TM. Bovine pericardium buttress limits recanalization of the uncut Roux-en-Y in a porcine model. J Gastrointest Surg. 2004;8:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Shibata C, Kakyo M, Kinouchi M, Tanaka N, Miura K, Naitoh T, Ogawa H, Yazaki N, Haneda S, Watanabe K. Results of modified uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Hepatogastroenterology. 2013;60:1797-1799. [PubMed] |