Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6315
Peer-review started: November 4, 2016
First decision: January 19, 2017
Revised: March 21, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: September 14, 2017
Processing time: 315 Days and 8.3 Hours
To detect the existence of isolated cancer cells in the mesentery of colorectum (named as Metastasis V), and investigate its clinical significance in colorectal cancer (CRC) patients.
Sixty-three CRC patients who received radical excision between January 2012 and September 2015 were included. All the patients underwent laparoscopy-assisted radical colorectomy or proctectomy [with complete mesocolic excision (CME) or total mesorectal excision (TME)] with R0 dissections at the Department of Gastrointestinal Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The location and size of the primary lesions were recorded immediately after the tumor was removed, with the surrounding mesenterium completely separated along the intestinal wall. Each dissected mesentery sample was analyzed for hematoxylin-eosin staining and immunohistochemistry using cytokeratin 19 antibody. Image Pro Plus Software 6.0 (Media Cybernetics, CA, United States) was used to semi-quantitatively measure the concentration of the cytokeratin 19 immunohistochemistry. The correlation between metastasis found in mesentery and clinicopathological characteristics was examined. The prognosis of patients was also evaluated by preoperative serum CEA level.
Metastasis V was detected in 14 of 63 (22.2%) CRC patients who underwent laparoscopy-assisted radical colorectomy or proctectomy (with CME or TME) with R0 dissection in our hospital between January 2012 and September 2015. There was no significant difference in age, gender, tumor size, and tumor location in patients with Metastasis V (P > 0.05). Metastasis V was more likely to occur in poorly differentiated tumor (5/11; 45.5%) than moderately (8/46; 17.4%) and well- differentiated one (1/6; 16.7%). The Metastasis V in N2 stage (9/14; 64.3%) was more frequent that in the N0 stage (3/35; 8.6%) or N1 stages (2/14; 14.3%). In addition, Metastasis V was positively related to the tumor invasive depth (T1:0/1, 0%; T2:1/12, 8.3%; T3:7/39, 17.9%; T4:6/11, 54.5%). Furthermore, preoperative serum CEA level in Metastasis V-positive patients was significantly higher than in Metastasis V-negative patients (4.27 ng/mL vs 3.00 ng/mL).
Metastasis V might be associated with a poor prognosis of CRC patients.
Core tip: Local-regional recurrence of colorectal cancer (CRC) is common in patients who received R0 resection. Our previous study proposed a novel type of metastasis designated as “Metastasis V” in gastric cancer. Metastasis V is defined as the appearance of cancer cells in the mesentery in broad sense, and may be a risk factor for the poor prognosis after radical surgery. In this study, Metastasis V was also detected in the mesentery of colorectum, and it might be associated with the poor prognosis of CRC patients.
- Citation: Luo XL, Xie DX, Wu JX, Wu AD, Ge ZQ, Li HJ, Hu JB, Cao ZX, Gong JP. Detection of metastatic cancer cells in mesentery of colorectal cancer patients. World J Gastroenterol 2017; 23(34): 6315-6320
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6315.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6315
Three decades ago, RJ Heald introduced the total mesorectal excision (TME) in the treatment of rectal cancer[1]. Subsequently, complete mesocolic excision (CME) was popularized by W Hoehenbuerg to reduce tumor relapse and improve prognosis in colon cancer patients[2]. These new approaches greatly contributed to the complete mesentery excision and improved lymph node harvest. Furthermore, with the development of surgical technology, neo-adjuvant chemotherapy and efficient perioperative management, the prognosis of patients with colorectal cancer (CRC) has been improved. However, local-regional recurrence of CRC is still often noted in the patients who received radical R0-resection with no lymphatic metastasis[3].
Direct invasion, lymphatic drainage, hematogenous spread and peritoneal dissemination are the four classical routes for local-regional recurrence or distant implantion of cancer cells. In addition to these pathways, the existence of metastatic cancer cells in the mesentery of colorectum has also been reported, and this type of metastasis was named as “extra-capsular spreads” or “extra-nodal metastasis”[4-7]. In our previous study, we proposed a novel type of tumor metastasis designated as Metastasis V in gastric cancer. Metastasis V is defined as the appearance of cancer cells in the mesogastrium with perigastric adipose tissue, and it may be a risk factor for patient survival after radical surgery[8]. Here, in this study, we further determined whether Metastasis V can be detected in the mesocolorectum, and examined its clinical significance.
Sixty-three patients who underwent surgery for CRC at the Department of Gastrointestinal Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology between January 2012 and September 2015 were included in this study. The patients consisted of 25 female patients and 38 male patients. All of these patients received laparoscopy-assisted radical colorectomy or proctectomy (with CME or TME) with R0 dissection. All participants signed informed written consents in this research. This study was approved by the Tongji Hospital Ethics Committee.
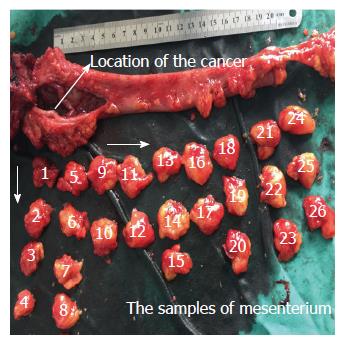
Clinical samples of CRC were obtained from the resected colorectal tissue. The location and size of the primary lesions were recorded immediately after the tumor was removed, and then the mesenterium was completely separated from the intestinal wall along with the anterior edge. All specimens were fixed in 10% formaldehyde before processing. The lymph nodes were obtained from the mesenterium. Next, the mesentery was cut into strip-shape with a width of 2 cm in an outward-to-inward manner, vertical to the intestinal wall. The strip-shaped samples were cut into cube samples with a mean length of 2 cm. All of the small samples were numbered from inside (proximal end of the cancer) to outside (Figure 1). These samples were embedded in 10% formaldehyde. Demographic characteristics of the 63 patients are described in Table 1. The clinicopathological stage was determined by the 7th edition of the AJCC Cancer Staging Manual.
| Parameters | Results |
| Gender | |
| Female | 25 |
| Male | 38 |
| Age (yr, mean, range) | 55.5 (28-86) |
| Location of cancer | |
| Colon | 11 |
| Rectum | 52 |
| Differentiated grade | |
| Well | 6 |
| Moderately | 46 |
| Poorly | 11 |
| Affected lymph nodes | |
| N0 | 35 |
| N1 | 14 |
| N2 | 14 |
| Invasive depth | |
| T1 | 1 |
| T2 | 12 |
| T3 | 39 |
| T4 | 11 |
Immunohistochemistry was performed using the cytokeratin 19 antibody. Cytokeratin 19 is specifically expressed in intestine epithelia cells and CRC cells[9]. Hence, it has been proven to be a highly sensitive marker for CRCs.
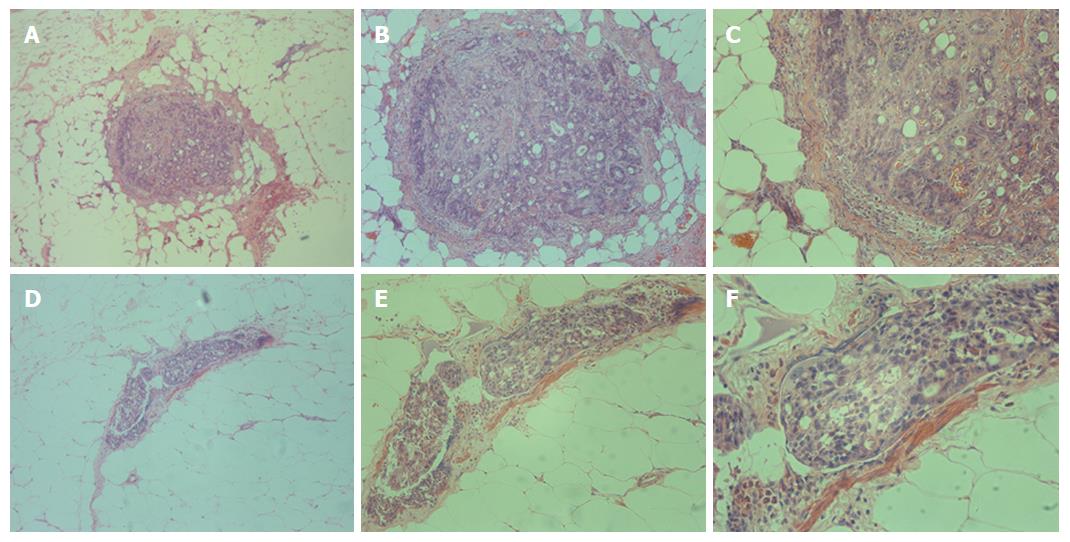
Immunostaining was performed by the standard streptavidin-biotin method. First, the slices were de-paraffinized and rehydrated. To retrieve antigenicity, the slices were boiled in 0.01 mol/L, pH 6.0 sodium citrate buffer for approximately 15 min. Endogenous peroxidase was restrained with 0.3% hydrogen peroxide for roughly 25 min. After blocking with 3% BSA for 30 min, the anti-cytokeratin 19 monoclonal antibody was arranged in blocking buffers and the slices were incubated at 4 °C overnight. After being washed in pH 7.4 PBS 3 times (5 min each time), the corresponding secondary antibody, retained at room temperature for approximately 50 min and marked by HRP was added. After one more wash with pH 7.4 PBS for 15 min, the chromogenic reagent DAB was added to the colorate. The slices were counterstained with Harris hematoxylin, dehydrated in a graded alcohol series (75% alcohol for 6 min, 85% alcohol for 6 min, absolute ethyl alcohol I for 6 min, and absolute ethyl alcohol II for 6 min), cleared in xylene for 5 min, and mounted. Individual tumor cells in mesenteries, separated from primary lesion and lymph nodes, were identified to be Metastasis V.
Image Pro Plus Software 6.0 (Media Cybernetics, CA, United States) was used to semi-quantitatively measure the concentration of the cytokeratin 19 immunohistochemistry. This procedure, was split into six steps: (1) discovering and surveying the section of interest; (2) adjusting the optical densities; (3) obtaining, transforming and preserving images; (4) amending the background and background staining; (5) configuration of the section of interest to determine the optical density; and (6) examining optical densities.
The Fisher’s exact test, χ² test, and Mann-Whitney U test were used to inspect the significance of the differences between the covariates. We considered a P value of less than 0.05 statistically significant. Standard statistical analyses were performed by SPSS version 20.0.
Metastasis V was detected in 14 of the 63 (22.2%) patients by immunohistochemistry staining. These isolated cancer cells separated from the primary lesion and lymph nodes were observed in the same slice (Figure 2). In terms of “T” stage, only one case showed limited muscular layer invasion, seven cases had sub-serosal invasion, and the rest had serosal invasion. In these patients, there were three lymph node negative patients with different invasive depths (Table 2).
| Patients | Sex | Age (yr) | Cancer size (cm) | Cancer location | Differentiated grade | Invasive depth | Affected lymph nodes | TNM |
| 1 | F | 37 | 2.5 | Rectum | Poorly | Serosal | 8/15 | pT4N2M0 |
| 2 | F | 42 | 3 | Rectum | Moderately | Subserosal | 5/17 | pT3N2M0 |
| 3 | M | 33 | 2 | Rectum | Moderately | Serosal | 5/15 | pT4N2M0 |
| 4 | M | 48 | 6 | Sigmoid | Well | Serosal | 12/21 | pT4N2M0 |
| 5 | F | 63 | 3 | Rectum | Poorly | Muscularis | 0/15 | pT2N2M0 |
| 6 | F | 45 | 3 | Rectum | Moderately | Serosal | 5/28 | pT4N2M0 |
| 7 | M | 58 | 3 | Rectum | Poorly | Subserosal | 8/12 | pT3N2M0 |
| 8 | M | 64 | 4 | Rectum | Poorly | Subserosal | 7/15 | pT3N2M1 |
| 9 | M | 54 | 6 | Rectum | Moderately | Subserosal | 2/13 | pT3N1M0 |
| 10 | F | 60 | 2 | Descending colon | Moderately | Subserosal | 0/17 | pT3NxM0 |
| 11 | M | 54 | 3 | Rectum | Moderately | Serosal | 5/12 | pT4N2M0 |
| 12 | M | 58 | 3 | Rectum | Moderately | Subserosal | 0/16 | pT3N0M0 |
| 13 | F | 57 | 3 | Rectum | Poorly | Serosal | 2/21 | pT4N1M1 |
| 14 | M | 47 | 4 | Rectum | Moderately | Subserosal | 24/32 | pT3N2M0 |
The demographic characteristics of patients and the pathologic features of tumors with Metastasis V are shown in Table 3. There was no significant difference in age, gender, tumor size, and tumor location (P > 0.05). Metastasis V was more likely to occur in poorly differentiated tumor (5/11; 45.5%) than moderately (8/46; 17.4%) and well-differentiated one (1/6; 16.7%). The Metastasis V in N2 stage (9/14; 64.3%) was more frequent than that in the N0 stage (3/35; 8.6%) or N1 stages (2/14; 14.3%). In addition, Metastasis V was positively related to the tumor invasive depth (T1: 0/1, 0%; T2: 1/12, 8.3%; T3: 7/39, 17.9%; T4: 6/11, 54.5%).
| Parameters | Metastasis V | P value | |
| Negative | Positive | ||
| Age (yr) | NS | ||
| < 45 | 8 | 3 | |
| ≥ 45 and ≤ 59 | 19 | 8 | |
| ≥ 60 | 22 | 3 | |
| Cancer size (cm) | NS | ||
| ≤ 3 | 24 | 10 | |
| > 3 and < 5 | 14 | 2 | |
| ≥ 5 | 11 | 2 | |
| Gender | NS | ||
| Female | 19 | 6 | |
| Male | 30 | 8 | |
| Cancer location | NS | ||
| Colon | 9 | 2 | |
| Rectum | 40 | 12 | |
| Differentiated grade | NS | ||
| Well | 5 | 1 | |
| Moderately | 38 | 8 | |
| Poorly | 6 | 5 | |
| Affected lymph nodes | < 0.05 | ||
| N0 | 32 | 3 | |
| N1 | 12 | 2 | |
| N2 | 5 | 9 | |
| Invasive depth | < 0.05 | ||
| T1 | 1 | 0 | |
| T2 | 11 | 1 | |
| T3 | 32 | 7 | |
| T4 | 5 | 6 | |
Previous studies have demonstrated the correlation between increased preoperative CEA and the poor prognosis of rectal cancer patients[10,11]. Here, we further determined the correlation between Metastasis V and preoperative CEA levels. Totally 42 CRC patients who underwent operations in our department were retrospectively studied. Metastasis V was detected in nine patients according to the pathological reports. Six patients were excluded because their preoperative CEA data was missed. The CEA levels were divided into two grades. The CEA levels more than 3 times of normal range (15.00 ng/mL) were classified as high grade, while those less than that were designated as low grade. Our data indicated that preoperative serum CEA levels in Metastasis V-positive patients were significantly higher than in Metastasis V-negative patients (4.27 ng/mL vs 3.00 ng/mL) (Table 4).
| Parameters | Metastasis V | P value | |
| Negative | Positive | ||
| Median (ng/mL) | 3 | 4.27 | < 0.05 |
| Grade | < 0.05 | ||
| High (> 15.00 ng/mL) | 5 | 5 | |
| Low (≤ 15.00 ng/mL) | 73 | 16 | |
In this study, we have shown that metastatic cancer cells can reside in the mesentery of the colorectum of CRC patients. These tumor cells showed no direct attachment to the primary lesions, with no involvement of vessel-lymphatic drainage system, and are confined to the fatty tissues enveloped by the fascia proper (Figure 1). In our previous study, we proposed a novel type of tumor metastasis designated as Metastasis V in gastric cancer patients[8]. Thus, we believe that Metastasis V could be detected in the mesenteries of both gastric and CRC patients. Similar to our previous studies, the incidence of Metastasis V was closely related to the tumor invasion of CRC patients, which was mainly detected in the T3 and T4 tumors (Table 2). Metastasis V was also correlated with lymphatic metastasis. Lymphatic nodes staging N1-N3 had larger percentages of Metastasis V than N0 (Table 2). Therefore, immunohistochemical analysis of Metastasis V in these patients is strongly recommended in clinical practice.
More interestingly, the preoperative serum CEA levels in Metastasis V-positive patients were significantly higher than in Metastasis V-negative patients (4.27 ng/mL vs 3.00 ng/mL) (Table 4). These data indicated that the prognosis of the patients with Metastasis V-positive tumors was worse than that of patients with Metastasis V-negative tumors. Due to the limited number of patients in the current study, a larger sample of cohort study should be enrolled for further analysis.
Little is known about the exact route or mechanism of Metastasis V. It was hypothesized that primary tumor lesions penetrates the intestinal wall, and then the cancer cells could probably scatter into the fatty tissues enveloped by proper fascia. In this way, Metastasis V occurs not only in CRC and gastric cancers, but also in oral head-neck, pancreas and papilla of Vater tumors[12-20].
It has been well known in practice that radical surgery for cancers should include en bloc resections of the primary tumor and neighboring tissues. However, it is difficult to determine the exact boundaries of en bloc resection within gastrointestinal tumors. “TME” or “CME” can reduce the tumor recurrence and increase patient survival, however, the possible reason is still unknown. In our opinion, the mesenteries of colorectum enveloped by the proper fascia not only propose a precise boundary for resection, but also function as pathologic barriers of Metastasis V spreading. Thus, our findings are clinically significant because TME or CME should be a useful choice for surgical excision of Metastasis V. Further studies on the efficiency of Metastasis V excision via TME or CME will be investigated.
We thank Drs. Chao-Ran Yu and Jie Shen for their helpful discussion and paper revision work.
Local-regional recurrence of colorectal cancer (CRC) is still often noted in the patients who received radical R0-resection with no lymph-node metastasis.
In addition to the classical four metastatic patterns, the existence of metastatic cancer cells in the mesentery of colorectum has also been reported, and this type of metastasis was named as “extra-capsular spreads” or “extra-nodal metastasis”. Examination of the clinical and oncological significance of isolated tumor cells found in mesentery of colorectum in CRC patients is one of the main frontiers in surgical oncology.
Metastasis V is defined as the appearance of cancer cells in the mesogastrium, and may be a risk factor for the poor prognosis after radical surgery. This study demonstrated that Metastasis V was also detected in the mesentery of colorectum, and it might be associated with the poor prognosis of CRC.
It is clinically useful to be assisted in evaluation of prognosis of CRC patients.
In this paper, the authors well described an interesting study about isolated cancer cells in the mesentery of colorectum (named as Metastasis V). This is an interesting point, and the issue of metastasis in patients with CRC is of main importance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Luchini C, Wittmann T S- Editor: Gong ZM L- Editor: Ma JY E- Editor: Zhang FF
| 1. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1937] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 2. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-364; discussion 364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1106] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 3. | Bouvier AM, Launoy G, Bouvier V, Rollot F, Manfredi S, Faivre J, Cottet V, Jooste V. Incidence and patterns of late recurrences in colon cancer patients. Int J Cancer. 2015;137:2133-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. 2001;92:3030-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Nakamura K, Ozaki N, Yamada T, Hata T, Sugimoto S, Hikino H, Kanazawa A, Tokuka A, Nagaoka S. Evaluation of prognostic significance in extracapsular spread of lymph node metastasis in patients with gastric cancer. Surgery. 2005;137:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Etoh T, Sasako M, Ishikawa K, Katai H, Sano T, Shimoda T. Extranodal metastasis is an indicator of poor prognosis in patients with gastric carcinoma. Br J Surg. 2006;93:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Johnson JT, Barnes EL, Myers EN, Schramm VL Jr, Borochovitz D, Sigler BA. The extracapsular spread of tumors in cervical node metastasis. Arch Otolaryngol. 1981;107:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 235] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Xie D, Osaiweran H, Liu L, Wang X, Yu C, Tong Y, Hu J, Gong J. Mesogastrium: a fifth route of metastasis in gastric cancer? Med Hypotheses. 2013;80:498-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3859] [Cited by in RCA: 3874] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 10. | Sisik A, Kaya M, Bas G, Basak F, Alimoglu O. CEA and CA 19-9 are still valuable markers for the prognosis of colorectal and gastric cancer patients. Asian Pac J Cancer Prev. 2013;14:4289-4294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Basbug M, Arikanoglu Z, Bulbuller N, Cetinkaya Z, Aygen E, Akbulut S, Satici O. Prognostic value of preoperative CEA and CA 19-9 levels in patients with colorectal cancer. Hepatogastroenterology. 2011;58:400-405. [PubMed] |
| 12. | Alvi A, Johnson JT. Extracapsular spread in the clinically negative neck (N0): implications and outcome. Otolaryngol Head Neck Surg. 1996;114:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 138] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003;39:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Hirabayashi H, Koshii K, Uno K, Ohgaki H, Nakasone Y, Fujisawa T, Syouno N, Hinohara T, Hirabayashi K. Extracapsular spread of squamous cell carcinoma in neck lymph nodes: prognostic factor of laryngeal cancer. Laryngoscope. 1991;101:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Veronese N, Luchini C, Nottegar A, Kaneko T, Sergi G, Manzato E, Solmi M, Scarpa A. Prognostic impact of extra-nodal extension in thyroid cancer: A meta-analysis. J Surg Oncol. 2015;112:828-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Luchini C, Wood LD, Cheng L, Nottegar A, Stubbs B, Solmi M, Capelli P, Pea A, Sergi G, Manzato E. Extranodal extension of lymph node metastasis is a marker of poor prognosis in oesophageal cancer: a systematic review with meta-analysis. J Clin Pathol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Veronese N, Fassan M, Wood LD, Stubbs B, Solmi M, Capelli P, Pea A, Nottegar A, Sergi G, Manzato E. Extranodal Extension of Nodal Metastases Is a Poor Prognostic Indicator in Gastric Cancer: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2016;20:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Veronese N, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, Sergi G, Manzato E, Fassan M, Wood LD. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol. 2016;27:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Luchini C, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, Sergi G, Manzato E, Fassan M, Wood LD. Extranodal extension is an important prognostic parameter for both colonic and rectal cancer. Ann Oncol. 2016;27:955-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Luchini C, Veronese N, Pea A, Sergi G, Manzato E, Nottegar A, Solmi M, Capelli P, Scarpa A. Extranodal extension in N1-adenocarcinoma of the pancreas and papilla of Vater: a systematic review and meta-analysis of its prognostic significance. Eur J Gastroenterol Hepatol. 2016;28:205-209. [PubMed] |