Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6273
Peer-review started: May 17, 2017
First decision: June 22, 2017
Revised: July 4, 2017
Accepted: August 8, 2017
Article in press: August 8, 2017
Published online: September 14, 2017
Processing time: 121 Days and 3.4 Hours
To investigate the factors predictive of failure when placing a second biliary self-expandable metallic stents (SEMSs).
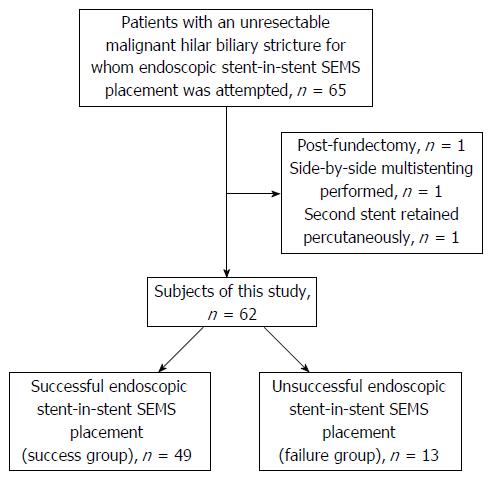
This study evaluated 65 patients with an unresectable malignant hilar biliary obstruction who were examined in our hospital. Sixty-two of these patients were recruited to the study and divided into two groups: the success group, which consisted of patients in whom a stent-in-stent SEMS had been placed successfully, and the failure group, which consisted of patients in whom the stent-in-stent SEMS had not been placed successfully. We compared the characteristics of the patients, the stricture state of their biliary ducts, and the implemented endoscopic retrograde cholangiopancreatography (ERCP) procedures between the two groups.
The angle between the target biliary duct stricture and the first implanted SEMS was significantly larger in the failure group than in the success group. There were significantly fewer wire or dilation devices (ERCP catheter, dilator, or balloon catheter) passing the first SEMS cell in the failure group than in the success group. The cut-off value of the angle predicting stent-in-stent SEMS placement failure was 49.7 degrees according to the ROC curve (sensitivity 91.7%, specificity 61.2%). Furthermore, the angle was significantly smaller in patients with wire or dilation devices passing the first SEMS cell than in patients without wire or dilation devices passing the first SEMS cell.
A large angle was identified as a predictive factor for failure of stent-in-stent SEMS placement.
Core tip: We investigated the factors predictive of failure when placing multiple endoscopic self-expandable metallic stents (SEMSs) to relieve a malignant biliary obstruction. The angle between the target biliary duct stricture and the first implanted SEMS was significantly higher in the failure than in the success group. There were significantly fewer wire or dilation devices passing the first SEMS cell in the failure group than in the success group. The angle was significantly smaller in patients with wire or dilation devices passing the first SEMS cell. In conclusion, a large angle was identified as a predictive factor for failure of stent-in-stent SEMS placement.
- Citation: Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Watanabe K, Nakamura J, Kikuchi H, Waragai Y, Takasumi M, Sato Y, Hikichi T, Ohira H. Predictive factors for the failure of endoscopic stent-in-stent self-expandable metallic stent placement to treat malignant hilar biliary obstruction. World J Gastroenterol 2017; 23(34): 6273-6280
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6273.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6273
A malignant hilar biliary stricture can be caused by several different types of cancer, such as cholangiocarcinoma, pancreatic cancer, liver cancer, and lymph node metastases. A malignant hilar biliary stricture causes obstructive jaundice, and biliary drainage must be accomplished prior to surgical or chemotherapeutic treatment. Two procedures can be used to achieve biliary drainage to treat malignant obstructive jaundice: endoscopic therapy and percutaneous transhepatic bile drainage. Due to its safety and efficacy, endoscopic therapy is the first-choice treatment[1].
Generally, self-expandable metallic stents (SEMSs) are superior to plastic stents in terms of stent patency, cost, and length of hospital stay[2-7]. SEMSs are likewise superior for the treatment of a malignant hilar biliary stricture[8]. Whether it is necessary to multistent a malignant hilar biliary stricture remains a topic of discussion[8-15]. In fact, some patients require multistenting to treat cholangitis and liver abscesses. However, it is sometimes difficult to place multiple metallic stents to relieve a malignant hilar biliary stricture. Moreover, the predictive factors for the failure of biliary multistenting have not been identified.
Therefore, the aim of the present study was to determine the predictive factors for the failure of biliary multistenting.
This study was retrospective in design. To evaluate the predictive factors for the failure to place an endoscopic stent-in-stent SEMS, we compared the patient characteristics and the therapeutic endoscopy factors of the groups with successful and unsuccessful stent-in-stent SEMS placement. This study was approved by the ethics committee of Fukushima Medical University.
We examined 65 patients with an unresectable malignant hilar biliary stricture in whom an endoscopic stent-in-stent biliary SEMS placement was attempted between April 2003 and August 2016 in our hospital. The patients were not required to give informed consent to participate in this study because the analysis used anonymised data obtained after each patient provided written consent to undergo the medical examination. We included 62 of the 65 patients in our study, all of whom had Bismuth type II-IV strictures, had not received upper gastrointestinal tract surgery, and had underwent multiple stent-in-stent placements (Figure 1). Moreover, all patients retained the first metallic stent that was placed. We divided the patients into two groups. The 49 patients in whom a stent-in-stent SEMS was successfully placed at the hilar biliary stricture were included in the success group. The 13 patients in whom only one metallic stent was placed at the hilar biliary stricture were included in the failure group.
Endoscopic retrograde cholangiopancreatography (ERCP) was performed by specialists of pancreaticobiliary endoscopy who had experience performing at least 2000 ERCP procedures or by trainees under the guidance of specialists. Before the ERCP procedure was initiated, all patients were sufficiently sedated with midazolam. After the ERCP endoscope was placed in the descending portion of the duodenum, biliary duct cannulation was performed, and the strictured region was observed using cholangiography. Endoscopic sphincterotomy (EST) was performed as required. The first metallic stent was placed at the hilar biliary stricture, and a wire was passed to the second targeted biliary duct. If a wire was not passed to the second targeted biliary duct, the procedure was finished. After the mesh was dilated using an ERCP catheter, a dilator catheter, or a balloon catheter, a second metallic stent was placed. Dilation devices were selected randomly by each endoscopist. JF240, TJF240, or JF260V ERCP endoscopes (Olympus, Tokyo, Japan) were used. The guidewires used in this study were Visiglide 1, Visiglide 2 (Olympus, Tokyo, Japan), Jagwire, Hydra Jagwire (Boston Scientific Japan, Tokyo, Japan), RevoWave (Piolax, Kanagawa, Japan), or Tracer Metro (Cook Japan, Tokyo, Japan). A CleverCut 3V or a Needle Knife device (Boston Scientific Japan, Tokyo, Japan) was used for EST. A SMART (Johnson & Johnson, Tokyo, Japan), JOSTENT (Zeon Medical, Tokyo, Japan), Zilver, Zilver 635 (Cook Japan, Tokyo, Japan), Niti-S D-type, Niti-S large-cell D-type (Taewoong-Medical, Gyeoenggi-do, Korea), Wall, or Wall Flex (Boston Scientific Japan, Tokyo, Japan) device was used as a biliary uncovered metal stent deployment system. A Tandem XL or CONTOUR taper tip, CONTOUR ultra-taper tip, CONTOUR 5-4-3 tip (Boston Scientific Japan), PR-233Q (Olympus), or MTW ERCP catheter taper (MTW Endoskopie, Wesel, Germany) was used as the ERCP catheter. A Soehendra biliary 6-Fr or 7-Fr dilation catheter (Cook Japan) was used as the biliary dilation catheter. An RX Hurricane balloon dilatation catheter (Boston Scientific Japan), Zara EPBD catheter (Kaneka Corporation, Tokyo, Japan), or REN biliary dilation catheter (Kaneka Corporation) was used to dilate the first SEMS lumen or mesh.
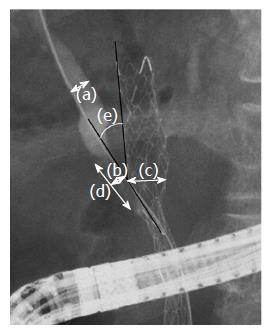
The following variables of the success and failure groups were considered: age, gender, diagnoses causing the biliary stricture (primary lesions or metastases), bismuth classification, diameter of the targeted biliary duct (Figure 2-a), diameter of the targeted biliary stricture (Figure 2-b), diameter of the first implanted SEMS (Figure 2-c), length of the targeted biliary duct stricture (Figure 2-d), angle between the target biliary duct stricture and the first implanted SEMS (Figure 2-e), length of the procedure, clinically effective rate, adverse effects, wire passage of the first SEMS cell, catheter usage to dilate the first SEMS cell, catheter passage of the first SEMS cell, dilator usage to dilate the first SEMS cell, dilator passage of the first SEMS cell, balloon catheter usage to dilate the first SEMS lumen, balloon usage to dilate the first SEMS cell, balloon passage of the first SEMS cell, the number of used dilation devices (0: no dilation device was used-3: catheter, dilator and balloon catheter were all used), type of first SEMS used (braided or laser-cutting type), stenting order, number of ERCP sessions, and the area of the first SEMS cell. Improvement in liver or biliary function (ALT or ALP or bilirubin) within 2 wk after ERCP was determined as “clinically effective”. The area of the first SEMS cell was not available for two patients in the failure group and three patients in the success group.
Student’s t test or Mann-Whitney U test and Fisher’s exact test were used to calculate the significance of the differences between the continuous variables and the nominal scales of the two groups. The Bismuth classifications were analysed using the Mann-Whitney U test. A P value of < 0.05 was considered to indicate a significant difference. All statistical analyses were performed using the EZR platform (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of R commander that was designed to perform functions that are frequently used in biostatistics[16].
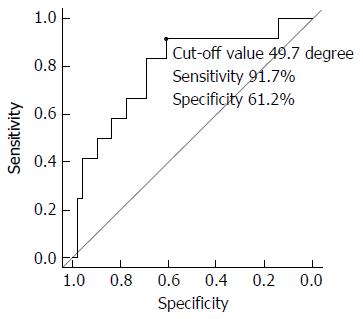
No significant differences in the characteristics of the patients (Table 1) or the details of the target biliary ducts of the success and failure groups (Table 1) were observed with the exception of the following: the angle between the target biliary duct stricture and the first implanted SEMS was significantly different between the success group and the failure group [44.4 (7-119) degree vs 75.3 (28-109.3) degree, P < 0.01]. The ROC curve of the angle between the target biliary duct stricture and the first implanted SEMS revealed a cut-off value of 49.7 degrees with a sensitivity of 91.7% and a specificity of 61.2% for predicting stent-in-stent SEMS placement failure (Figure 3).
| Success (n = 49) | Failure (n = 13) | P value | |
| Age, median (range), yr | 74 (42-88) | 71 (16-76) | 0.45 |
| Sex (male), n (%) | 33 (67.3) | 8 (61.5) | 0.75 |
| Diagnoses | |||
| Primary lesion (Biliary tract or pancreatic cancer) | 40 | 10 | 0.70 |
| Metastases | 9 | 3 | |
| Pancreatic cancer | 1 | ||
| Lung cancer | 1 | ||
| Tracheal cancer | 1 | ||
| Colon cancer | 3 | 2 | |
| Uterine cancer | 1 | ||
| Gastric cancer | 1 | 1 | |
| Prostatic cancer | 1 | ||
| Bismuth classification | 0.98 | ||
| II | 12 | 3 | |
| III | 18 | 5 | |
| IV | 19 | 5 | |
| Target biliary duct status, | |||
| Diameter of the target biliary duct, median (range), mm | 6.4 (2.0-15.9) | 6.6 (4.4-17.3)1 | 0.45 |
| Diameter of the target biliary stricture, median (range), mm | 0 (0-1.6) | 0 (0-0.9) | 0.94 |
| Diameter of the first implanted SEMS, median (range), mm | 5.8 (3.1-11.7) | 6.7 (3.4-12.6) | 0.25 |
| Length of the target biliary stricture, median (range), mm | 11.0 (3.0-69.6) | 7.9 (1.7-34.2)1 | 0.44 |
| Angle between the target biliary duct stricture and the first implanted SEMS, median (range), degree | 44.4 (7-119) | 75.3 (28-109.3)1 | < 0.01 |
Regarding the ERCP procedures and outcomes used for the patients in the two groups, clinically effective rate, the rates of wire passage through the first SEMS cell, catheter passage through the first SEMS cell, dilator passage through the first SEMS cell, and balloon catheter passage through the first SEMS cell in the failure group were significantly lower than in the success group (Table 2). No other variables for the ERCP procedures used for the members of the success and failure groups were significantly different.
| Success (n = 49) | Failure (n = 13) | P value | |
| Procedure time, median (range), min | 70 (20-160) | 90 (40-150)1 | 0.30 |
| Clinically effective rate | 49 (100) | 11 (84.6) | 0.04 |
| Adverse effects | 1 (2) | 1 (7.7) | 0.38 |
| Post-ERCP pancreatitis | 1 | 0 | |
| Perforation of biliary duct | 0 | 1 | |
| Wire passage of the first SEMS cell | 49 (100) | 9 (69.2) | 0.006 |
| Diameter of wire (0.025/0.035) | 32/142 | 6/61 | 0.31 |
| Catheter usage to dilate the a first SEMS cell | 24 | 8 | 0.54 |
| Catheter passage of the first SEMS cell | 22 (92) | 4 (50) | 0.02 |
| Dilator usage to dilate first SEMS cell | 18 | 5 | 1.00 |
| Dilator passage of the first SEMS cell | 17 (94) | 2 (40) | 0.02 |
| Balloon catheter usage to dilate the first SEMS lumen | 5 | 1 | 1.00 |
| Balloon catheter usage to dilate the first SEMS cell | 18 | 3 | 0.51 |
| Balloon catheter passage of the first SEMS cell | 18 (100) | 0 (0) | < 0.001 |
| The number of used dilation devices, median (range) | 1 (0-3) | 1 (0-3) | 0.79 |
| Type of first SEMS used (braided/laser), n | 41/8 | 9/4 | 0.26 |
| Stenting order | 0.22 | ||
| Left→Left | 2 | 1 | |
| Left→Right | 28 | 6 | |
| Right→Left | 12 | 6 | |
| Right→Right | 7 | 0 | |
| Procedure sessions | 0.328 | ||
| 1 | 42 | 13 | |
| 2 | 7 | 0 | |
| Area of first SEMS cell, median (range), mm2 | 18.3 (3.5-39.3) | 18.3 (3.5-18.3)3 | 0.59 |
In this study, we investigated the factors predictive of failure in placing a second SEMS. The angle between the target biliary duct stricture and the first implanted SEMS was significantly associated with failure to place a second SEMS. In fact, failure to pass the devices was significantly associated with unsuccessful endoscopic stent-in-stent SEMS placement.
Few reports have examined the relationship between failure to place an endoscopic stent-in-stent SEMS and the characteristics of the patients. In one report, metastatic disease was found to be related to the failure to place an endoscopic stent-in-stent SEMS[17]. Although a similar number of patients who were evaluated in the previous investigation were analysed in this study, different results were obtained. However, the relationship between the failure to place an endoscopic stent-in-stent SEMS and the obstructive state of the biliary duct has not been reported. In this study, the effect of the precise details of the biliary obstructive state on the placement of an endoscopic stent-in-stent SEMS was evaluated. Among several features regarding the biliary obstructive state, only the angle between the target biliary duct stricture and the first implanted SEMS was significantly different between the success and failure groups. An angle less than 49.7 degrees, according to the ROC curve, predicted high probability of successful placement of a second SEMS.
Several reports have considered the devices used to treat patients with a malignant hilar biliary obstruction. The efficacy of using a slimmer stent or a large-cell metal stent for bilateral stent-in-stent placement has been reported[18-21]; the large-cell metal stent that was used was in these studies was a Niti-S large-cell stent (Taewoong-Medical), and the slimmer stent that was used was a Zilver stent (Cook Japan). We generally used these types of stents to initially treat the patients recruited in this study. The areas of the first SEMS cell placed in the patients of the two groups were not significantly different. This result is consistent with those of a previous study showing that the cell size did not affect successful bilateral drainage[22].
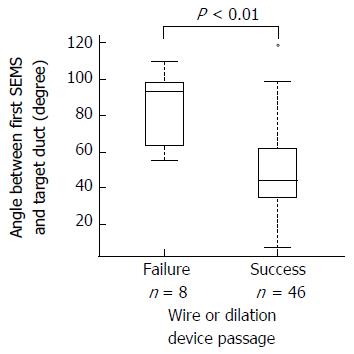
In studies reporting a high success rate for bilateral drainage, a 7-Fr Soehendra dilator (Cook Japan), a 6- to 8-mm CRE wire-guided oesophageal/pyloric balloon dilatation catheter, or a 6- or 8-mm Hurricane RX dilatation catheter (Boston Scientific Japan) was used[18,21]. In this study, these devices and ERCP catheters, such as the Tandem XL cannula (Boston Scientific Japan), were used to pass the first SEMS. To identify the cause underlying the failure of these devices to pass, we evaluated the influence of the angle between the target biliary duct stricture and the first implanted SEMS on wire or dilation catheter passage using the Mann-Whitney U test (Figure 4). The angle was statistically higher in patients in whom the wire or dilation device was unable to pass the first implanted SEMS than in patients in whom the wire or dilation catheter passed the first implanted SEMS [93.0 degrees (55-109.3) vs 44.2 degrees (7-119.0), P < 0.01, median (range)]. Therefore, the findings demonstrate that the angle influences not only the second SEMS placement but also the passage of the wire or dilation device through the first SEMS cell.
Finally, we considered how to increase the success rate of stent-in-stent deployment of SEMSs in patients with larger angles. Upon comparing patients in the success and failure groups with larger angles (Table 3), the diameter of the first implanted SEMS was not significantly different. According to this result, the cause of the failure of the second SEMS insertion was not radial force. The passage of dilation devices was significantly different between the two groups. As described above, we used SEMSs recommended in past reports. Based on this study, we believe that improvements in dilation devices have contributed to overcoming such difficult cases. As the second SEMS insertion was effective in improving liver or biliary function in some patients (clinically effective rate from Table 2), an improvement in dilation devices is desired.
| Success (n = 19) | Failure (n = 12) | P value | |
| Diameter of the first implanted SEMS, median (range), mm | 6.2 (3.1-15.9) | 6.5 (4.4-17.3) | 0.16 |
| Wire passage of the first SEMS cell | 19 (100) | 8 (69.2) | 0.02 |
| Diameter of wire (0.025/0.035) | 12/61 | 5/61 | 0.44 |
| Catheter usage to dilate the a first SEMS cell | 8 | 7 | 0.47 |
| Catheter passage of the first SEMS cell | 87.5 (7/8) | 42.9 (3/7) | 0.12 |
| Dilator usage to dilate first SEMS cell | 6 | 4 | 1.00 |
| Dilator passage of the first SEMS cell | 6 (100) | 1 (25) | 0.03 |
| Balloon catheter usage to dilate the first SEMS lumen | 1 | 1 | 1.00 |
| Balloon catheter usage to dilate the first SEMS cell | 7 | 2 | 0.42 |
| Balloon catheter passage of the first SEMS cell | 7 (100) | 0 (0) | 0.03 |
| The number of used dilation devices, median (range) | 1 (0-2) | 1 (0-3) | 0.76 |
| Type of first SEMS used (braided/laser) | 13/6 | 8/4 | 1.00 |
| Stenting order | 0.53 | ||
| Left→Left | 1 | 1 | |
| Left→Right | 12 | 5 | |
| Right→Left | 6 | 6 | |
| Procedure sessions | 0.27 | ||
| 1 | 16 | 12 | |
| 2 | 3 | 0 | |
| Area of first SEMS cell, median (range), mm2 | 18.3 (3.5-18.3) | 18.3 (3.5-18.3)2 | 0.96 |
A limitation of this study is that it was a retrospective study that involved a small number of patients at one institution. Most reports regarding stenting to treat malignant hilar biliary obstruction consider the devices used here. It would be difficult to conduct a prospective study of the predictive factors for failure to place an endoscopic stent-in-stent SEMS. However, a larger multicentre study should be performed in the future.
In conclusion, we revealed that the angle between the target biliary duct stricture and the first implanted SEMS has an impact on the passing of both wire and dilation devices through the first SEMS cell and the placement of the second SEMS. We determined that a large angle between the target biliary duct stricture and the first implanted SEMS is a predictive risk factor for endoscopic stent-in-stent SEMS placement failure.
We thank all of the staff members of the Department of Gastroenterology of Fukushima Medical University, the Department of Endoscopy of Fukushima Medical University Hospital, and the gastroenterology ward of Fukushima Medical University Hospital. We also thank American Journal Experts for their English proofreading services.
Endoscopic biliary drainage is the first choice for biliary drainage in unresectable malignant biliary obstruction patients. Among drainage stents, metallic stents are more effective than plastic stents. The placement of multiple endoscopic self-expandable metallic stents (SEMSs) to relieve a malignant biliary obstruction is often challenging. Therefore, we investigated the factors predictive of failure when placing a second SEMS.
Regarding endoscopic stent-in-stent SEMS insertion, which stents should be used is major topic of discussion. On the other hand, predictive risk factors of endoscopic stent-in-stent SEMS placement failure have not been sufficiently validated.
This is the first report to investigate the relationship between details of the biliary obstructive state and the failure of endoscopic stent-in-stent SEMS placement. We found that a large angle between the target biliary duct stricture and the first implanted SEMS is a predictive risk factor for endoscopic stent-in-stent SEMS placement failure.
If the risk factors of endoscopic stent-in-stent SEMS placement failure are known, time spent on procedures will not be wasted. The procedure is completed as a unilateral biliary drainage procedure or another rapidly drainage technique, such as percutaneous transhepatic biliary drainage.
Endoscopic retrograde cholangiopancreatography: An endoscope is inserted into the descending portion of the duodenum. After a catheter is inserted into the biliary duct or pancreatic duct, a contrast agent is injected to visualize these ducts under X-ray fluoroscopy. Images of the biliary duct or pancreatic duct are observed. SEMSs: This stent is a metallic stent that can expand after it is placed in the malignant biliary stricture.
This is an interesting retrospective study attempting to identify predictive factors for unsuccessful deployment of a second stent when placement of multiple metal stents (MS) was attempted in the stent-in-stent manner for unresectable malignant perihilar biliary obstruction. The authors evaluated many factors, including etiology, findings of cholangiography, and procedural factors, and concluded that the duller (larger) angle between the first deployed stent and the target duct for next placement was the important factor. This is a very interesting issue and your conclusion seems informative.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kanno Y S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Speer AG, Cotton PB, Russell RC, Mason RR, Hatfield AR, Leung JW, MacRae KD, Houghton J, Lennon CA. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2:57-62. [PubMed] |
| 2. | Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488-1492. [PubMed] |
| 3. | Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 358] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993;25:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 234] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Lammer J, Hausegger KA, Flückiger F, Winkelbauer FW, Wildling R, Klein GE, Thurnher SA, Havelec L. Common bile duct obstruction due to malignancy: treatment with plastic versus metal stents. Radiology. 1996;201:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 163] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 7. | Moses PL, Alnaamani KM, Barkun AN, Gordon SR, Mitty RD, Branch MS, Kowalski TE, Martel M, Adam V. Randomized trial in malignant biliary obstruction: plastic vs partially covered metal stents. World J Gastroenterol. 2013;19:8638-8646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Mukai T, Yasuda I, Nakashima M, Doi S, Iwashita T, Iwata K, Kato T, Tomita E, Moriwaki H. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013;20:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354-362. [PubMed] |
| 10. | De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-553. [PubMed] |
| 11. | De Palma GD, Pezzullo A, Rega M, Persico M, Patrone F, Mastantuono L, Persico G. Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc. 2003;58:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003;58:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Naitoh I, Ohara H, Nakazawa T, Ando T, Hayashi K, Okumura F, Okayama Y, Sano H, Kitajima Y, Hirai M. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Iwano H, Ryozawa S, Ishigaki N, Taba K, Senyo M, Yoshida K, Sakaida I. Unilateral versus bilateral drainage using self-expandable metallic stent for unresectable hilar biliary obstruction. Dig Endosc. 2011;23:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Yasuda I, Mukai T, Moriwaki H. Unilateral versus bilateral endoscopic biliary stenting for malignant hilar biliary strictures. Dig Endosc. 2013;25 Suppl 2:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13141] [Article Influence: 1095.1] [Reference Citation Analysis (0)] |
| 17. | Kawakubo K, Kawakami H, Toyokawa Y, Otani K, Kuwatani M, Abe Y, Kawahata S, Kubo K, Kubota Y, Sakamoto N. Risk factors for technical failure of endoscopic double self-expandable metallic stent placement by partial stent-in-stent method. J Hepatobiliary Pancreat Sci. 2015;22:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kim JY, Kang DH, Kim HW, Choi CW, Kim ID, Hwang JH, Kim DU, Eum JS, Bae YM. Usefulness of slimmer and open-cell-design stents for endoscopic bilateral stenting and endoscopic revision in patients with hilar cholangiocarcinoma (with video). Gastrointest Endosc. 2009;70:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Kogure H, Isayama H, Kawakubo K, Sasaki T, Yamamoto N, Hirano K, Sasahira N, Tsujino T, Tada M, Koike K. Endoscopic bilateral metallic stenting for malignant hilar obstruction using newly designed stents. J Hepatobiliary Pancreat Sci. 2011;18:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Kogure H, Isayama H, Nakai Y, Tsujino T, Ito Y, Yamamoto K, Mizuno S, Yagioka H, Kawakubo K, Sasaki T. Newly designed large cell Niti-S stent for malignant hilar biliary obstruction: a pilot study. Surg Endosc. 2011;25:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Kogure H, Isayama H, Nakai Y, Tsujino T, Matsubara S, Yashima Y, Ito Y, Hamada T, Takahara N, Miyabayashi K. High single-session success rate of endoscopic bilateral stent-in-stent placement with modified large cell Niti-S stents for malignant hilar biliary obstruction. Dig Endosc. 2014;26:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Lee JM, Lee SH, Chung KH, Park JM, Paik WH, Woo SM, Lee WJ, Ryu JK, Kim YT. Small cell- versus large cell-sized metal stent in endoscopic bilateral stent-in-stent placement for malignant hilar biliary obstruction. Dig Endosc. 2015;27:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |