Published online Sep 14, 2017. doi: 10.3748/wjg.v23.i34.6212
Peer-review started: February 9, 2017
First decision: April 21, 2017
Revised: May 12, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: September 14, 2017
Processing time: 222 Days and 5.1 Hours
To investigate association of circulating inflammatory factors at the time of colorectal cancer (CRC) surgery with survival.
Plasma levels from 174 CRC patients (69 females and 105 men), with median age 70 years (range 29-90), localized in the colon (n = 105) or rectum (n = 69), with stage I (n = 24), stage II (n = 54), stage III (n = 67) and stage IV (n = 29) were measured using commercially available Bio-Plex Pro™ Human Chemokine Panel 40-Plex, including 40 different chemokines, cytokines and interleukins. The prognostic association of each inflammatory factor was analysed as CRC-specific and total mortality.
Out of 174 patients, 66 died during the follow-up, 40 because of CRC specific mortality. High tertile levels of 8 factors were significantly associated with increased CRC-specific mortality, of which CCL1, CCL20, CCL24, CX3CL1, IL-4 and TNF-α remained significant in a multivariate Cox regression analysis. High tertile levels of 14 factors were associated with increased total mortality, of which CCL1, CCL15, CCL20, CX3CL1, CXCL13, IFN-γ, IL-2, IL-4 and IL-10 remained significant after adjustment for clinical covariates. For most of the inflammatory factors the association between higher tertile levels and an increased mortality in general appeared two years after surgery. High tertile levels of TNF-α and CCL24 were exclusively associated with CRC-specific mortality. The distribution of these factors were not associated with TNM stage with exception for CCL20.
High plasma levels of inflammatory factors are associated with increased risk of mortality among CRC patients and could be potential biomarkers for revealing prognosis.
Core tip: Plasma levels of 40 different cytokines, chemokines and interleukins were analyzed in colorectal cancer (CRC) patients of which high tertile levels of nine factors were associated with total mortality and six factors with CRC-specific mortality. For most of the inflammatory factors the association between higher tertile levels and an increased mortality in general appeared two years after surgery.
- Citation: Olsen RS, Nijm J, Andersson RE, Dimberg J, Wågsäter D. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol 2017; 23(34): 6212-6219
- URL: https://www.wjgnet.com/1007-9327/full/v23/i34/6212.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i34.6212
Inflammation is of importance in cancer development, and many tumours develop due to prolonged or chronic inflammation throughout their progression[1]. Carcinogenesis in colorectal cancer (CRC) is a multistep process maintained by accumulation of genetic and epigenetic aberrations in several pathways[2,3]. Also, local immunoregulation mediated by inflammatory cells, such as white blood cells, of the tumour microenvironment are involved in the release of inflammatory factors that are able to activate local immune networks to promote both the development and growth of malignant CRC cells by increasing their proliferation, survival and angiogenesis[1,4]. Inflammatory factors such as cytokines together with angiogenic factors are able to trigger the development of invasive abilities as they increase the migration and motility of tumour cells, resulting in the occurrence of metastasis[1]. Cytokines, which include chemokines and interleukins, are a broad and loose category of small proteins produced by white blood cells, stromal cells and cancer cells[1,5]. They are able to regulate the intensity and duration of the immune response by either stimulating or inhibiting the activation, proliferation and/or differentiation of various cells and are also able to regulate the secretion of antibodies and other cytokines[5]. By targeting selected cytokine networks or pathways one may be able to restrain CRC tumorigenesis or even improve the response rate in CRC tumours to chemotherapies, and there are several clinical trials that have focused on evaluating the blockage of different cytokines[6-8].
Increased levels of inflammatory factors have been associated with increased mortality in CRC patients, also in stage I which has a good oncological prognosis, but also in asymptomatic assumed healthy individuals[9-12].
The aim of this paper was to study the association of plasma levels of cytokines, chemokines and interleukins in CRC patients at the time of surgery with survival. The hypothesis is that strong inflammation at the time of surgery is associated with worse prognosis.
This study involved analysis of plasma samples from 174 CRC patients from southeastern Sweden who had undergone surgical resections for primary colorectal adenocarcinoma between 2006-2013 at the Department of Surgery, County Hospital Ryhov, Region of Jönköping County, Jönköping, Sweden. The clinicopathological characteristics of the patients were obtained from surgical and pathological records. Follow-up was performed by consulting the medical records from all hospital departments and the primary care up to January 31, 2016. The date of an eventual cancer recurrence and the date and cause of death as related to CRC-specific mortality or not were determined from a review of the patient’s files. The study was approved by the Regional Ethical Review Board in Linköping, Linköping, Sweden, and written informed consent was obtained from each patient.
Venous blood samples were collected at the time of surgery and centrifuged to separate plasma and blood cells. Plasma was stored at -80 °C until analysis. Plasma samples were available from 174 patients (69 females and 105 men), and their median age was 70 years (range 29-90). The patients’ tumours were localized in the colon (n = 105) or rectum (n = 69) and were classified as stage I (n = 24), stage II (n = 54), stage III (n = 67) and stage IV (n = 29).
Diluted plasma (1:4) from 174 CRC patients and an eight-point standard curve were analysed for each of the 40 factors using a commercial Bio-Plex Pro™ Human Chemokine Panel 40-Plex (Bio-Rad Laboratories, Inc., CA, United States) including chemokines, cytokines and interleukins according to the manufacturer’s recommendations. Magnetic separation was performed using the Bio-Plex Pro Wash Station (Bio-Rad Laboratories). Bead fluorescence readings were taken using the Bio-Plex Manager version 6.1.0.727 (Bio-Rad Laboratories) with Low PMT (Low RP1) setting on the Bio-Plex 200 System (Bio-Rad Laboratories). The results are presented as pg/mL and grouped into tertiles defined as low, middle or high tertile.
A Shapiro-Wilk test was used to determine the normal distribution. The Pearson χ2 test was used to determine differences in distribution of covariates; age, gender, localization, cancer recurrence, radical surgery, TNM stage, preoperative treatment, and adjuvant treatment between patients with CRC-specific mortality compared to survivors or deceased by other causes. An association of the inflammatory variables with TNM stage was analysed by comparing the distribution of inflammatory factors using Jonkheere Terpstra test. The association of age, sex, tumour localization, TNM stage, local radical resection, pre- and postoperative adjuvant treatment and tertiles of the examined inflammatory variables with CRC specific and total mortality were performed with Kaplan-Meier curves, log-rank test and Cox’s regression analysis. Both univariate and multivariate Cox regression analysis were performed. The proportional hazard assumption was verified by visual inspection of log-log plots. The statistical analyses were performed using the SPSS for Windows computer package (IBM® SPSS® Statistics, 2012, version 21, SPSS Inc., Chicago, IL, United States).
The clinical baseline characteristics of the total study population are presented in Table 1. Out of 174 patients, 40 died because of CRC. Thirty five of these were stage III and IV patients. Twenty-six of the 174 patients died from other causes and 108 were still alive at the end of follow-up. Frequency of radical surgery, TNM stage and adjuvant treatment differed significantly between patients with CRC-specific mortality compared with survivors or deceased by other causes. On the other hand, age, gender, localization of the cancer and preoperative treatment did not differ between the groups. A threshold of P < 0.20 was set for the covariates used in the adjustment of statistical analyses. Age was included in the adjusted model since mortality is highly associated with increased age in general.
| CRC patients | Others (survivors or deceased by other causes) | CRC specific mortality | P value | |
| n = 174 | n = 134 (77) | n = 40 (23) | ||
| Age | 70 (range 29-90) | 70 (29-90) | 72 (36-90) | 0.2641 |
| Gender | 105 men (60) | 83 men (62) | 22 men (55) | 0.431 |
| Localization | 105 colon (60) | 78 colon (58) | 27 colon (68) | 0.292 |
| Cancer recurrence | 34 (20) | 16 (12) | 18 (45) | < 0.001 |
| Radical surgery | 165 (95) | 130 (97) | 35 (88) | < 0.051 |
| TNM stage I/II/III/IV | 24/54/67/29 (14/31/38/17) | 22/51/54/7 (17/38/40/5) | 2/3/13/22 (5/8/32/55) | < 0.0011 |
| Preoperative treatment | 41 (24) | 36 (27) | 5 (13) | 0.0601 |
| Adjuvant treatment | 71 (41) | 47 (35) | 24 (60) | 0.0051 |
| Total mortality | 66 (38) | 26 (19) | 40 (100) | < 0.001 |
Each of the inflammatory factors were tested for any eventual association with TNM stage by the Jonkheere Terpstra test.Only CCL20, CCL27, IL-8 and MIF were associated with TNM stage (Table 2).
| Factor | TNM stage I (n = 24), Median1 (range) | TNM stage II (n = 54), Median1 (range) | TNM stage III (n = 67), Median1 (range) | TNM stage IV (n = 29), Median1 (range) | P value |
| CCL20 | 100 (77-242) | 119.5 (80-272) | 124 (89-183) | 149 (111-378) | 0.034 |
| CCL27 | 11956 (9975-15131) | 11293 (8799-14133) | 10308 (8173-13923) | 9461 (7619-12283) | 0.035 |
| IL-8 | 99 (81-439) | 113 (97-146) | 104 (85-153) | 166 (113-243) | 0.009 |
| MIF | 19565 (13546-33372) | 23720 (14452-43786) | 21900 (14146-33330) | 47235 (20723-89267) | 0.031 |
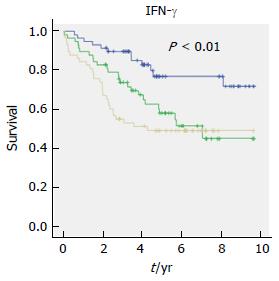
In a univariate Cox regression analysis, the highest tertile levels of 14 factors, CCL1, CCL3, CCL15, CCL20, CX3CL1, CXCL1, CXCL10, CXCL13, IFN-γ, IL-1β, IL-2, IL-4, IL-8/CXCL8 and IL-10, were significantly associated with total mortality (Table 3). CX3CL1 had the highest hazard ratio (HR) of 3.3 with a 95%CI of 1.8-6.1, P < 0.001, for the highest tertile. Nine of the factors in the univariate analysis, CCL1, CCL15, CCL20, CX3CL1, CXCL13, IFN-γ, IL-2, IL-4 and IL-10, remained significant after adjustment of clinical covariates with P < 0.20, such as TNM stage, radical surgery, preoperative- and adjuvant treatment and age. Figure 1 shows an example of a Kaplan-Meier analysis of plasma levels of IFN-γ and an increased risk of total mortality. The Kaplan-Meier curves illustrate mortality as a function of follow-up time in relation to tertile levels.
| Factor | Total mortality | Total mortality adjusted1 | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 1.0 (1.0-1.1) | 0.006 | 1.1 (1.0-1.1) | < 0.001 |
| TNM stage II | 1.5 (0.5-4.0) | 0.464 | 1.2 (0.4-3.2) | 0.777 |
| TNM stage III | 1.8 (0.7-4.8) | 0.228 | 2.2 (0.8-6.1) | 0.141 |
| TNM stage IV | 6.8 (2.6-17.9) | < 0.001 | 14.6 (6.0-42.6) | < 0.001 |
| Radical surgery | 0.2 (0.1-0.4) | < 0.001 | 0.3 (0.1-0.6) | 0.002 |
| Preoperative treatment | 0.8 (0.4-1.5) | 0.454 | 0.7 (0.4-1.4) | 0.338 |
| Adjuvant treatment | 1.1 (0.6-1.7) | 0.842 | 0.7 (0.4-1.3) | 0.226 |
| CCL1 | 3.2 (1.7-5.9) | < 0.001 | 2.7 (1.4-5.4) | 0.004 |
| CCL3 | 2.0 (1.1-3.6) | 0.030 | 1.2 (0.6-2.2) | 0.605 |
| CCL15 | 2.3 (1.2-4.3) | 0.010 | 1.9 (1.0-3.7) | 0.046 |
| CCL20 | 3.1 (1.6-6.0) | 0.001 | 2.2 (1.1-4.3) | 0.021 |
| CCL26 | 1.7 (1.0-3.0) | 0.055 | 1.7 (0.9-3.0) | 0.078 |
| CX3CL1 | 3.3 (1.8-6.1) | < 0.001 | 2.3 (1.2-4.5) | 0.014 |
| CXCL1 | 1.9 (1.0-3.4) | 0.038 | 1.4 (0.8-2.6) | 0.295 |
| CXCL10 | 2.1 (1.1-4.0) | 0.017 | 1.3 (0.7-2.6) | 0.387 |
| CXCL13 | 1.8 (1.1-3.2) | 0.033 | 2.0 (1.0-3.7) | 0.039 |
| CXCL16 | 1.6 (0.9-2.8) | 0.139 | 1.7 (0.9-3.1) | 0.113 |
| IFN-γ | 3.1 (1.6-6.1) | 0.001 | 3.5 (1.6-7.5) | 0.001 |
| IL-1β | 2.1 (1.2-3.8) | 0.012 | 1.7 (0.9-3.1) | 0.081 |
| IL-2 | 2.2 (1.2-4.2) | 0.011 | 2.7 (1.4-5.4) | 0.005 |
| IL-4 | 2.4 (1.2-4.6) | 0.014 | 2.3 (1.1-4.5) | 0.018 |
| IL-8/CXCL8 | 2.6 (1.4-4.6) | 0.002 | 1.6 (0.9-3.0) | 0.115 |
| IL-10 | 2.2 (1.2-4.1) | 0.014 | 2.3 (1.2-4.6) | 0.014 |
| TNF-α | 1.6 (0.9-2.8) | 0.104 | 1.5 (0.8-2.6) | 0.202 |
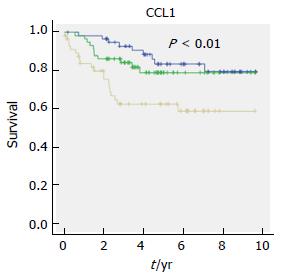
When investigating the CRC specific mortality among the inflammatory factors, the univariate Cox regression analysis revealed that the highest tertile levels of 8 factors, CCL1, CCL3, CCL15, CCL20, CX3CL1, CXCL16, IL-4 and IL-8/CXCL8, were significantly associated with CRC specific mortality (Table 4). CCL20 showed the highest HR of 4, CI of 1.6-10.1, P < 0.01. After adjustment for clinical covariates with P < 0.20, only 4 factors remained significant, CCL1, CCL20, CX3CL1 and IL-4. In addition, TNF-α and CCL24 became significant after this adjustment. Kaplan-Meier analysis of CRC specific mortality and tertile levels of CCL1 is shown in Figure 2.
| Factor | CRC specific mortality | CRC specific mortality adjusted1 | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age | 1.0 (1.0-1.0) | 0.532 | 1.1 (1.0-1.1) | 0.006 |
| TNM stage II | 0.7 (0.1-4.1) | 0.674 | 0.5 (0.1-3.0) | 0.444 |
| TNM stage III | 2.6 (0.6-11.7) | 0.201 | 2.7 (0.6-12.9) | 0.209 |
| TNM stage IV | 15.5 (3.6-66.3) | < 0.001 | 27.6 (5.8-131.1) | < 0.001 |
| Radical surgery | 0.2 (0.1-0.5) | 0.001 | 0.19 (0.1-0.5) | 0.001 |
| Preoperative treatment | 0.5 (0.2-1.2) | 0.106 | 0.4 (0.1-0.9) | 0.359 |
| Adjuvant treatment | 2.2 (1.2-4.2) | 0.013 | 0.9 (0.5-2.0) | 0.947 |
| CCL1 | 3.2 (1.4-7.0) | 0.004 | 2.8 (1.1-7.1) | 0.025 |
| CCL3 | 2.4 (1.1-5.3) | 0.036 | 1.2 (0.5-2.8) | 0.653 |
| CCL15 | 2.8 (1.3-6.1) | 0.011 | 2.0 (0.9-4.9) | 0.107 |
| CCL20 | 4.0 (1.6-10.1) | 0.003 | 2.7 (1.0-7.0) | 0.046 |
| CCL24 | 2.2 (1.0-4.8) | 0.061 | 2.5 (1.1-5.7) | 0.037 |
| CX3CL1 | 3.7 (1.6-8.3) | 0.002 | 2.6 (1.1-6.4) | 0.036 |
| CXCL16 | 2.5 (1.1-5.4) | 0.022 | 2.0 (0.8-4.8) | 0.138 |
| IFN-γ | 1.8 (0.8-3.9) | 0.138 | 1.7 (0.7-4.1) | 0.253 |
| IL-1β | 1.9 (0.9-3.8) | 0.090 | 1.7 (0.8-3.6) | 0.193 |
| IL-4 | 2.5 (1.1-5.7) | 0.033 | 2.4 (1.0-5.5) | 0.048 |
| IL-8/CXCL8 | 3.3 (1.5-7.3) | 0.003 | 1.5 (0.6-3.5) | 0.344 |
| TNF-α | 2.0 (0.9-4.2) | 0.078 | 2.3 (1.0-5.4) | 0.047 |
In summary, higher tertile levels of the inflammatory factors CCL1, CCL20, CX3CL1 and IL-4 were all associated with increased risk of both total and CRC-specific mortality after Cox regression analysis when adjusted for clinical covariates. Higher tertile levels of TNF-α and CCL24 were exclusively associated with CRC-specific mortality. For most of the inflammatory factors the association between higher tertile levels and an increased mortality in general appeared two years after surgery. Twenty three inflammatory factors did not have any association with CRC-specific or total mortality when studying the follow-up time in relation to the tertile levels of these factors (Table 5).
| Factor | Factor |
| CCL2 | CCL27 |
| CCL7 | CXCL2 |
| CCL8 | CXCL5 |
| CCL11 | CXCL6 |
| CCL13 | CXCL9 |
| CCL17 | CXCL11 |
| CCL19 | CXCL12 |
| CCL21 | GM-CSF |
| CCL22 | IL-6 |
| CCL23 | IL-16 |
| CCL25 | MIF |
| CCL26 |
In this study, we screened plasma samples from 174 CRC patients for 40 different inflammatory factors using a commercial multiplex kit, which allow simultaneous quantification of several factors of interest, to determine whether the plasma levels of these factors were associated with CRC prognosis.
We found that high tertile levels of CCL1, CCL20, CX3CL1 and IL-4 were associated with increased CRC-specific mortality in a multivariate Cox regression analysis. We also found an increased total mortality in association of high tertile levels of CCL1, CCL15, CCL20, CX3CL1, CXCL13, IFN-γ, IL-2, IL-4 and IL-10.
Several studies have focused on higher or lower levels of some of the inflammatory factors included in our study such as CCL15, CCL20, CX3CL1, CXCL13, CXCL16, IL-4, IL-8/CXCL8, IL-10 and TNF-α by either comparing expression levels in tissue, in serum or in plasma from CRC patients and controls or among patients only[13-22]. Also, expression levels of some of these factors have been studied in relation to survival[13-15,17,18,20-22].
More recent studies have found an association of increased levels of inflammatory markers and increased mortality in CRC patients, but also in CRC patients stage I and in asymptomatic healthy individuals[9-12]. Our findings of a worse prognosis in association with increased level of inflammation may therefore be a more general phenomenon and not directly related to the cancer disease. As mentioned, the local immunoregulation mediated by inflammatory cells, such as white blood cells, of the tumour microenvironment are important for the release of inflammatory factors such as cytokines, which are able to activate local immune networks to promote both the development and growth of malignant CRC cells[1,4]. White blood cells play an important part in immunoregulation but play dual roles. They are supposed to defeat the cancer development by producing cytokines and activating inflammatory signalling pathways enabling necrosis or apoptosis of cancer cells. But the cancer itself may also induce an immunomodulation of the white blood cells, making them unable to produce cytokines and other inflammatory factors. In this way, the cancer cells avoid inflammatory recognition and are then able to continue their development[1,4].
The CX3CL1 chemokine is expressed by epithelial cells in both CRC and normal colorectal mucosa. Higher levels of this chemokine have been associated with better prognosis and higher survival rate in CRC patients, depending on anti-tumour immunity through a higher number of attracted lymphocytes[16]. This is contradictory to our findings in the univariate analysis, which show an association between higher tertile levels of CX3CL1 and a > 3.5-fold increased risk for CRC-specific mortality among our CRC patients.
The CCL15 chemokine has a strong chemotactic activity for myeloid cells such as dendritic cells, monocytes, neutrophils and some T-lymphocytes[23]. In a study by Inamoto et al[13] a trend between higher levels of CCL15 and poor survival among CRC patients was observed. In this study we confirm that a higher inflammatory tertile level of CCL15 is related to CRC-specific mortality when we do not include age as a covariate in the statistical analysis.
Expression of the CCL20 chemokine has been demonstrated in dendritic cells, macrophages, eosinophilic granulocytes and in B- and T-cell lymphocytes, as reviewed by Schutyser et al[24]. In CRC, higher serum levels of CCL20 may serve as a potential biomarker for prognosis. It may also be useful for identification of patients with increased risk of disease recurrence in stage II CRC[14]. In the present investigation, higher tertile levels of CCL20 in plasma is associated with a 4-fold increased risk for CRC-specific mortality in the univariate analysis, which could be explained by a relation to TNM stage.
IL-4 is produced by basophils, activated T-lymphocytes and mast cells and seems to be upregulated in CRC[25,26]. It has been suggested that IL-4 might be involved in the process of supporting the tumour-initiating cells, enabling them to escape immune surveillance and in turn promote CRC progression[19]. Our data show that the highest tertile level of IL-4 is associated with an increased risk of CRC specific mortality as a result of ongoing CRC progression over time.
The CCL1 chemokine is secreted from fibroblasts[27] and Th2 cells[28]. CCL1 has been implicated in other types of cancer but little is known about its effects on CRC[27]. The Th2-cells express the CCR8 receptor[29,30], which is activated by CCL1[31], and it mediates Th2 cell recruitment to sites of inflammation[32,33]. In cancer, the CCL1-CCR8 autocrine loop has been shown to have a protective function by enabling lymphoma and T cell leukaemia cells to avoid apoptosis in vitro[34,35] and to play a role in T cell transformation[36]. In this study, higher tertile levels of this chemokine were associated with a 2.8 fold increased risk for CRC-specific mortality and one might speculate that the CCL1-CCR8 autocrine loop may help CRC cells to progress their development and spread. Due to our results CCL1 might be a new important factor to consider in further studies regarding its implications on CRC.
There are several limitations identified in our study that need to be taken into consideration. First, a control group was not included in the present work since our focus was on survival among CRC patients, making us unable to study differences and/or associations in levels of the inflammatory factors among patients and healthy individuals. However, there are already several studies that have investigated differences in levels of cytokines among both patients and healthy control subjects[37,38]. Second, our cohort was relatively small influencing the statistical evaluation and especially associations with stage, which was weak due to low power. This also makes it difficult to stratify the patients into respect to more variables such as type of surgery, with inflammatory complicated CRC such as peritumorous abscess, perforation or peritonitis, or inflammatory bowel disease. Third, it is also important to realize that the level of inflammatory factors in the circulation might reflect the release of factors during cancer carcinogenesis, due to other underlying diseases or by systemic inflammation in general. In this study we did not have the possibility to investigate this.
Future aspects should focus on studying these inflammatory factors in a larger CRC patient cohort to see if they might have the potential as biomarkers that can be measured through a rapid, simple, non-invasive and less costly plasma analysis enabling the identification of CRC patients with worse prognosis. Functional studies are needed to elucidate weather these are causative factors for tumour progression or a biomarker for CRC prognosis or a marker for a general fragility.
In summary, high tertile levels of a range of chemokines, cytokines and interleukins are associated with a worse prognosis in patients operated for CRC, both as expressed as cancer specific mortality (CCL1, CCL20, CCL24, CX3CL1, IL-4 and TNF-α) and total mortality (CCL1, CCL15, CCL20, CX3CL1, CXCL13, IFN-γ, IL-2, IL-4 and IL-10). This suggests that the observed association of a worse prognosis in CRC patients with an increased level of inflammation may not only be associated to the cancer disease but expression of a fragile host.
Inflammation is of importance in cancer development, and many tumours develop due to prolonged or chronic inflammation throughout their progression.
Several studies have previously investigated expression of some inflammatory factors in circulation of patients with colorectal cancer (CRC).
This study analyzed 40 different inflammatory factors at the same time in plasma from CRC patients which was investigated in multivariate Cox regression analysis taken into account clinical co-variates.
The identification of high levels of several factors associated with CRC specific mortality makes them interesting targets for further studies and as potential targets for immune therapy. The results further support that CRC progression is an inflammatory active condition.
Chemokines are a family of small cytokines that are secreted by a variety of cells and can attract cells expressing the corresponding receptor, normally leukocytes.
The authors in this study investigated whether the circulating inflammatory factors were associated with worse long-term prognosis in CRC. The results showed that high plasma levels of inflammatory factors were associated with an increased risk of total and CRC specific mortality among CRC patients. The whole manuscript is well designed and used a fluency style.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hua D, Ju SQ, Sokolov M S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5745] [Article Influence: 239.4] [Reference Citation Analysis (0)] |
| 2. | Lengauer C, Kinzler KW, Vogelstein B. DNA methylation and genetic instability in colorectal cancer cells. Proc Natl Acad Sci USA. 1997;94:2545-2550. [PubMed] |
| 3. | Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 1606] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 4. | Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727-740. [PubMed] |
| 5. | Gallin J, Snyderman R, Fearon DT, Haynes BF, Nathan C. Inflammation: Basic Principles and Clinical Correlates. 3rd ed. Philadelphia: Lippincott William Wilkins, 1999. . |
| 6. | Jatoi A, Dakhil SR, Nguyen PL, Sloan JA, Kugler JW, Rowland KM Jr, Soori GS, Wender DB, Fitch TR, Novotny PJ, Loprinzi CL. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer. 2007;110:1396-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Angevin E, Tabernero J, Elez E, Cohen SJ, Bahleda R, van Laethem JL, Ottensmeier C, Lopez-Martin JA, Clive S, Joly F. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2192-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Shantha Kumara HM, Myers EA, Herath SA, Jang JH, Njoh L, Yan X, Kirchoff D, Cekic V, Luchtefeld M, Whelan RL. Plasma monocyte chemotactic protein-1 remains elevated after minimally invasive colorectal cancer resection. World J Gastrointest Oncol. 2014;6:413-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Mansouri D, Powell AG, Park JH, McMillan DC, Horgan PG. Long-Term Follow-Up of Patients Undergoing Resection of TNM Stage I Colorectal Cancer: An Analysis of Tumour and Host Determinants of Outcome. World J Surg. 2016;40:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Watt DG, McSorley ST, Park JH, Horgan PG, McMillan DC. A Postoperative Systemic Inflammation Score Predicts Short- and Long-Term Outcomes in Patients Undergoing Surgery for Colorectal Cancer. Ann Surg Oncol. 2017;24:1100-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative Systemic Inflammatory Response, Complication Severity, and Survival Following Surgery for Colorectal Cancer. Ann Surg Oncol. 2016;23:2832-2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Singh-Manoux A, Shipley MJ, Bell JA, Canonico M, Elbaz A, Kivimäki M. Association between inflammatory biomarkers and all-cause, cardiovascular and cancer-related mortality. CMAJ. 2017;189:E384-E390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Inamoto S, Itatani Y, Yamamoto T, Minamiguchi S, Hirai H, Iwamoto M, Hasegawa S, Taketo MM, Sakai Y, Kawada K. Loss of SMAD4 Promotes Colorectal Cancer Progression by Accumulation of Myeloid-Derived Suppressor Cells through the CCL15-CCR1 Chemokine Axis. Clin Cancer Res. 2016;22:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Iwata T, Tanaka K, Inoue Y, Toiyama Y, Hiro J, Fujikawa H, Okugawa Y, Uchida K, Mohri Y, Kusunoki M. Macrophage inflammatory protein-3 alpha (MIP-3a) is a novel serum prognostic marker in patients with colorectal cancer. J Surg Oncol. 2013;107:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Dimberg J, Dienus O, Löfgren S, Hugander A, Wågsäter D. Polymorphisms of Fractalkine receptor CX3CR1 and plasma levels of its ligand CX3CL1 in colorectal cancer patients. Int J Colorectal Dis. 2007;22:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Ohta M, Tanaka F, Yamaguchi H, Sadanaga N, Inoue H, Mori M. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. Int J Oncol. 2005;26:41-47. [PubMed] |
| 17. | Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D, Wu HR. Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer. Eur Rev Med Pharmacol Sci. 2014;18:1916-1924. [PubMed] |
| 18. | Matsushita K, Toiyama Y, Tanaka K, Saigusa S, Hiro J, Uchida K, Inoue Y, Kusunoki M. Soluble CXCL16 in preoperative serum is a novel prognostic marker and predicts recurrence of liver metastases in colorectal cancer patients. Ann Surg Oncol. 2012;19 Suppl 3:S518-S527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Volonté A, Di Tomaso T, Spinelli M, Todaro M, Sanvito F, Albarello L, Bissolati M, Ghirardelli L, Orsenigo E, Ferrone S. Cancer-initiating cells from colorectal cancer patients escape from T cell-mediated immunosurveillance in vitro through membrane-bound IL-4. J Immunol. 2014;192:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Di Caro G, Carvello M, Pesce S, Erreni M, Marchesi F, Todoric J, Sacchi M, Montorsi M, Allavena P, Spinelli A. Circulating Inflammatory Mediators as Potential Prognostic Markers of Human Colorectal Cancer. PLoS One. 2016;11:e0148186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Al Obeed OA, Alkhayal KA, Al Sheikh A, Zubaidi AM, Vaali-Mohammed MA, Boushey R, Mckerrow JH, Abdulla MH. Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J Gastroenterol. 2014;20:18390-18396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 22. | Stanilov N, Miteva L, Dobreva Z, Stanilova S. Colorectal cancer severity and survival in correlation with tumour necrosis factor-alpha. Biotechnol Biotechnol Equip. 2014;28:911-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Forssmann U, Mägert HJ, Adermann K, Escher SE, Forssmann WG. Hemofiltrate CC chemokines with unique biochemical properties: HCC-1/CCL14a and HCC-2/CCL15. J Leukoc Biol. 2001;70:357-366. [PubMed] |
| 24. | Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409-426. [PubMed] |
| 25. | Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 321] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 4896] [Article Influence: 257.7] [Reference Citation Analysis (0)] |
| 27. | Yeh CR, Hsu I, Song W, Chang H, Miyamoto H, Xiao GQ, Li L, Yeh S. Fibroblast ERα promotes bladder cancer invasion via increasing the CCL1 and IL-6 signals in the tumor microenvironment. Am J Cancer Res. 2015;5:1146-1157. [PubMed] |
| 28. | Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547-551. [PubMed] |
| 29. | Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med. 2004;200:725-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 30. | Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875-883. [PubMed] |
| 31. | Roos RS, Loetscher M, Legler DF, Clark-Lewis I, Baggiolini M, Moser B. Identification of CCR8, the receptor for the human CC chemokine I-309. J Biol Chem. 1997;272:17251-17254. [PubMed] |
| 32. | Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med. 2001;193:573-584. [PubMed] |
| 33. | Islam SA, Chang DS, Colvin RA, Byrne MH, McCully ML, Moser B, Lira SA, Charo IF, Luster AD. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nat Immunol. 2011;12:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 34. | Van Snick J, Houssiau F, Proost P, Van Damme J, Renauld JC. I-309/T cell activation gene-3 chemokine protects murine T cell lymphomas against dexamethasone-induced apoptosis. J Immunol. 1996;157:2570-2576. [PubMed] |
| 35. | Ruckes T, Saul D, Van Snick J, Hermine O, Grassmann R. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood. 2001;98:1150-1159. [PubMed] |
| 36. | Tamgüney G, Van Snick J, Fickenscher H. Autocrine stimulation of rhadinovirus-transformed T cells by the chemokine CCL1/I-309. Oncogene. 2004;23:8475-8485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Hillenbrand A, Fassler J, Huber N, Xu P, Henne-Bruns D, Templin M, Schrezenmeier H, Wolf AM, Knippschild U. Changed adipocytokine concentrations in colorectal tumor patients and morbidly obese patients compared to healthy controls. BMC Cancer. 2012;12:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Thorsen SB, Lundberg M, Villablanca A, Christensen SL, Belling KC, Nielsen BS, Knowles M, Gee N, Nielsen HJ, Brünner N. Detection of serological biomarkers by proximity extension assay for detection of colorectal neoplasias in symptomatic individuals. J Transl Med. 2013;11:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |