INTRODUCTION
Double balloon enteroscopy (DBE) is an endoscopic technique for diagnosis and treatment of small bowel diseases[1]. This variety of deep enteroscopy allows a complete exploration of the small bowel by alternatively combining the inflation and deflation of two balloons placed at the tip of the tube and the overtube following a precise sequence of push and pull manoeuvres, which allow the endoscope to progress deep into the small bowel[2]. DBE is not only exploratory but also a therapeutic technique which after more than fifteen years has even been considered as the “gold standard” technique for the endoscopic exploration of the small bowel. Furthermore, the combination of the endoscopic capsule and DBE has been highlighted as the most effective tool for diagnosis and treatment of diseases affecting the small bowel[3]. This was openly stated in the “guideline” published by the ESGE in 2008[4,5] and has also been largely reported in the scientific literature. However, as in any other endoscopic technique complications can appear, and among them post-procedure pancreatitis (post-DBE) is within the most severe.
There is a large series of studies describing direct and indirect complications associated to DBE[6]. In a multicentric study involving 10 endoscopy units from four different continents[7], a total of 40 complications from a total of 2362 DBE were reported (1.7%). This number is within the range of complication currently associated to the therapeutic conventional endoscopy techniques. Concerning the post-DBE pancreatitis several case-series published in Europe[8,9], Asia[7,10,11] and United States[12] refer figures as low as 0.3%, and in the opposite, as high as 3%[13-15] or even 12.5%[16]. Overall, in a recent paper, Kopacova reported a historic accumulation of more than 100 post-DBE pancreatitis already published[17].
According to the international consensus of classification of acute pancreatitis[18,19], the diagnosis requires two of the following three items: (1) abdominal pain consistent with acute pancreatitis; (2) serum lipase activity or amylase activity at least three times greater than the upper limit of normal; and (3) characteristic findings of acute pancreatitis on contrast-enhanced computed tomography (CT) and less commonly magnetic resonance imaging (MRI) or transabdominal ultrasonography. In clinical practice suspicion of post-DBE pancreatitis must always be ruled out before patients are delivered home. Severe pancreatitis is not difficult to recognize, however the mild form may be a problem. Mild acute pancreatitis is characterized by the absence of organ failure and the absence of local or systemic complications. Patients with subclinical mild acute pancreatitis are usually discharged during the early phase and usually do not require pancreatic imaging, and mortality is very rare. Besides many of mild pancreatitis could be missed in patients with DBE performed on an outpatient basis[17]. It is unknown how many patients with self-controlled abdominal pain have been able to have mild acute pancreatitis post-DBE[17]. This circumstance of lack of patient follow-up suggests to some authors a generalized underestimation of the number of post-DBE pancreatitis[13].
Increased levels of one or more of the pancreatic serum markers is very common after oral DBE[16,20,21]. Thus, amylase concentration below the double of the basal levels has been found in 39%[15], 46%[21] or even 58%[20] of oral DBE at 24 h of procedure. Hyperlipasemia has been described from as soon as 4 h[16,17,20,22], to up 12 h[16] or 24 h[16,17,20] after DBE. Also, in patients where pancreatic disease was diagnosed lipase levels remained increased even after amylase returned to normal figures[23]. The C-reactive protein (CRP) can also be related to either non-specific inflammation of the bowel mucosa or pancreatic inflammation, thus its diagnostic significance very much depends on the concomitant appearance of increased levels of amylase and/or lipase[14,16]. Regarding the evolution of these three serum markers, in a recent study, we determined pre- and post-procedure (4 h) levels of amylase, lipase and CRP in a series of 55 randomly selected patients for oral DBE (non-published data). Results in demonstrate a significant increase of the three markers after 4h of DBE. Contrastingly, neither the age, sex, geographical origin, insertion depth and duration of the procedure, nor the type of procedure (diagnostic vs therapeutic) showed any significant correlation with the levels of the pancreatic markers.
Up to here, the most common evidences for suspicion and diagnosis of the oral post-DBE pancreatitis have been summarized. However, despite more than 15 years of worldwide DBE practice the ethiopathogenesis of the post-DBE pancreatitis is not yet completely clarified. A shared belief in the literature is that the cause of this grave complication is associated to the technique itself[14,20,21,24,25]. Thus, a combination of different circumstances such as mechanical stress by the instruments (balloons, mainly) or the push and pull manoeuvres, anatomical features of the pancreas and its vascularization, and undetermined conditions of the patient could be implicated in each case[2,10,20,24-26]. Nevertheless, up to three different hypotheses have been formulated. One refers to a reduced blood perfusion of the pancreas and the bowel (vascular etiology)[14,24,27], another to obstruction of the duodenal papilla by mechanical pressure or damage (papilla injury etiology)[13,15,16,21,25,28-30], and the third one to potential pancreatic reflux due to increased intraluminal pressure within the small bowel (reflux etiology)[24,31]. To better understanding the problem in the last decade we have carried out several of experiments in porcine model[32-39] where oral DBE were systematically monitored. Although the porcine model was previously used both ex vivo and in vivo for DBE training and to increase the security conditions in translational studies[2,35,36], no previous in vivo studies were directly aimed at finding out the etiology of the post-DBE pancreatitis. In the present work, we are summarizing our findings[33,34,39], with addition of some unpublished results, and reviewing the related literature in order to gather substantial evidences supporting the vascular etiology as the most likely explanation for the potential appearance of pancreatitis after oral DBE.
VASCULAR ETIOLOGY: REDUCED PANCREATIC AND INTESTINAL PERFUSION DURING DBE BY ORAL APPROACH
A broad number of scientific studies have suggested this cause[14,24,27]. This hypothesis establishes that during DBE the continuous repetition of push and pull manoeuvres progressively recoils the root of the mesentery such that the vascularization supplying the pancreas and the first portion of the small bowel is significantly reduced[14,24,27]. The increasing ischemia causes different degrees of cellular damage and a reactive condition leading to subclinical or occasionally clinical pancreatitis after the procedure. Also, the potential compression of the pancreas and its vascularization against the vertebral spine during the exploration may intensify the damage to this organ and increase the risk of inflammation[26]. The tail and body of the pancreas are the portions less mobile and more susceptible to such an ischemic and mechanical stress, which may explain why these portions are the most frequently affected in many cases of clinically diagnosed post-DBE pancreatitis[20,21,25,40].
Evidences of structural disorders and hypoxia in the pancreas
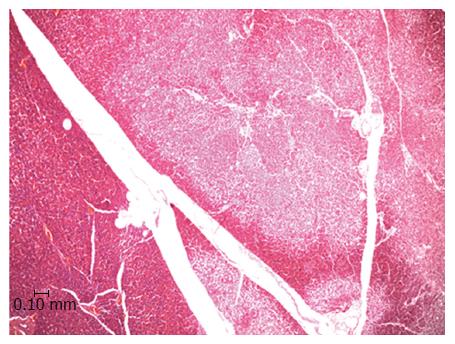
In an experiment set up already published oral DBE were performed in Large White pigs[33]. Serum levels of pancreatic markers (amylase, lipase and CRP) were monitored before, during and after (24 h and 7 d) the procedure, which lasted for either 90 (n = 10) or 140 min (n = 10). In order to avoid any pressure over the duodenal papilla the balloons were never inflated until the Treitz ligament was reached. Amylase, lipase and CRP levels at 24 h post-DBE were significantly increased, however none of the pigs showed any clinical symptom of pancreatitis after the procedure. Besides, no association between the serum markers and the duration of the procedure or the total explored distance was found. Seven days post-DBE the pigs were euthanized and the pancreases removed and processed for histopathological study. A remarkable result was that 47.4% of the animals showed pancreatic lesions which consisted in disseminated focal areas of ischemic type tissue necrosis with inflammatory cell infiltration around the necrotic foci. There was a lack of dilation of the pancreatic ducts. The lesions mainly affected the exocrine component and always affected the tail of the pancreas (Figure 1). The pancreatic tail (left lobe in pigs) was preferentially damaged because it is the portion with the most limited blood supply. In pigs, it is supplied by a single artery, which is a branch of the splenic artery, and a similar situation is found in humans where the main artery of the tail of the pancreas is the major pancreatic artery[41]. Such anatomical particularity predisposes the left pancreatic lobe to suffer from hypoxia or even ischemia if blood supply is mechanically restricted. It is interesting to highlight that CT has revealed that human pancreatitis is predominantly located in the tail of pancreas[14,40,42]. This preferential location of the histopathological lesions and the lack of clinical symptoms in spite of substantial elevations of serological markers is a clue to assume a similar etiopathogeny for the appearance of the lesions in the pigs and humans[15,26].
Figure 1 Light microscopy image of the porcine pancreas after double-balloon enteroscopy showing located ischemic necrosis in pancreatic interlobular tissue.
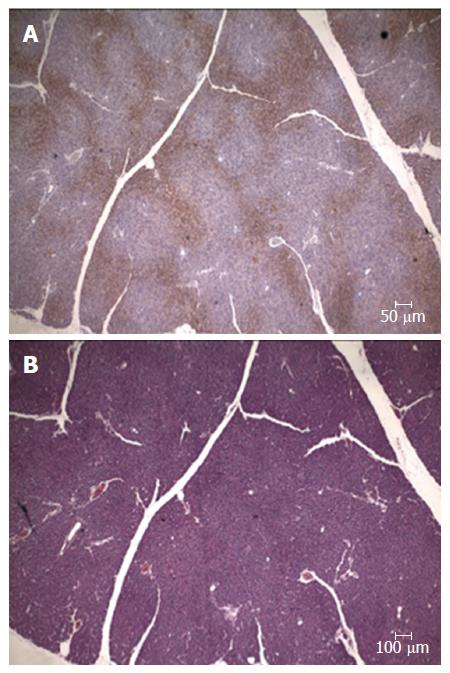
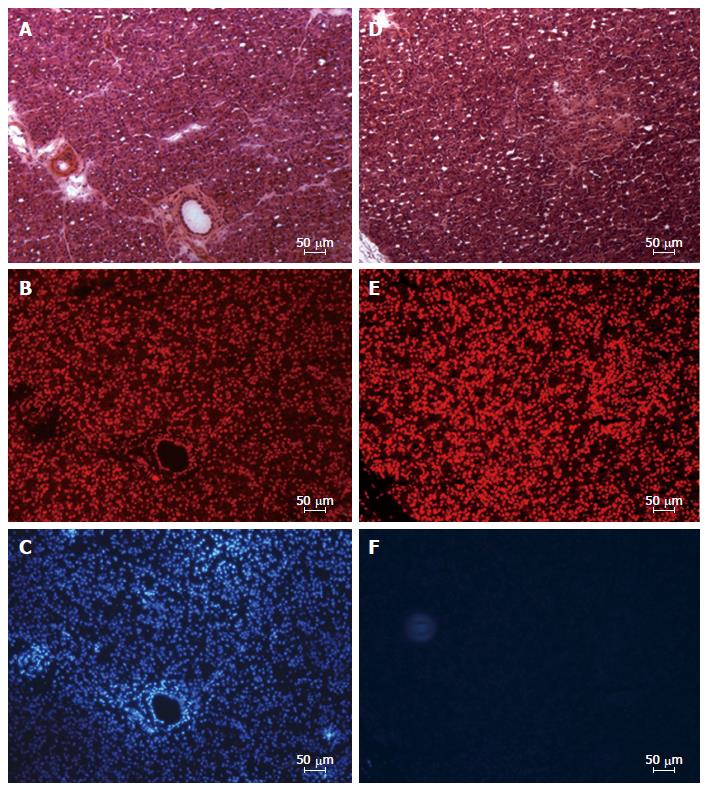
To further investigate whether a pancreatic ischemia during DBE was in the origin of the histopathological findings immunohistochemistry of hypoxia markers was carried out. The vascular endothelial growth factor (VEGF) is an endogenous factor involved in vascular permeation and angiogenesis which may be over expressed when blood supply is reduced[43]. In the endocrine component of the pancreas an increased expression of VEGF has been observed in experimental situations reproducing a hypoxic scenario[44-46]. Likewise, in the pancreases of our pigs (same animals of the previous experiment) a positive immunostaining for VEGF was observed in both the endocrine and exocrine components. No VEGF expression was found in the control group while in the experimental group there were areas where VEGF was not expressed alongside others where it was highly expressed. In the necrotic areas low or nule VEGF expression was observed, as corresponds to areas with damaged cells. Around these necrotic areas however high VEGF expression was found, which correspond to a reactive expression due to ischemia. Overall the highest expression of VEGF was in the tail and body of the pancreas (Figure 2).
Figure 2 Pancreas immunohistochemistry.
A: Expression of the VEGF in a normal parenchyma; B: Pancreatic acini with normal structure and nuclei, which express VEGF. In upper right corner a ischemic zone where less VEGF expression is shown, the pancreatic acini structure has been lost and nuclei are pyknotic. VEGF: Vascular endothelial growth factor.
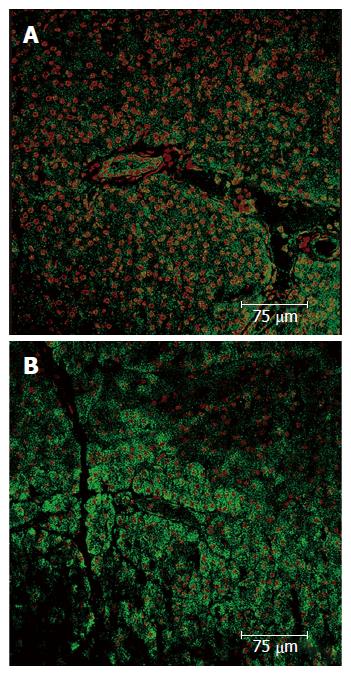
Complementarily, the exogenous marker pimonidazole hydrochloride (Hypoxyprobe®) was used in another set of pigs (non-published results). This compound is a derivative of the 2-nitroimidazole, which forms irreversible adducts with thiol groups in proteins, peptides and amino acids in tissues with oxygen tension of below 10 mmHg[47]. This exogenous marker has been largely tested in animal models and humans for immunodetection of hypoxic tissues harvested from both normal organs and malignant tumors[48,49]. In our experiment Hypoxyprobe® was administered endovenously to 5 anaesthetized Large White pigs (70-90 kg). Then, an oral DBE was performed for 60 min, and after euthanasia, the presence of hypoxic areas in the pancreas assessed by immunostaining. Results demonstrated the presence of focal areas positively stained for pimonidazole adducts (Figure 3), which was an unequivocal demonstration of pancreatic hypoxia (proof of concept). Like in the previous experiment the hypoxic areas were preferentially located (more abundant and wider) in the tail of the pancreas, and affected the exocrine and endocrine components. Although pancreatic hypoxia associated to oral DBE was conceptually demonstrated in this study, it could not be concluded that the direct cause of the hypoxia suffered by the pancreas was DBE. In theory, the presence of pimonidazole adducts could either be a consequence of reduced blood flow or increased oxygen demands by the pancreatic tissue[49], thus further studies were needed to directly assess if a reduced perfusion of the pancreas do really exist during oral DBE.
Figure 3 Pancreas immunohistochemistry.
A: Expression of the pimonidazole hydrochloride (Hypoxyprobe®) presence of focal areas positively stained; B: Serial section stained with hematoxyline-eosine.
Evidences for reduced perfusion of the pancreas in the oral DBE
Blood flow to the human and porcine pancreas is primarily supplied by the celiac and superior mesenteric arteries. These two main vessels supply the head and body of the pancreas by a vascular arcade resulting from anastomosis of the cranial and caudal pancreaticoduodenal arteries. The left pancreatic lobe, as mentioned above, is supplied by a single, terminal, and constant major pancreatic artery which arises from the first portion of the splenic artery, a branch of the celiac artery[41,50-52].
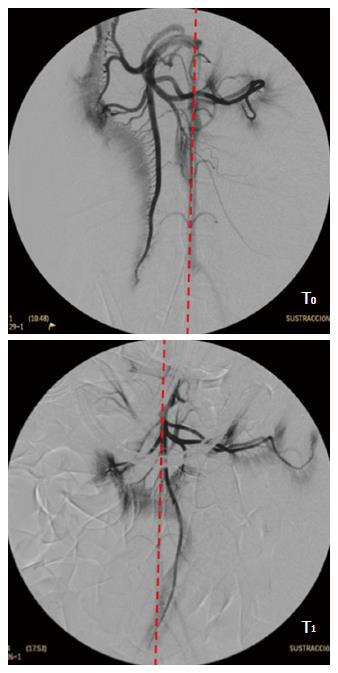
To assess whether a reduced perfusion of the pancreas is associated with the oral DBE two experiments were designed in porcine model. In a former study[39] an endovascular approach under fluoroscopic guidance was carried out in 10 Large White pigs of 45-50 kg. The femoral artery was approached and after selective catheterization angiographies of the superior mesenteric and celiac arteries obtained (time T0). Then, conventional oral DBE was performed for 90 min, and at the end of procedure, with the endoscope inserted at maximum depth the angiographic study was repeated (time T1). During the procedure continuous estimation of the explored distance was done[35], but to avoid potential interference of the vascular catheterization neither serum markers of pancreatitis nor histopathological study were done. At T0, the cranial mesenteric vascular axis was located mainly to the right of the spine, in its usual anatomical position, but at T1 it was completely to the left (Figure 4), which was related with the intestinal folding and the pull-and-push manoeuvres. Besides, at T1 a lack of vascular repletion was seen in the caudal pancreaticoduodenal artery. There was a reduction in the level of opacification of the branches of the cranial mesenteric artery addressed to the pancreas and bowel. As revealed by the injected contrast the perfusion of the jejunal arteries, the network of straight arteries, and the capillary network in the antimesenteric border of the small intestine was significantly reduced. With regards to the selective angiography of the celiac trunk, differences between T0 and T1 were also relevant. As it was common in all the experiments, none of the animals presented signs of post-DBE pancreatitis. The study described was most surely the only angiographic evaluation of the pancreas and small bowel carried out simultaneously to DBE. The results give radiological evidences of a reduced perfusion to these organs and agree with the recommendation of handling the overtube gently during the push-and-pull manoeuvres to avoid excessive restrain of the mesentery and the traction force on the pancreas[27].
Figure 4 Selective angiogram of the cranial mesenteric artery.
T0: Before the DBE; T1: With the endoscope inserted at maximum during DBE. Red line shows the aorta topography. DBE: Double balloon enteroscopy.
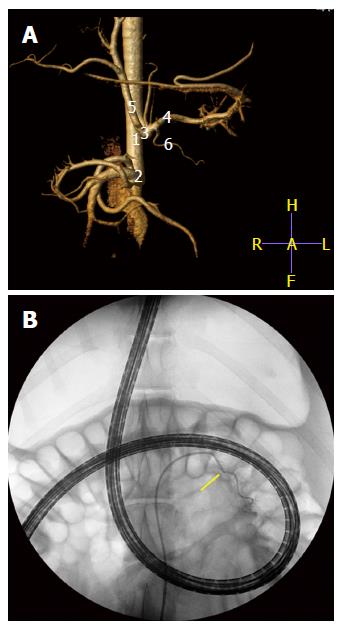
A step further in the evaluation of the vascularization of the pancreas during DBE was achieved in a second experiment (non-published results). The similar and genuine vascular pattern of the tail of the pancreas in pigs and humans supplied by a major pancreatic artery from the splenic artery, lead us to design an endovascular super-selective approach to this vessel at the end of an oral DBE. 5 pigs (40-50 kg) were anaesthetised and, first of all, a CT angiogram (Philips Brillance CT-6) with vascular 3D reconstruction obtained from each animal so as to monitor his individual vascular anatomy before angiographic procedure (Figure 5). The images displayed the whole pattern of ramifications of the celiac artery, the pancreatic branch for the tail of pancreas included. Then, an oral DBE was currently done for 60 min, and by the end of procedure, when the endoscope was inserted at the maximum depth, a super-selective angiography of the major pancreatic artery achieved. In brief, this was the followed protocol. Under fluoroscopic guidance (BV-300; Philips, Best, the Nertherlands), a 0.035- inch hydrophilic guide wire (Radifocus guidewire, Terumo, Tokyo, Japan) and a 5-French cobra catheter (Radiofocus Cobra Small; Terumo, Tokyo, Japan) were selectively addressed towards the celiac artery. Celiac arteriograms clearly depicted the vascular anatomy of the hepatic and splenic arteries and their branches. By road-map guidance, the cobra catheter was selectively positioned and wedged into the major pancreatic artery. The super-selective angiography of this artery was conducted by manual injection of 3-5 mL contrast medium to ensure that the tip of the catheter was at the desired site[53]. The nuclear dye Hoechst (Bizbenzimida H 33258 fluorochrome) was injected through that vessel to allow its contact with the cells in the tail of the pancreas (Figure 5). Hoechst is a membrane-permeable, adenine-thymine specific fluorescent dye useful for in vivo staining of DNA. It has been proved very useful as a marker of tumour cells and to assess the vascular supply to tumours[54-56] as well as vascular perfusion[57] and hypoxia[58]. After euthanasia the pancreases were collected, the tail trimmed in 1 cm × 1 cm × 1 cm blocks, which were identified by zone and frozen in liquid nitrogen. Criosections (10 μm thick) were obtained and stained with the nuclear marker TO-PRO®3 iodide (Molecular Probes) at a dilution of 1:100 in PBS. To-Pro3 was used as a control dye of the whole cellular population in the tissue sections. Fluorescence microscopy was used to evaluate the nuclear DNA staining with the two dyes.
Figure 5 Computed tomography angiogram (A) with vascular 3D reconstruction to monitor his individual vascular anatomy (1) abdominal aorta; (2) cranial mesenteric artery; (3) celiac artery; (4) splenic artery; (5) hepatic artery; and (6) major pancreatic artery.
B: Super-selective angiography of the major pancreatic artery (arrow) with the endoscope inserted at maximum during DBE. The nuclear dye Hoechst (Bizbenzimida H 33258 fluorochrome) is injected through that vessel to allow its contact with the cells in the tail of the pancreas. DBE: Double balloon enteroscopy.
The quantification of staining for both dyes in a digital analyser allowed to directly asses potential changes in the perfusion of the tail of pancreas during DBE (Figure 6). In average, from the total number of nuclei (To-Pro staining) only 35% of them were Hoechst positive. Moreover, the sequenced study in proximo-distal and latero-medial direction of the tail of the pancreas allowed to identify the proximo-lateral portion as the one with the lowest level of Hoechst staining (25%) and the disto-medial portion the one with the highest level (42%). The other two portions, proximo-medial and disto-lateral, showed a 35% and 38% of positive Hoechst cells, respectively. According to these results, a positive gradient in proximo-distal direction was observed in terms of potential blood supply to the tail of the pancreas during oral DBE.
Figure 6 Fluorescence microscopy image of cryosections from left lobe of the pancreas showing distribution of endocrine and exocrine cells with nuclei stained in disto-medial (A-C) and proximo-lateral (D-F) portions.
A and D: Serial sections stained with hematoxyline-eosine; B-E: Serial sections stained with the nuclear marker TO-PRO®3 iodide; C and F: Serial sections showing the location the nuclear dye Hoechst (Bizbenzimida H 33258 fluorochrome).
These conclusive results, which directly associated the oral DBE with a reduced vascular supply to the tail of the pancreas, together with all the previous evidences give consistent clues to support the vascular etiology as a likely cause of post-DBE pancreatic inflammation in the porcine model. The anatomical similarities between the human and porcine pancreas supports a translation of results which may help to better understand the pathogeny and clinics of post-DBE pancreatic disorders.
OCCLUSIVE ETIOLOGY: MANIPULATION AND OCCLUSION OF THE DUODENAL PAPILLA
The potential occlusion of the duodenal papilla by the balloons during oral DBE is a risk largely mentioned in the literature[13,15,16,21,25,28-30]. Evidences of pancreatic disorders during Endoscopic Retrograde Cholangiopancreatography (ERCP) practice are conclusive. For this reason, there is a recommendation of avoiding the inflation of the balloons in the pancreato-biliar area until the Treitz ligament has been reached[16,31,36]. However, awareness of this anatomical landmark by the endoscopist is not always guaranteed[14], especially during the learning curve of DBE. Some studies have related the increase of amylase and lipase levels after DBE with the distance of the first balloon inflation from the Treitz ligament[14,16,59,60]. The longer the distance the lower the figures of the serum markers. Nevertheless, a correspondence between this trend and the incidence of persistent hyperamylasemia or pancreatitis has not been concluded[9].
In the experimental field, we used a porcine model to demonstrate if the direct contact of the inflated balloons with the duodenal papilla was responsible for significant and persistent changes in the serum markers as well as the appearance of structural disorders in the pancreatic tissue[34]. The results were conclusive. The longer the contact of the inflated overtube’s balloon with the papilla the higher the levels of amylase (up to 22.7% increase), lipase (up to 7.5 times higher) and CRP (up to 6.3 times higher). Contrastingly, in the DBE control group, where the balloons were inflated after the ligament of Treitz, the serum markers did not increase more than two times the baseline.
Moreover, in those pigs where the overtube’s balloon was inflated and kept around the duodenal papilla the histopathological study showed moderate to severe acinar vacuolization, oedema and even necrosis. The histopathological pattern resembled the structural disorders currently found in experimental studies of pancreatitis caused by forced obstruction of the pancreatic duct[61,62]. On the other hand, it has been described that slight vacuolization and/or oedema are reversible if the cause of obstruction disappears, which is compatible with a complete functional and structural recovery of the pancreas[63]. This could explain the absence of symptoms of pancreatitis in any of the pigs despite the persistent contact of the balloon with the duodenal papilla.
These results in animal model clearly support the theory that avoiding contact with the duodenal papilla during DBE insertion decreases the risk of iatrogenic lesions in the pancreas[14,16,59,60]. However, even when the pancreato-biliar area is avoided hyperamylasemia and symptoms of pancreatitis can still appear after DBE. Therefore, avoiding the obstructive picture does not seems to be the only solution, although it reduces the risk of post-DBE pancreatitis[2,33].
THE REFLUX ETIOLOGY
This hypothesis is based on the fact that the endoluminal pressure within the small bowel and particularly, around the pancreato-biliar area rises while DBE progresses. The increased pressure within the duodenal lumen leads to reduced drainage, retrograde circulation or even reflux of the pancreatic secretion[25,31], which cause a severe pancreatic lesion[24]. Accordingly, the pancreatic structural disorders and correspondent inflammation are expected to affect the whole organ in a diffuse pattern. However, that is not the case of the post-DBE pancreatitis, as revealed by imaging diagnosis and histopathology, where the pancreatic affection is preferentially located in the tail of the pancreas in both human[7,13,14,40,42] and porcine models[33]. In addition, no significant dilatation of pancreatic ducts is currently found. Thus, rather than a primary cause of post-DBE pancreatitis this etiology should be considered as secondary or occasional. So, it seems difficult that the etiopathogeny of post-EDB pancreatitis is mainly due to a duodenal reflux mechanism[2].
ADDITIONAL RISK FACTORS FOR POST-DBE PANCREATITIS
In addition to reviewing the etiologies of the post-DBE pancreatitis, a discussion about potential risks involved in this complication is pertinent. The time length of the procedure, the final depth of endoscope insertion and the purpose of the DBE (diagnostic vs therapeutic) have been mentioned[14,16,17,20,31,40]. Again, the use of a validated animal model for DBE allows a more accurate approach to this topic[32-35].
The question about how long can DBE take to avoid complications such as pancreatitis has not received yet a unanimous answer. A large variety of figures are found in the literature. Explorations as short as 30 min or as long as 240 min[64] have been referred. Between those limits, DBE of 148 min[14,20], 115 min[16] and 95 min[25] are mentioned. Some authors agree a time window between 70 and 100 min as the criteria to avoid DBE complications such as perforation[2,65,66], while in relation with the learning curve other authors highlight the important reduction in time after approximately 10 DBEs, which in average was quantified from 109 min to 92 min[67]. From another perspective, some works define the maximum effective time of exploration as the period from the beginning of procedure till the moment when no more than 1-2 cm are progressed after several push-and-pull cycles. Such a time of effective exploration, which in humans and pigs takes around 60 min, has been referred as “the gold hour” of DBE practice. However, the relevance of this reference is uncertain. While it is noticeable that oral DBE longer than one hour have been associated with increases of pancreatic lesion or pancreatitis[14,16,17,20,31,40], other studies do not find any consistent reason to consider the time as a direct risk factor[14,21]. This was also the overall conclusion of an experimental study in porcine model[33]. The time and length of each push-and-pull manoeuvre was carefully monitored and no significant association between DBE duration (90 min vs 140 min) and the risk of pancreatitis (amylase and lipase levels) or the grade of histopathological disorders was found.
The final insertion depth of the endoscope is another controversial risk associated to DBE, especially because the estimation of the explored distance is always subjective. Several methods to assess insertion depth have been proposed[2,68], but the most frequently used was presented by May in 2005[2]. This method was later validated for experimental purposes in a porcine model[35]. On the other hand, evaluating how insertion depth may be a risk of pancreatitis is neither easy nor accurate in DBE practice. The influence of this variable cannot be isolated from other factors associated to the patient and/or the pathology, which strongly condition how deep the endoscope is addressed. Even under experimental conditions the total insertion depth of the endoscope was not correlated with the level of pancreatic markers or the appearance of structural disorders in the pancreas[33]. Nevertheless, when both the time length DBE interval and the depth of insertion are combined potential complications are more likely.
Several other risks for post-DBE pancreatitis have been suggested[17]. The indication (purpose) of DBE, whether diagnostic or therapeutic is one of them. Diagnostic DBE are less invasive and have a lower risk of major complications (around 1%), such as pancreatitis, haemorrhage or perforation, than therapeutic (up to 5%)[17,69]. The learning curve has also been suggested as a risk for major complications[14]. Up to a minimum of 50 DBE are needed to finish the learning curve[69] but this was never related with higher amylase or lipase levels, or post-DBE pancreatitis[20,59,70]. On the other hand, the use of CO2 insufflation during DBE is highly recommended as it prevents over-inflation of the small bowel. CO2 is helpful for an easier and deeper insertion of the endoscope, and has a possible preventive effect to post-DBE pancreatitis[14,17].
CONCLUSION
Based on a growing number of indirect clinical evidences and supported by experimental studies in porcine model, a vascular distress is suggested as the most likely etiology of post-DBE pancreatitis. During oral DBE the push-and-pull manoeuvres cause mechanical strength on the small bowel, pancreas and mesentery, which reduce the normal blood supply. The resulting situation of pancreatic ischemia commonly determines transient cellular hypoxia, with a lack of clinical signs of pancreatitis, however it may occasionally drive severe hypoxia, necrosis and clinical pancreatitis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Du YQ, Figueiredo PN S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF