Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6128
Peer-review started: February 9, 2017
First decision: April 21, 2017
Revised: May 9, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: September 7, 2017
Processing time: 210 Days and 6.3 Hours
To describe real-world treatment patterns of gastrointestinal neuroendocrine tumors (GI NET).
In this retrospective cohort study, we used 2009-2014 data from 2 United States commercial claims databases to examine newly pharmacologically treated patients using tabular and graphical techniques. Treatments included somatostatin analogues (SSA), cytotoxic chemotherapy (CC), targeted therapy (TT), interferon (IF) and combinations. We identified patients at least 18 years of age, with ≥ 1 inpatient or ≥ 2 outpatient claims for GI NET who initiated pharmacologic treatment from 7/1/09-6/30/14. A 6 mo clean period prior to first treatment ensured patients were newly treated. Patients were followed until end of enrollment or the study end date, whichever was first.
We identified 2258 newly treated GI NET patients: mean (SD) age was 55.6 years (SD = 9.7), 47.2% of the patients were between 55 and 64 years, and 48.8% were female. All regions of the United States were represented. 59.6% started first-line therapy with SSA monotherapy (964 with octreotide LAR, 380 with octreotide SA, and 1 with lanreotide), 33.3% CC, 3.6% TT, and 0.5% IF. The remainder received combinations. Mean follow up was 576 d. Overall mean first-line therapy duration was 361 d (449 d for SSA, 215 for CC, 267 for TT). 58.9% of patients had no pharmacological treatment beyond first line. The most common second-line was combination therapy with SSA. In graphical pattern analysis, there was no clear pattern visible after first line therapy.
In this study, 60% of patients initiated treatment with SSA alone or in combination. The relatively long time to discontinuation suggests possible sustained effectiveness and tolerability.
Core tip: In this retrospective study of real-world treatment patterns, somatostatin analogues were the most common initial pharmacologic treatment in patients with gastrointestinal neuroendocrine tumors, and most of the remaining patients began treatment with chemotherapy. However, despite the many treatment options, over half of the patients discontinued treatments after first line and only less than 10% of patients received any second-line pharmacotherapy. Given limitations of claims data to elucidate reasons for this lack of continued treatment, a study using more detailed clinical information such as medical charts or physician surveys is warranted.
- Citation: Benson III AB, Broder MS, Cai B, Chang E, Neary MP, Papoyan E. Real-world treatment patterns of gastrointestinal neuroendocrine tumors: A claims database analysis. World J Gastroenterol 2017; 23(33): 6128-6136
- URL: https://www.wjgnet.com/1007-9327/full/v23/i33/6128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i33.6128
Neuroendocrine tumors (NET) comprise a broad family of rare and often slow growing malignancies. NET can develop anywhere in the body and arise from neuroendocrine cells throughout the endocrine system[1,2]. Approximately two-thirds of NET tumors occur in the gastrointestinal (GI) tract. These sites include the stomach, small intestine, appendix, colon, and rectum[3]. NET secrete peptides and neuroamines that cause distinct syndromes (e.g., carcinoid syndrome), in which case they are referred to as “functional” tumors. Clinical presentation depends on the site of the primary tumor and whether they are functional. Surgery may be curative in the early stages, but delayed diagnosis is typical.
While rare, the incidence and prevalence of NET appear to be increasing worldwide[4-8]. The incidence of NET in the United States increased from 10.9 cases per million person-years (PMPY) in 1973 to 52.5 PMPY in 2004, and to 69.8 PMPY in 2012 as reported using the United States Surveillance Epidemiology and End Results database[4,9]. Prevalence also increased and was reported as 216 per million per year for GI NET in the United States.
The management of GI NET is based on a variety of factors including stage, anatomic location, and the presence and type of symptoms. The most recent NCCN guidelines for unresectable and metastatic GI NET recommend somatostatin analogues (SSA) as first-line treatment, but do not recommend a particular treatment sequence for the remaining therapies[10]. Considering the heterogeneity of GI NET tumors and the resultant lack of specificity in guidelines, we aimed to describe the current real-world treatment patterns of GI NET in a large sample of patients.
We conducted a longitudinal, retrospective cohort analysis of newly pharmacologically treated GI NET patients using two large United States commercial claims databases. Data from the Truven Health Analytics MarketScan database and the IMS PharMetrics database (both using dates from January 1, 2009 to December 31, 2014) were combined to increase sample size. To prevent duplicate records, patients with the same age, gender, region, and date of first GI NET diagnosis in a calendar year found in both databases were randomly removed from one of the databases. Both databases are Health Insurance Portability and Accountability Act compliant administrative claims databases that contain de-identified adjudicated medical claims (e.g., inpatient and outpatient services) and pharmacy claims (e.g., outpatient prescriptions) submitted for payment by providers, healthcare facilities, and pharmacies. For both data sources, claims include information on each physician visit, medical procedure, hospitalization, drug dispensed, date of service, number of days of medication supplied, test performed, and complete payment information. Each medical claim has a principal diagnosis and secondary diagnoses codes associated with it. Available patient demographic information includes age, gender, and geographic region. Dates of enrollment and disenrollment are also recorded. As the data were fully de-identified, this study was considered exempt from approval by the Institutional Review Board.
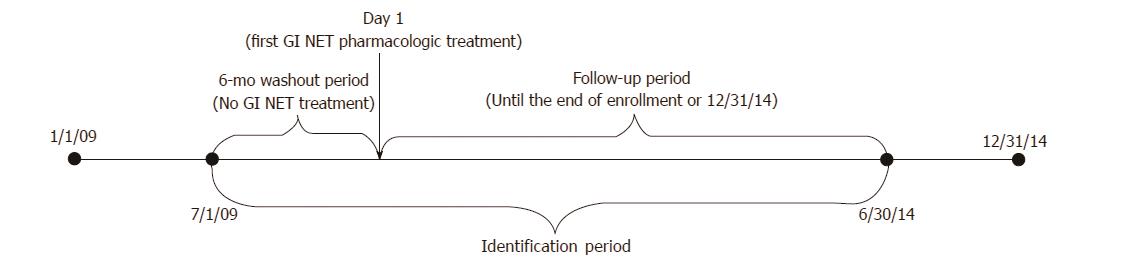
Patients at least 18 years of age were identified from each dataset if they had at least 1 inpatient or 2 outpatient claims with an International Statistical Classification of Disease-9-Clinical Modification (ICD-9-CM) for GI NET (209.00-209.03, 209.10-209.17, 209.23, 209.24-209.27, 209.40-209.43, 209.50-209.57, 209.62, 209.65-209.67) during the study period (1/1/2009-12/31/2014). The first GI NET pharmacologic treatment claim on or after the appearance of the GI NET diagnosis code and within the ID period (7/1/2009 to 6/30/2014) was considered to be the index date. Patients were required to be enrolled for a baseline period of at least six months before the index date. To ensure new treatment, patients with any evidence of pharmacologic treatment during this baseline period were excluded. In order not to include the same patient twice, we searched for any patients with the same age, gender, region, and date of GI NET diagnosis who could be found in both databases, but we found none. Patient follow-up was variable and continued until the end of enrollment or the study end date (12/31/2014), whichever was first (Figure 1).
The primary outcome measure was the use of pharmacologic or liver directed therapy. Pharmacotherapy was divided into four groups: SSA, TT, CC and IF. SSA included octreotide and lanreotide, TT included everolimus and sunitinib, and CC included temozolomide, streptozotocin, doxorubicin, liposomal doxorubicin, fluorouracil, capecitabine, dacarbazine, oxaliplatin and thalidomide. Pharmacologic therapy was identified in claims using both the Healthcare Common Procedure Coding System (HCPCS) and National Drug Codes (NDC). Liver directed therapies comprised liver resection, transplant, lesion ablation (using radiotherapy, cryotherapy, microwave and thermal energy, and including laparoscopic, open and percutaneous routes), embolization (including bland, radioisotope, and chemotherapy), and radiation therapy. Liver directed therapies were identified in claims using HCPCS, ICD-9-CM, and Current Procedural Terminology (CPT) codes. Chemotherapy observed only once and on the same date as embolization was considered chemoembolization and not part of a pharmacologic regimen.
First-line therapy was defined as the pharmacologic treatment regimen observed on, or within three months of, the index date. Therapy included monotherapy or combination therapies. A three-month period after the index date was used to identify pharmacologic therapy intended as first-line but not administered on the index date. This would include, for example, combination chemotherapy where the second agent is given after some delay. Second-line therapy was defined as beginning when treatment was switched from one category of pharmacotherapy to another (e.g., from SSA alone to CC alone), or when a new category of treatment was added (e.g., from SSA alone to SSA plus CC). Changes from one cytotoxic agent to another, or one SSA to another, were not considered a switch. The first day of treatment switch or addition was defined as the initiation date of second-line therapy.
Means and proportions were presented in tabular analyses. An inverse Kaplan-Meier curve was used to show duration of first-line therapy. All data transformations and statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC). Graphical analyses were conducted using GRAPHx™, a proprietary graphics-based algorithm. The GRAPHx method uses multi-colored line segments to represent various treatments, plotting them over time. The images are reviewed visually for the presence and length of segments and change in colors and patterns over time.
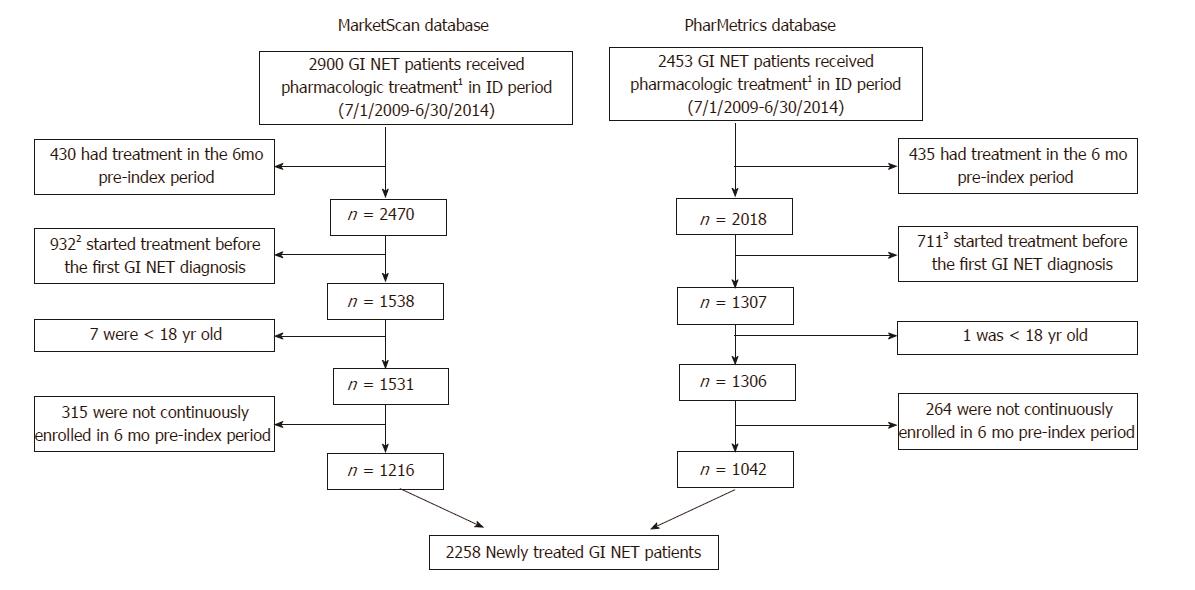
There were 2900 and 2453 patients meeting the definition of GI NET who also had a claim for pharmacologic treatment between 7/1/2009 and 6/30/2014 in the MarketScan and PharMetrics databases, respectively. After excluding patients who had treatment during a 6-mo pre-index period (and therefore were considered to be continuing, rather than initiating, treatment); received treatment before receiving a diagnosis of GI NET; were < 18 years old; or were not continuously enrolled in the 6-mo pre-index period, there remained 2258 newly treated GI NET patients who were included in the study (Figure 2).
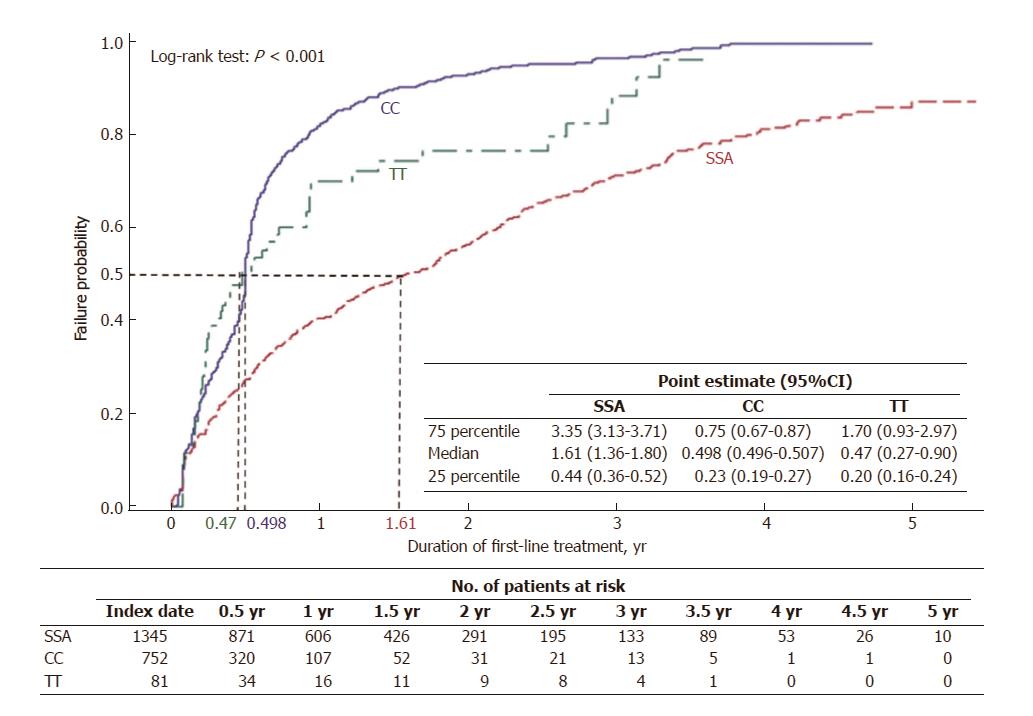
Gender was evenly split with n = 1103 (48.8%) female patients and n = 1155 (51.2%) male. The average age was 55.6 years (SD = 9.7) and 47.2% of the patients were between 55 and 64 years. All regions of the United States were represented. More than half of patients, n = 1345 (59.6%), were treated with SSA as first-line monotherapy, 964 with octreotide LAR, 380 with octreotide SA, and 1 with lanreotide. An additional 75 patients (3.3%) received SSA in combination with other either CC, TT or IF. The second largest group, n = 752 (33.3%), was treated with CC monotherapy, and n = 81 (3.6%) received TT monotherapy (Table 1). Mean duration of first-line therapy was 361 d (SD = 385) for all newly treated patients. The mean observed duration of treatment for first-line SSA monotherapy users was 449 d (SD = 434.2). It was 215 d (SD = 228.8) for first-line CC monotherapy and 267 d (SD = 325.7) for first-line TT monotherapy (Table 2). By 588 d of treatment (1.61 years), half of SSA initiators had discontinued treatment, compared to 182 d (0.498 years) for half of CC users and 171 d (0.47 years) for half of TT users to discontinue treatment (Figure 3). Liver directed therapy was used by 12.5% during first-line pharmacologic therapy; another 3.7% received it sometime after the first-line (Table 2).
| First-line treatment | All newly treated patients | |||||||||
| SSA | CC | TT | SSA + CC | SSA + TT | TT + CC | IF | SSA + IF | SSA + TT + CC | ||
| n | 13451 | 752 | 81 | 42 | 31 | 3 | 2 | 1 | 1 | 2258 |
| 59.6% | 33.3% | 3.6% | 1.9% | 1.4% | 0.1% | 0.1% | 0.0 | 0.0 | 100.0% | |
| Age (mean ± SD, yr) | 56.3 ± 9.5 | 54.7 ± 9.9 | 54.9 ± 10.5 | 53.5 ± 10.9 | 53.8 ± 10.1 | 59.3 ± 3.8 | 58.5 ± 3.5 | N/A | N/A | 55.6 ± 9.7 |
| 18-24 | 6 (0.4) | 2 (0.3) | 0 (0) | 1 (2.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (0.4) |
| 25-34 | 20 (1.5) | 25 (3.3) | 4 (4.9) | 3 (7.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 52 (2.3) |
| 35-44 | 121 (9.0) | 92 (12.2) | 9 (11.1) | 4 (9.5) | 4 (12.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 230 (10.2) |
| 45-54 | 371 (27.6) | 225 (29.9) | 18 (22.2) | 12 (28.6) | 12 (38.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 638 (28.3) |
| 55-64 | 651 (48.4) | 338 (44.9) | 39 (48.1) | 20 (47.6) | 11 (35.5) | 3 (100.0) | 2 (100.0) | 1 (100.0) | 1 (100.0) | 1066 (47.2) |
| 65+ | 176 (13.1) | 70 (9.3) | 11 (13.6) | 2 (4.8) | 4 (12.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 263 (11.6) |
| Female | 677 (50.3) | 341 (45.3) | 40 (49.4) | 26 (61.9) | 17 (54.8) | 1 (33.3) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1103 (48.8) |
| Region | ||||||||||
| Midwest | 321 (23.9) | 183 (24.3) | 20 (24.7) | 13 (31.0) | 7 (22.6) | 0 (0) | 1 (50.0) | 0 (0) | 0 (0) | 545 (24.1) |
| Northeast | 261 (19.4) | 150 (19.9) | 12 (14.8) | 11 (26.2) | 7 (22.6) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 442 (19.6) |
| South | 563 (41.9) | 323 (43.0) | 36 (44.4) | 12 (28.6) | 15 (48.4) | 2 (66.7) | 1 (50.0) | 0 (0) | 1 (100.0) | 953 (42.2) |
| West | 200 (14.9) | 96 (12.8) | 13 (16.0) | 6 (14.3) | 2 (6.5) | 0 (0) | 0 (0) | 1 (100.0) | 0 (0) | 318 (14.1) |
| Year of treatment initiation | 130 (9.7) | 41 (5.5) | 4 (4.9) | 2 (4.8) | 1 (3.2) | 0 (0) | 0 (0) | 1 (100.0) | 0 (0) | 179 (7.9) |
| 2009 | ||||||||||
| 2010 | 271 (20.1) | 122 (16.2) | 4 (4.9) | 11 (26.2) | 7 (22.6) | 0 (0) | 1 (50.0) | 0 (0) | 0 (0) | 416 (18.4) |
| 2011 | 282 (21.0) | 159 (21.1) | 15 (18.5) | 17 (40.5) | 5 (16.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 478 (21.2) |
| 2012 | 270 (20.1) | 168 (22.3) | 33 (40.7) | 4 (9.5) | 10 (32.3) | 0 (0) | 1 (50.0) | 0 (0) | 1 (100.0) | 487 (21.6) |
| 2013 | 268 (19.9) | 174 (23.1) | 16 (19.8) | 3 (7.1) | 4 (12.9) | 2 (66.7) | 0 (0) | 0 (0) | 0 (0) | 467 (20.7) |
| 2014 | 124 (9.2) | 88 (11.7) | 9 (11.1) | 5 (11.9) | 4 (12.9) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 231 (10.2) |
| Days of follow-up | ||||||||||
| Mean | 621 | 514 | 454 | 588 | 425 | 244 | 675 | 836 | 496 | 576 |
| (SD) | (468.5) | (409.1) | (403.6) | (424.1) | (269.6) | (140.7) | (145.7) | N/A | N/A | (447.1) |
| Median | 500 | 393 | 290 | 455 | 360 | 216 | 675 | 836 | 496 | 454 |
| First-line | All newly treated patients | |||||||||
| SSA | CC | TT | SSA + CC | SSA + TT | TT + CC | IF | SSA + IF | SSA + TT + CC | ||
| n | 1345 | 752 | 81 | 42 | 31 | 3 | 2 | 1 | 1 | 2258 |
| 59.6% | 33.3% | 3.6% | 1.9% | 1.4% | 0.1% | 0.1% | 0.0% | 0.0% | 100.0% | |
| Duration of first-line treatment (mean ± SD, d) | 449 ± 434.2 | 215 ± 228.8 | 267 ± 325.7 | 408 ± 327.9 | 276 ± 189.5 | 208 ± 165.6 | 251 ± 285.0 | 836 ± 0 | 426 ± 0 | 361 ± 385.0 |
| First-line ending status | 635 (47.2) | 609 (81.0) | 44 (54.3) | 26 (61.9) | 14 (45.2) | 1 (33.3) | 1 (50.0) | 0 (0) | 1 (100.0) | 1331 (58.9) |
| Stop | ||||||||||
| Switch | 128 (9.5) | 33 (4.4) | 14 (17.3) | 5 (11.9) | 7 (22.6) | 1 (33.3) | 1 (50.0) | 0 (0) | 0 (0) | 189 (8.4) |
| End of enrollment | 582 (43.3) | 110 (14.6) | 23 (28.4) | 11 (26.2) | 10 (32.3) | 1 (33.3) | 0 (0) | 1 (100.0) | 0 (0) | 738 (32.7) |
| Liver directed therapy timing | ||||||||||
| During first-line | 171 (12.7) | 87 (11.6) | 9 (11.1) | 10 (23.8) | 4 (12.9) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 282 (12.5) |
| After first-line | 36 (2.7) | 39 (5.2) | 7 (8.6) | 0 (0.0) | 2 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 84 (3.7) |
| +/- 30 d after stopping first line therapy1 | 635 | 609 | 44 | 26 | 14 | 1 | 1 | 0 | 1 | 1331 |
| 29 (4.6) | 42 (6.9) | 2 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 73 (5.5) | |
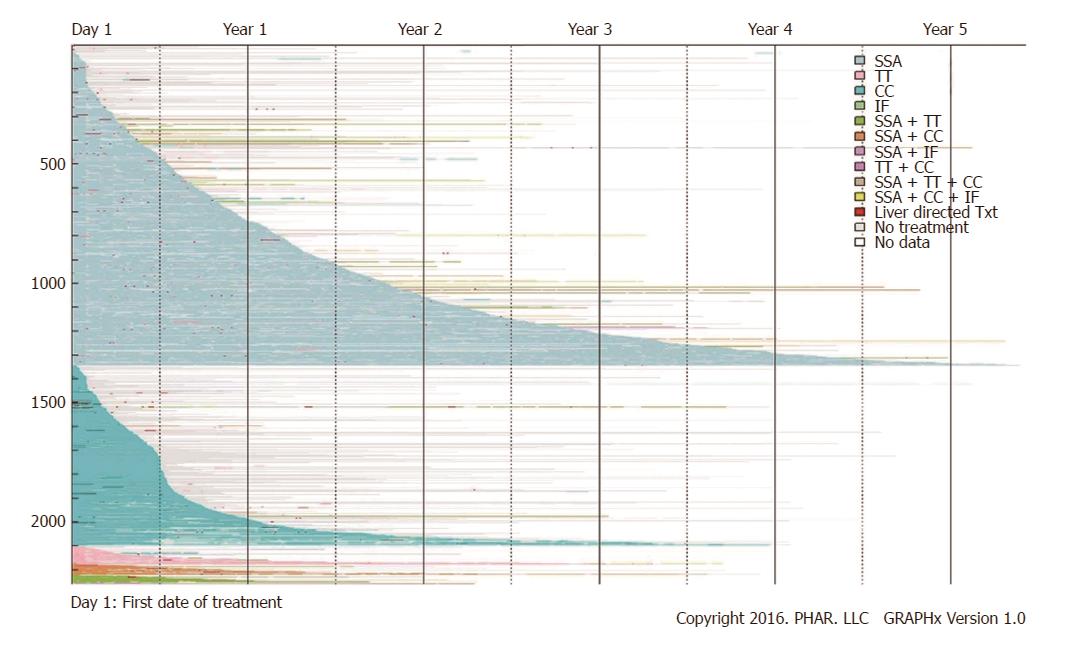
By the end of the study follow-up period [mean (SD, median) of 576 d (447.1, 454)] 58.9% (n = 1331) patients had stopped pharmacologic therapy completely. These patients continued to be enrolled in one of the databases but no longer had claims for pharmacologic treatment. In Figure 4, these patients can be identified as colored line segments that terminate in gray segments of variable length, with the gray representing the period of no treatment. An additional 32.7% (n = 738) continued their initial therapy until the end of their enrollment; these patients were still receiving their first-line therapy at the time they left a covered plan or reached the end of study. This pattern is shown in Figure 4 as a colored segment terminating in white. The remaining 8.4% (n = 189) were observed to change pharmacologic treatment during the follow-up period (a colored segment terminating in different colored segment) (Figure 4 and Table 2). Among these 189 patients who were observed to begin second-line therapy, 128 (67.7%) had initially been treated with SSA. Among these 128 first-line SSA users, 89 (69.5%) added additional therapy (e.g., CC or TT) as their second-line treatment. In patients who did not begin therapy with SSA, most received SSA monotherapy or combination therapy as second-line (Table 3). Liver directed therapy (short, red segments) appears dispersed throughout periods of both pharmacologic treatment (colored segments) and periods of no pharmacologic treatment (gray segments) (Figure 4). Among 1331 patients who stopped pharmacologic treatment and did not begin a second-line treatment, 5.5% were treated with liver-directed therapy within 30 d before or after they stopped.
| First-line treatment | Patients withsecond-line treatment | |||||||
| SSA | CC | TT | SSA + CC | SSA + TT | TT + CC | IF | ||
| n | 128 | 33 | 14 | 5 | 7 | 1 | 1 | 189 |
| 67.7% | 17.5% | 7.4% | 2.6% | 3.7% | 0.5% | 0.5% | 100.0% | |
| Second-line treatment | ||||||||
| SSA + TT | 51 (39.8) | 4 (12.1) | 4 (28.6) | 3 (60.0) | 0 (0) | 1 (100.0) | 63 (33.3) | |
| SSA + CC | 33 (25.8) | 6 (18.2) | 0 (0) | 5 (71.4) | 0 (0) | 0 (0) | 44 (23.3) | |
| CC | 24 (18.8) | 4 (28.6) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 29 (15.3) | |
| SSA | 16 (48.5) | 5 (35.7) | 0 (0) | 0 (0) | 1 (100.0) | 0 (0) | 22 (11.6) | |
| TT | 12 (9.4) | 7 (21.2) | 2 (40.0) | 0 (0) | 0 (0) | 0 (0) | 21 (11.1) | |
| SSA + IF | 3 (2.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1.6) |
| IF | 2 (1.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.1) |
| TT + CC | 1 (0.8) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 2 (1.1) | |
| SSA + TT + CC | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 2 (1.1) |
| SSA + CC + IF | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.5) |
This study used two very large, nationally representative claims databases, which together represent up to 100 million covered lives, to describe real-world treatment of GI NET. Three findings were of particular interest. First, the most common initial pharmacologic treatment was with SSA, with average duration of use of just over 18 mo. Second, although 60% of patients initiated treatment with SSA alone or in combination, most of the remainder began treatment with CC, therapy recommended by NCCN only if no other options (SSA, TT, or liver directed treatment) are feasible. Third, despite the many available treatment options, less than one in 10 patients was observed to receive treatment with second-line pharmacotherapy of any type.
Each of these findings must be considered in light of what is known about the disease as well as the inherent limitations of the data source. Treating NET patients is a complex process. Treatments are individualized based on tumor size, location, and pathology, as well as whether the tumor is functional, type and extent of symptoms, and speed of progression. Our data did not include this level of detail. Insurance companies aggregate information on inpatient and outpatient services (generally reported as ICD-9-CM or ICD-10), procedures (ICD-9 procedure codes and CPT codes) and pharmacy claims (NDC) as claims are submitted for payment by providers, healthcare facilities, and pharmacies. So for example, while GI NET can be identified by using a list of ICD-9-CM codes, presence of advanced disease must be inferred by observing the use of pharmacologic treatment. Another limitation of the date source is that the available length of time for analysis is relatively brief, so conclusions regarding average duration of use may not be representative of long-term treatment patterns.
Our primary finding that 60% of patients initiated pharmacologic therapy with SSA is consistent with the NCCN recommendation of these drugs for initial treatment of clinically significant and progressive NET. Consistent with the clinical observation that SSA tend to be well tolerated, patients initiating treatment with SSA remained on first-line therapy longer than the patients who began treatment with CC and TT. The second finding noted above, that 1/3 of GI NET patients began treatment with CC, is more surprising. CC is relatively ineffective in these patients, and as a result is recommended only if other options are not feasible.
There are several possible explanations for the frequent use of CC. Patients observed to initiate cytotoxic treatment may have been treated in the past with other agents and either progressed or were intolerant to those agents. We reviewed data for 6 mo before the first pharmacologic treatment, but treatments more than 6 mo in the past would have been missed. It may also be that some of the patients had a pathology finding suggesting chemotherapy would be beneficial, or a different type of GI tumor that was incorrectly coded as NET. Our data were de-identified, meaning we could not confirm the diagnosis in medical records, physician or patient surveys, or by other means. Finally, some clinicians may have been unfamiliar with either best practice recommendations or the available, albeit limited, data on GI NET treatment. Our findings are consistent with a recent large case series from a tertiary referral center that found SSA and CC were the two most common treatment strategies used for gastroenteropancreatic NET[11].
Previous studies have found significant divergence between clinical guidelines and treatment in other, more common, cancers. Chagpar and colleagues found that while Stage I colon cancer patients were treated according to guidelines by 95%, higher stage patients were less likely to be treated according to guidelines. Stage II high-risk patients showed the lowest concordance with only 36% being treated per recommendation[12]. In a prospective study of women with breast cancer, Giorano and colleagues found that 83% of patients 55-64 years old received care concordant with chemotherapy guidelines compared with 29% of patients 75 or older[13].
The finding that only a small proportion of patients are observed to receive second-line treatment is also surprising. The 5-year survival of patients with advanced GI NET is more than 70%. Most surviving patients would be expected to receive continued treatment, whether in the form of liver-directed treatment or pharmacotherapy. We considered multiple explanations for this finding. First, although median follow-up was over 15 mo, many patients were eventually lost to follow-up when they disenrolled from a plan included in our databases. One third of patients were continuing to use their index treatment when they were lost to follow-up. Whether (or when) these individuals progressed to second-line treatment cannot be determined using these databases. If these patients were systematically different from the ones who remained under observation, our results would be biased.
Despite this significant loss to follow-up, nearly 60% of patients were observed to continue enrollment but stop therapy. That is, they survived and remained in the data set, but no second-line pharmacotherapy use could be identified. We considered the possibility that these patients received some liver-directed treatment that alleviated their symptoms or controlled their disease, obviating the need for second-line treatment. However, we found no evidence of this: liver-directed treatment was observed in only 5.5% of patients around the time they stopped first-line treatment. We also considered whether some patients may have had a secondary source of payment, such that their claims for pharmacotherapy did not appear in our databases. Just over 11% of patients were 65 and older and would have been eligible for Medicare. Payment rules regarding patients with both commercial coverage and Medicare are complex[14] but generally require the commercial payer (for which we did have data) to be primarily responsible for payment. In cases where Medicare had primary responsibility, we would have missed claims for pharmacologic or liver-directed therapy and thus underestimated treatment. The magnitude of this problem is impossible to know using our current data source. A study using Medicare data and examining patients over 65 only might be less likely to suffer from this bias. Finally, it may indeed be the case that some patients stop therapy completely. Such patients may be terminal and choose not to undergo further treatment, or they may be relatively asymptomatic and decline to be treated on that basis. Further research with detailed clinical data would be needed to confirm which, if any, of these explanations is the most accurate.
In this large, claims-based, retrospective study of real-world pharmacologic treatment patterns, we found that 60% of GI NET patients began therapy with SSA and about one-third with CC. The relatively long time to discontinuation of SSA, as well as their use in combination with other agents, suggests they may be well tolerated and potentially have sustained effectiveness. We also found that over half of the patients discontinued treatment after first-line and only less than 10% of the patients received second-line treatment despite the availability of a number of different options. To address the limitations of this study and expand knowledge of real-world treatment patterns, a study using more detailed clinical information such as medical charts or physician surveys is warranted. In addition, future studies should consider using databases that would allow for greater longitudinal follow-up, such as registries, to assist in the further understanding of treatment patterns and length of therapy.
Neuroendocrine tumors (NET) comprise a broad set of rare tumors. Almost 2/3 arise in the gastrointestinal (GI) tract. The management of GI NET is based on a variety of factors including stage, anatomic location, and the presence and type of symptoms. The most recent NCCN guidelines for unresectable and metastatic GI NET recommend somatostatin analogues (SSA) as first-line treatment, but do not recommend a particular treatment sequence for the remaining therapies.
This study’s results add to the limited knowledge about real-world treatment patterns for GI NET, which is especially significant in light of the lack of treatment guidelines regarding treatment sequences beyond first-line therapy.
This study used two very large, nationally representative claims databases to describe real-world treatment of GI NET. The three key findings were: first, the most common initial pharmacologic treatment was with SSA, with average duration of use of just over 18 mo; second, although 60% of patients initiated treatment with SSA alone or in combination, most of the remainder began treatment with cytotoxic chemotherapy, therapy recommended by NCCN only if no other options (SSA, targeted therapy, or liver directed treatment) are feasible; and third, despite the many available treatment options, less than one in 10 patients was observed to receive treatment with second-line pharmacotherapy of any type. The authors findings are consistent with a recent large case series from a tertiary referral center that found SSA and CC were the two most common treatment strategies used for gastroenteropancreatic NET. Previous studies have also found significant divergence between clinical guidelines and treatment in other, more common, cancers.
This study suggests that there is frequent use of CC in GI NET treatment, although CC is relatively ineffective in these patients and recommended only if other options are not feasible. This may be a result of clinicians unfamiliarity with either best practice recommendations or the available, albeit limited, data on GI NET treatment. To address the limitations of this study and expand knowledge of real-world treatment patterns, a study using more detailed clinical information such as medical charts or physician surveys is warranted. In addition, future studies should consider using databases that would allow for greater longitudinal follow-up, such as registries, to assist in the further understanding of treatment patterns and length of therapy.
NET arise from cells that release hormones in response to nerve stimulation. Insurance claims databases compile coded information related to charges for medical care for large populations, but they do not contain clinically detailed records.
The article aims to describe real-world treatment patterns of GI NET.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amornyotin S, Gazouli M S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Korse CM, Taal BG, van Velthuysen ML, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. Eur J Cancer. 2013;49:1975-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1183] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 3. | Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a seer analysis. J Cancer. 2012;3:292-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3246] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 5. | Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 6. | Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (1)] |
| 7. | Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in Taiwan: a nation-wide cancer registry-based study. PLoS One. 2013;8:e62487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Fraenkel M, Kim M, Faggiano A, de Herder WW, Valk GD; Knowledge NETwork. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer. 2014;21:R153-R163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Shen C, Zhao B, Zhou S, Halperin D, Xu Y, Shih Y, Yao J. Incidence and Prevalence of Neuroendocrine Tumors in the United States 1973-2012. Poster presentation at the. 2016;2016. |
| 10. | NCCN. Neuroendocrine Tumors. National Comprehensive Cancer Network;. 2016; Available from: https://www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf. |
| 11. | Jalbert JJ, Casciano R, Tao B, Dutton T, Brais LK, Pulgar SJ, Berthon A, Gabriel S, Dinet J, Kulke M. Treatment Patterns Among Metastic GEP-NET Patients Treated at a Tertiary Referral Center. Poster presentation at the. 2017;2017. |
| 12. | Chagpar R, Xing Y, Chiang YJ, Feig BW, Chang GJ, You YN, Cormier JN. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. 2012;30:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Giordano SH, Hortobagyi GN, Kau SW, Theriault RL, Bondy ML. Breast cancer treatment guidelines in older women. J Clin Oncol. 2005;23:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Centers for Medicare & Medicaid Services. Which insurance pays first [Internet]. Medicare.gov. Available from: https://www.medicare.gov/supplement-other-insurance/how-medicare-works-with-other-insurance/who-pays-first/which-insurance-pays.html. |