Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6088
Peer-review started: April 27, 2017
First decision: June 8, 2017
Revised: July 21, 2017
Accepted: August 2, 2017
Article in press: August 2, 2017
Published online: September 7, 2017
Processing time: 133 Days and 13.2 Hours
Influence of chronic hyperglycemia on chemical coding of enteric neurons in stomach using pig as a model for human diabetic complications.
Ten pigs were divided into two groups: diabetic (D group, n = 5) and control (C group, n = 5). Pigs constituting the experimental group were given streptozotocin (150 mg/kg). Animals were euthanized six weeks after the induction of diabetes. The samples of stomach were collected from animals of both groups. The cryostat sections were processed for double immunofluorescence staining using primary antisera directed towards pan-neuronal marker (Hu C/D) proteins and/or neuronal isoform of nitric oxide synthase (nNOS), vasoactive intestinal peptide (VIP) and galanin (GAL).
In the control group in the myenteric ganglia (MG) of the corpus we have noted 22.28% ± 1.19% of nNOS positive neurons, while in diabetic group we have found 40.74% ± 2.22% of nNOS immunoreactive perikarya (increase by 82.85 %). In turn in the pylorus we have observed 15.91% ± 0.58% nNOS containing neurons in control animals and 35.38% ± 1.54% in the diabetes group (increase by 122.37%). In the MG of the antrum and submucosal ganglion (SG) in the corpus hyperglycemia did not cause statistically significant changes. With regard to VIP-positive cell bodies in the antrum MG in the control animals we have noted 18.38 ± 1.39% and 40.74% ± 1.77% in the experimental group (increase by 121.65%). While in the corpus we have observed 23.20% ± 0.23% in the control and 30.93% ± 0.86% in the diabetes group (increase by 33.31%). In turn in the pylorus VIP positive cells bodies constituted 23.64% ± 1.56% in the control group and 31.20% ± 1.10% in the experimental group (increase by 31.97%). In the submucosal ganglion in the corpus we have noted 43.61% ± 1.06% in the control animals and 37.00% ± 1.77% in the experimental group (decrease by 15.15%). Expression of GAL-positive perikarya showed statistically significant changes only in the MG of the antrum and pylorus. In the antrum GAL positive perykarya constituted 26.53% ± 1.52% in the control and 36.67% ± 1.02% in the experimental animals (increase by 38.22%). While in the pylorus GAL positive neurons in the control group constituted 16.32% ± 0.92% and 17.99% ± 0.38% in the experimental animals (increase by 10.23%).
Our results support the hypothesis that in the course of diabetes, long term episodes of high glucose serum level may influence the chemical phenotyping of enteric neurons.
Core tip: Our results revealed the neuronal plasticity of enteric neurons within porcine stomach in response to chronic hyperglycemia. We used the pig as a model for human gastrointestinal disorders occurring in people with diabetes. Our study highlights the important role of the enteric nervous system in response to high glucose serum level. We observed a substantial increase in the expression of nitric oxide, galanin and vasoactive intestinal peptide inside the enteric neurons. Since all of the investigated molecules have inhibitory properties, they may be involved in the impairment of the motor function of the stomach occurring in people with long-term diabetes.
- Citation: Bulc M, Palus K, Zielonka Ł, Gajęcka M, Całka J. Changes in expression of inhibitory substances in the intramural neurons of the stomach following streptozotocin- induced diabetes in the pig. World J Gastroenterol 2017; 23(33): 6088-6099
- URL: https://www.wjgnet.com/1007-9327/full/v23/i33/6088.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i33.6088
Diabetes is one of the most frequently diagnosed endocrinopathies worldwide[1,2]. Long-term episodes of hyperglycemia often occur during course of diabetes. As a consequence of poorly-controlled glucose serum level, numerous tissue and organ dysfunctions can occur[3]. The central, peripheral and autonomic neurons are particularly vulnerable to carboxylic stress, which is a consequence of hyperglycemia[4]. One of the many consequences of autonomic system damage are numerous disorders in the gastrointestinal tract[5-7]. They can involve any organ in the digestive system, such as the esophagus, stomach, gallbladder tract, pancreas, small and large intestines. Generally, they are referred to as diabetic gastrointestinal autonomic neuropathy[5,8]. These disorders are not directly life-threatening, but often lead to a significant impairment in the quality of life[9]. Up to 75% of patients with long-term diabetes experience gastric motor dysfunction, leading to the following symptoms: constipation, nausea, heartburn, post-prandial fullness, abdominal pain as well as diarrhea[10,11].
In physiological conditions, the enteric nervous system (ENS) regulates the function of the gastrointestinal tract. ENS (called the “enteric brain”) is independent of the central nervous system control[12]. This unique arrangement of neurons is organized in the muscular and submucosal plexuses and can control secretion processes, absorption and gastrointestinal motility[13]. The functioning of enteric neurons is based on the secretion of a broad spectrum of neurotransmitters, which are structurally and chemically differentiated[14]. The most useful division of bioactive substances of enteric neurons include two classes of neurons: inhibitory and excitatory[12,13]. The nitric oxide (NO) produced by enteric neurons is a major non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmitter which mediates smooth muscle relaxation in the gastrointestinal tract and is, therefore, an important factor in the stomach and gut contractility[14]. Vasoactive intestinal peptide (VIP), together with NO comprise the primary inhibitory NANC neurotransmitters[15]. The biological activity of VIP leads to hyperpolarization of the smooth muscle fibers. Moreover, the relaxation function of VIP involves increased production of NO in smooth muscle cells[14,15]. Consequently, VIP acts as an inhibition factor of gastric emptying and reduced gastric acid secretion. Simultaneously, VIP is involved in the regulation of blood flow in the submucosal layer[16]. It is also essential that this substance has an effective neuroprotective function. This has been confirmed by the increasing survival of neurons previously damaged by lipopolysaccharide, as well as in the case of axotomy[17,18]. Galanin (GAL) can play both inhibitory and excitatory functions, depending on the digestive tract region, as well as the animal species. For example, in the dog ileum galanin is an excitatory factor while in the guinea pig it exhibits an opposite action[19].
Notably, in the field of metabolic disorders, the pig has gained appreciable interest as its metabolic, biochemical and pathophysiological response to diabetes partly mimics that observed in humans[20-22]. It has been shown that the blood supply of the porcine pancreas is similar to the human pancreas and the number of insulin-producing cells is within a similar range as observed in humans, making it a valuable model for the study of diabetes[23]. The most suitable and generally accepted pig model of hyperglycemia is streptozotocine-induced type 1 diabetes, where carbohydrate metabolism and pancreatic insulin secretion remain at a very similar level to humans with long-term diabetes[20]. Thus, taking advantage of its close similarity to humans, we raised the question of how the porcine gastric enteric neurons adapt to hyperglycemia. Therefore, the aim of this study was to provide data on the adaptive changes in chemical phenotyping, including inhibitory substances [vasoactive intestinal polypeptide, galanin and neuronal isoform of nitric oxide synthase (nNOS)] in the gastric enteric neurons of streptozotocin-induced hyperglycemic pigs.
Ten juvenile both sex pigs of the White Large Polish breed, weighing from 17.0 kg to 20 kg, were used in this study. The pigs were randomly divided into two groups: diabetic (D group, n = 5) and control (C group, n = 5) and were housed in cages suitable for pigs. Prior to the experiment initiation, the animals were given one week of acclimatization to observe their general health, to minimize physiological stress and to ensure the proper conduct of the study. All treatment of animals was conducted in compliance with the instructions of the Local Ethical Committee in Olsztyn (Poland) (decision number 13/2015/DTN) with special attention paid to minimizing any stress reaction.
After acclimatization, hyperglycemia in the diabetic group was induced by use of streptozotocin (STZ) (Sigma-Aldrich, St Louis, MO, United States, S0130) 150 mg/kg of body weight, dissolved in a freshly-prepared disodium citrate buffer solution (pH = 4.23, 1 g streptozotocin/10 mL solution). For this purpose, pigs were anesthetized and the solution was administrated via intravenous needle inserted into an ear with continuous infusion for approximately 5 min. To avoid nausea and vomiting after streptozotocin injection, animals were fasted for 18 h before the experiment and the control pigs were injected with equal amounts of vehicle (citrate buffer).
The pigs were continuously observed for 24 h after streptozotocin injection. In order to avoid temporary hypoglycemia, 250 mL of a 50% glucose solution per animal was administered intravenously. The pigs received a normal diet throughout the whole time of the experiment twice a day and tap water ad libitum. The blood glucose level was measured to confirm diabetes. The blood glucose concentration was estimated using an Aceent-200 (Cormay) biochemical analyzer, with the colorimetric measurement at a wavelength of 510 nm/670 nm. For this purpose, capillary blood from the ear was collected. The plasma glucose level was measured prior to the experiment initiation in both control and experimental groups. The next measurement was made 48 h after the induction of diabetes. Subsequent measurements of glucose levels were monitored weekly until the end of the experiment.
Six weeks after streptozotocin injection, animals were deeply anesthetized via intravenous administration of pentobarbital (Vetbutal, Biowet, Poland) and perfused transcardially via the ascending aorta with 4% paraformaldehyde in a 0.1 mol/L phosphate buffer (PB, pH 7.4). The samples were post-fixed by immersion in the same fixative for 1 h, rinsed several times with phosphatase buffer (PB) and then transferred into 30% sucrose solution and stored at 4 °C until sectioning. The tissue blocks were cut in frontal or sagittal planes using a Microm HM 560 cryostat (Carl Zeiss, Germany) at a thickness of 12 μm and mounted on gelatinized glass slides.
The sections were processed for a double immunofluorescence staining. Briefly, after air-drying at room temperature for 45 min and rinsing in 0.1 mol/L phosphate-buffered saline (PBS; pH 7.4; 3 × 10 min), the sections were incubated in a blocking buffer containing: 10% of normal goat serum (MP Biomedicals, United States), in 0.1 mol/L PBS, 0.1% donkey serum (Abcam, United Kingdom), 1% Triton X-100 (Sigma-Aldrich, United States), 0.05% Thimerosal (Sigma-Aldrich, United States) and 0.01% NaN3 for 1 h at room temperature to reduce non-specific background staining. Subsequently, after another wash in PBS (3 × 10 min) the sections were incubated overnight at 4 °C with primary antibodies raised in different species and directed towards general neuronal marker Hu C/D proteins (mouse polyclonal: Invitrogene United States; code A-212711:1000 working diluted 1:1000), anti-nNOS antibodies (rabbit polyclonal: Chemicon, Billerica, MA, United States, cat. No. AB 5380; working dilution 1:4000), VIP (rabbit polyclonal; Biomol, Hamburg, Germany, cat. No. VA1285; working dilution 1:6000) and GAL (rabbit polyclonal: Millipore, Billerica, MA, United States, cat. No. AB 2233; working dilution 1:1000). All antibodies were diluted in PBS containing 0.3% Triton X-100 and 1% BSA. On the following day, the sections were rinsed (PBS, 3 × 15 min) and incubated with secondary antibodies (donkey anti-mouse Alexa Fluor 488, 1:1000 Invitrogen United States; code A21202, and donkey anti-rabbit Alexa Fluor 546 1:1000 Invitrogen, United States; code A10040) diluted in PBS containing 0.25% BSA and 0.1% Triton X-100) for 4 h. The sections were then rinsed three times (PBS, 3 × 5 min) and mounted in fluorescent mounting medium (DAKO, Carpinteria, CA, United States). The prepared specimens were viewed and photographed using an Olympus BX51 microscope equipped with epi-fluorescence and appropriate filter sets, coupled with a digital monochromatic camera (Olympus XM 10) connected to a PC and analyzed with Cell Dimension software (Olympus, Tokyo, Japan). Standard controls, i.e., pre-absorption of the neuropeptide antisera with appropriate antigen, omission, and replacement of the primary antisera by non-immune sera, were performed to test the antibodies and specificity of the method. The test was performed as follows: sections of the stomach were incubated with “working” dilution of the primary immunoserum, which had been previously pre-absorbed for 18 h at 37 °C with 20 μg of appropriate purified protein VIP (064-24, Phoenix Pharmaceutical), GAL (026-06, Phoenix Pharmaceutical) and nNOS (N3033, Sigma, St Louis, MO, United States). Additional negative controls, such as omission and replacement of all primary antisera with non-immune sera, were also performed. This procedure completely eliminated specific staining.
The number of nNOS, VIP and GAL-like immunoreactive (LI) enteric neurons was expressed as a percentage of the total number of Hu C/D positive perikarya. At least 700 Hu C/D labeled cell bodies of intramural ganglia and each part of the stomach were examined. Only neurons with well-visible nucleus were counted. To prevent the double-counting of Hu C/D immunoreactive neurons, the sections were located at least 100 μm apart. Data pooled from all animals groups were statistically analyzed using Statistica 10 software (StatSoft Inc., Tulsa, OK, United States) and were expressed as a mean ± SE. Significant differences were evaluated using Student’s t-test for independent samples (aP < 0.05, bP < 0.01, and cP < 0.001).
The baseline plasma glucose level measured in animals before STZ treatment and the development of hyperglycemia were both within standard reference values for the pig (5.01 mmol/L ± 0.10 mmol/L). During the weeks following STZ implementation, a consistent increase in plasma glucose concentration was observed. A significant 3.4 -fold (17.36 mmol/L ± 0.38 mmol/L) increase in glucose level was observed on the 7th day after STZ injection and 4-5 wk after the injection the highest increase (4.4 fold) level of glucose was observed (22.26 mmol/L ± 1.21 mmol/L). In the last week of experiment, the baseline serum glucose level decreased slightly, reaching 21.24 mmol/L ± 1.11 mmol/L. The mean glucose concentration during the experimental period is presented in Table 1.
| Date | Control group(mmol/L) | SE | Experimental group (mmol/L) | SE |
| Before streptozotocin injection | 5.01 | 0.10 | 5.030 | 0.10 |
| 1 wk after streptozotocin injection | 5.08 | 0.10 | 17.36 | 0.38 |
| 2 wk after streptozotocin injection | 4.91 | 0.18 | 20.72 | 0.24 |
| 3 wk after streptozotocin injection | 5.19 | 0.06 | 21.58 | 0.27 |
| 4 wk after streptozotocin injection | 5.31 | 0.12 | 20.08 | 0.09 |
| 5 wk after streptozotocin injection | 4.84 | 0.32 | 22.26 | 1.21 |
| 6 wk after streptozotocin injection | 5.20 | 0.10 | 21.45 | 1.11 |
Despite significant hyperglycemia, none of the diabetic animals showed any abnormalities throughout the entire period of the experiment. Moreover, none of the animals required exogenous insulin injection.
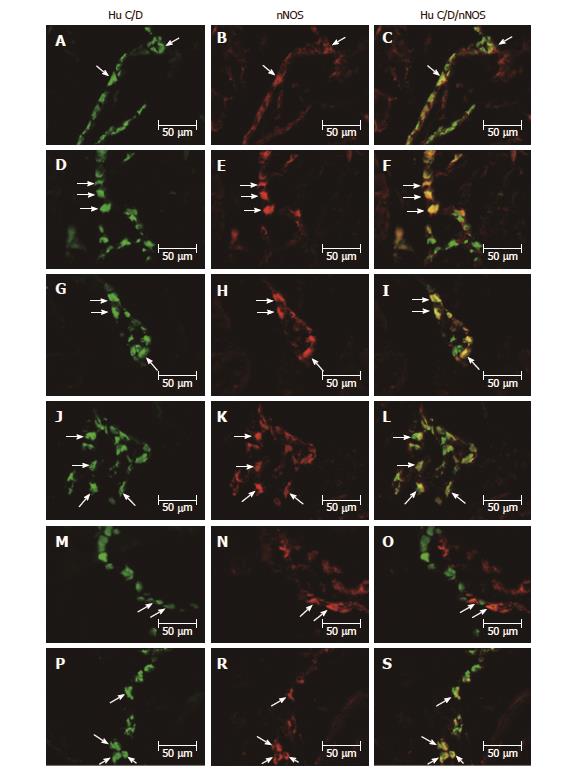
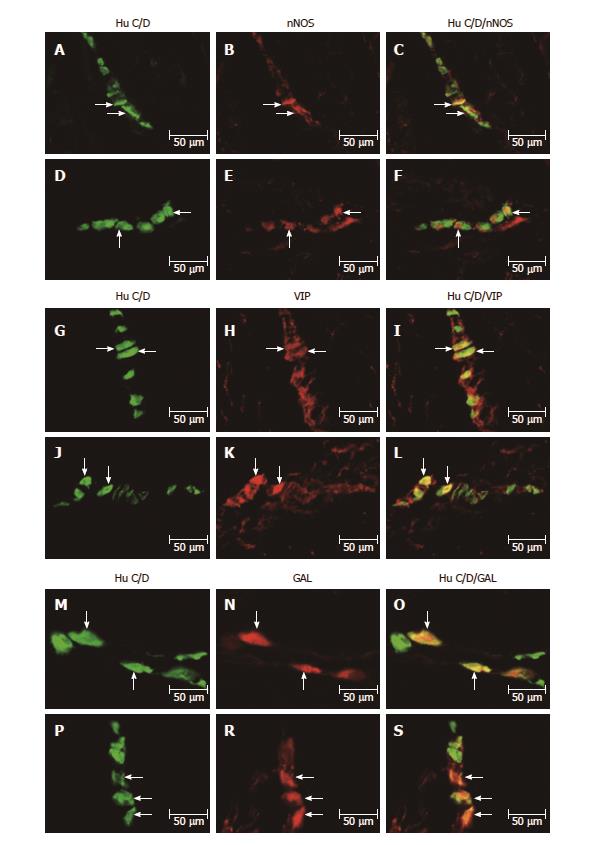
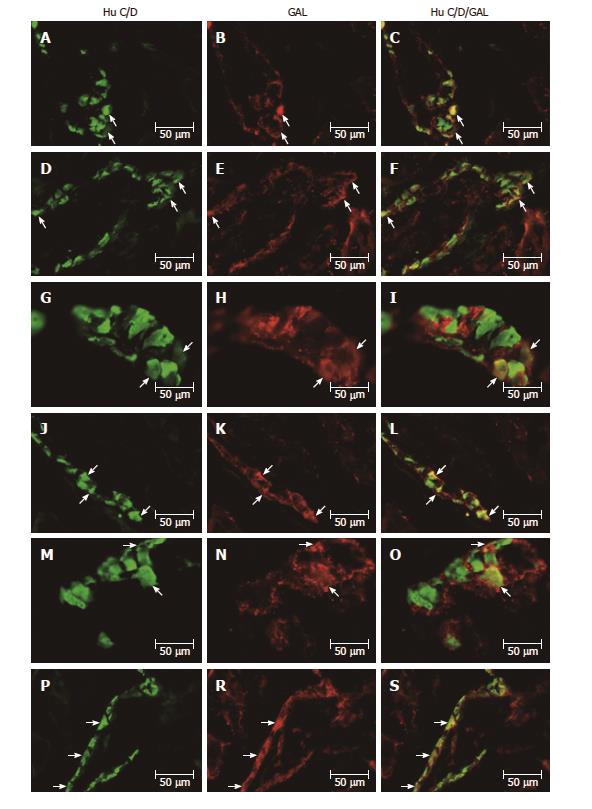
All biologically active substances studied were presented in the investigated area of the stomach (Table 2). In the control group, the nNOS distribution varied and clearly depended on the analyzed area of the stomach (Figure 1A-S and Figure 2A-F). In the myenteric ganglia (MG) of the antrum, the total number of nNOS neurons was 37.12% ± 2.81% (Figure 1A-C), while in the corpus, the quantity of nNOS positive cells bodies inside the MG was relatively lower 22.28% ± 1.19% (Figure 1G-I). Additionally, in submucosal ganglion (SG) in the corpus, nNOS-LI neurons were estimated at 18.62% ± 1.66% (Figure 2A-C). In the pylorus, in MG about 15.91% ± 0.58% nNOS-LI were observed (Figure 1M-O).
| Stomach part | Control group | Experimental group | ||||
| Antrum | Corpus | Pylorus | Antrum | Corpus | Pylorus | |
| nNOS1 | nNOS1 | |||||
| Myenteric ganglia | 37.12 ± 2.81 | 22.28 ± 1.19 | 15.91 ± 0.58 | 41.93 ± 2.34 | 40.74b ± 2.22 | 35.38c ± 1.54 |
| Submucosal ganglia | - | 18.62 ± 1.66 | - | - | 19.79 ± 1.51 | - |
| VIP1 | VIP1 | |||||
| Myenteric ganglia | 18.38 ± 1.39 | 23.2 ± 0.23 | 23.64 ± 1.56 | 40.74c ± 1.77 | 30.93c ± 0.86 | 31.20a ± 1.10 |
| Submucosal ganglia | - | 43.61 ± 1.06 | - | - | 37.00a ± 1.77 | - |
| GAL1 | GAL1 | |||||
| Myenteric ganglia | 26.53 ± 1.52 | 17.73 ± 1.12 | 16.32 ± 0.43 | 36.67b ± 1.02 | 16.51 ± 0.92 | 17.99a ± 0.38 |
| Submucosal ganglia | - | 41.42 ± 0.88 | - | - | 40.49 ± 0.63 | - |
Although experimentally-induced hyperglycemia contributed to the expression of nNOS in enteric neurons, the changes were differentiated. Statistically significant changes were observed in the MG in the corpus from 22.28% ± 1.19% to 40.74% ± 2.22% (increase by 82.85%) (Figure 1J-L) increased of 18.46% and in the pylorus from 15.91%±0.58% to 35.38% ± 1.54% (increase by 122.37%) (Figure 1P-S) increased of 19.47%. In contrast, in the MG in the antrum and SG in the corpus, hyperglycemia did not cause statistically significant changes (Figures 1D-F and 2D-F).
The other investigated substance was VIP. As in the case of nNOS, VIP-positive cells were presented in all studied areas, but clear differences were noted between the various regions of the stomach (Figures 2G-L and 3A-S). In the control group the largest number of VIP-positive cells bodies were noted within the SG in the corpus 43.61% ± 1.06% (Figure 2G-I). Definitely fewer VIP-positive perikarya were observed within the MG in the antrum 18.38% ± 1.39% (Figure 3A-C), pylorus 23.64% ± 1.56% (Figure 3M-O) and MG of the corpus 23.20% ± 0.23% (Figure 3G-I).
In the diabetic group, statistically significant changes were observed in all investigated areas. The highest increase in VIP-immunopositive perikarya was noted in the MG inside the corpus from 18.38% ± 1.39% to 40.74% ± 1.77% (increase by 121.65%) (Figure 3J-L). With regard to MG in the antrum and pylorus, the changes in chemical coding were relativity smaller from 23.20% ± 0.23% to 30.93% ± 0.86% (increase by 33.31%) inside to antrum (Figure 3D-F) and from 23.64 ± 1.56% to 30.47% ± 1.10% inside to pylorus, respectively (increase by 31.97%) (Figure 3P-S). However, in the SG of the corpus, a decrease in the number of VIP-LI neurons was noted from 43.61% ± 1.06% to 37.00% ± 1.77% (decrease by 15.15%) (Figure 2J-L).
As in the case of previously studied substances, the distribution of GAL varied and clearly depended on the area of the stomach (Figure 2M-S and 4A-S). In the control group, the largest population of cells were observed in the MG of antrum 26.53% ± 1.52% (Figure 4A-C). In the MG in the area of corpus and pylorus, a comparable number of GAL-positive neurons were observed 17.73% ± 1.12% in the corpus (Figure 4G-I) and 16.32% ± 0.43% in the pylorus (Figure 4M-O). The highest population of GAL-positive perikarya was noted in the SG in the corpus 41.42% ± 0.88% (Figure 2M-O).
In the diabetic group, statistically significant changes were noted only in the MG in the antrum and pylorus, whereas increased expression of GAL-LI in enteric neurons was observed from 26.53% ± 1.52% to 36.67% ± 1.02% in the antrum (increase by 38.22%) (Figure 4D-F) and from 17.73% ± 1.12% to 17.99% ± 0.38% in the pylorus (increase by 10.23%) (Figure 4P-S). However, in the corpus in both the MG and SG, the population of GAL-LI neurons changed in a statistically insignificant manner compared to the control group (Figures 4J-L and 2P-S).
Autonomic neuropathy is a commonly occurring complication in poorly-controlled blood glucose level patients. Studies of the development mechanisms of this pathology have been conducted in many research center[4,5,8,23]. Moreover, different animal models have been used to determinate the exact mechanism of diabetes complications[24]. Thus, there are doubts as to whether the pig is a suitable model for the study of diabetic autonomic neuropathy occurring in patients with long-term diabetes. Unfortunately, since spontaneous diabetes in large domestic animals such as the pig are extremely rare[25], it is important to select an appropriate method leading to the induction of diabetes and, thus, the development of hyperglycemia. Total pancreatectomy is a technique involving a considerable mortality rate of animals and is not presently used[25]. Therefore, in the present study we used streptozotocin administered intravenously in order to induce diabetes. This method is widely-accepted and used in many studies to determine the side-effects of elevated blood glucose[26]. In our study, the choice of streptozotocin as a diabetogenic substance also turned out to be the opposite, because all animals developed hypoglycemia during the first week of experiment and we did not note mortality falling during the study. Moreover, it is worth underlining that the type 1 STZ-induced hyperglycemic/diabetic pig model has been predominately validated in studies of the cardiovascular complications as well as in peripheral neuropathy[27,28]. On the other hand, diabetic gastroenteropathy, especially concerning the chemical phenotyping of enteric neurons in this model is unclear. The available data describing neurochemical changes in the ENS of hyperglycemic/diabetic animals are limited to the studies on small animal models, mainly rat and mice[29-34].
Our results demonstrated that the porcine ENS, exhibits alterations in the chemical coding of the inhibitory neurons after six weeks of sustained hyperglycemia. It is generally recognized that NO, together with a VIP, acts as inhibitory neurotransmitter in muscle and submucosal layer of gastrointestinal tract[14,15]. As shown in our results, a visible increase in nNOS expression was presented in the myenteric ganglion of the corpus and pylorus. Contrary to these results, in the MG of the antrum and in the SG of the corpus, changes in the synthesis of nNOS have not been noted. As mentioned above, the previous studies, of the chemical coding of enteric neurons were conducted mainly on rodents. The obtained results indicate a differential response of enteric neurons, depending on the duration time of hyperglycemia and the gastrointestinal tract region studied. Belai et al[29,30] described a decrease in nNOS expression in the antrum, while in the small intestine and colon the same authors did not find statistically important changes compared to the control group. Meanwhile, another study revealed an increased level of nNOS in the antrum during streptozotocin-induced diabetes in rats[32]. In turn, hyperglycemia evoked a visible increase in the number of myenteric neurons in all investigated areas and a slight decrease in VIP-LI immunoreactivity in the submucosal ganglia in the corpus. As previously reported in diabetic rats, chronic hyperglycemia caused a marked increase in the immunoreactivity of VIP, in both the submucosal and myenteric ganglion. However, in the myenteric plexus, the increase was preceded by a slight decrease in the intensity of VIP immunoreactivity[31,34]. It is worth underlining that since these results focused on the ileum, we cannot directly relate them to the stomach.
Galanin is a neurotransmitter which can play an inhibitory or excitatory role depending on the investigated area of gastrointestinal tract as well as animal species. Galanin is also a modulator regulating the activity of other neurotransmitters, including VIP and NO. In this study, although we have shown an essential increase of galanin expression in myenteric ganglion neurons in the antrum and pylorus, in the corpus of the stomach we did not observe statistically significant changes compared to the control group. To date, an increase in galanin immunoreactivity has only been observed in the colon of non-obese diabetic mice (NOD) and the ileum of 12-wk-old diabetic rats[31,35]. Only in obese mice with coexisting diabetes has a decrease of galanin in the colon been noted[35]. Taking into account our results and the available data on the expression of neuroactive substances in enteric neurons (especially on inhibitory properties) the common pattern of changes is generally acceptable, i.e. inhibitory neurotransmitters such as nNOS, VIP and GAL appear to decrease in the early stage of diabetes with an increase in later stages, possibly secondary to regeneration[7]. Our results clearly indicate that the neurotoxic effect of chronic hyperglycemia in pigs leads to neuronal regeneration, which is reflected by an up-regulation of the synthesis of NO, VIP and GAL. It is well-known that NO and VIP have strong neuroprotective activity[36-40]. Therefore, the ENS adapts to changes in environmental conditions, increasing the synthesis of biologically active substances which may play a protective role against cellular oxidative stress as a consequence of high glucose serum levels. Moreover, we have noted down-regulation of VIP expression in the corpus of the submucosal ganglion. The different reaction of submucosal neurons probably results from another function played by neurons which leads to regulation of secretory activity[16]. It is worth noting that since other investigated substances within the submucosal ganglion in the corpus of the stomach did not show statistically significant changes, we can conclude that these neurons have a different sensitivity to high glucose level.
Our results may partly explain the reason of gastrointestinal motility disorders observed in people with long-term diabetes. Increase of the expression of inhibitory substances may impair the function of the pyloric sphincter relaxation and the antrum contraction. Which, in turn, contributes to gastric emptying which has been observed in humans[11].
In conclusion, this is the first report describing changes in the expression of inhibitory substances in the intramural neurons of the stomach in hyperglycemic STZ-induced diabetic pigs. Our results indicate that after six weeks of high glucose serum level expression of inhibitory substances (such as NO), vasoactive intestinal polypeptide and galanin undergo significant changes (described as chemical phenotyping), which suggests that STZ-injected pigs might serve as a good model in early studies of autonomic nerve changes under hyperglycemic conditions. Furthermore, our data provide a background for more detailed studies on the contribution of enteric neurons to the pathogenesis of gastrointestinal disturbances as well as reference data for further, pre-clinical studies on hyperglycemia-related autonomic neuronal changes in a species more closely related to humans.
Diabetes mellitus is associated with several changes in gastrointestinal (GI) tract motility that lead to nausea, bloating, abdominal pain, diarrhoea and constipation. Up to 75% of patients with diabetes can experience these symptoms. The pathogenesis of altered GI functions in diabetes is multifactorial and the role of the enteric nervous system (ENS) in this respect has gained significant attention. The neuronal remodelling affects the ratio of inhibitory neurons, which in turn leads to impaired of nerve mediated muscle responses and can contribute to the motility dysfunction seen in diabetes patients.
Streptozotocin is widely used to induce experimental diabetes in animals. Its application leads to blood insulin levels decrease and result in development of hyperglycaemia. Long term elevated glucose level cause diabetic neuropathy.
This is the first study utilizing pig as a model species for human diabetes induced gastrointestinal complications. The results suggest that swine due to close physiological similarity to human especially concerning gastrointestinal tract function seems to be better model for biomedical research than rodents.
The results provide evidence that acute hyperglycaemia changes properties of neuromodulators/neurotransmitters in GI. Pharmacological modulation of selected biologically active substances can be potentially new approach for treatment of the diabetes evoked gastrointestinal complications.
Streptozotocin treatment supplies a substrate for xanthine oxidase resulting in the formation of superoxide radicals. Consequently, hydrogen peroxide and hydroxyl radicals are also generated. Furthermore, streptozotocin liberates toxic amounts of nitric oxide that inhibits aconitase activity and participates in DNA damage. As a result of the streptozotocin action B cells undergo the destruction by necrosis.
The authors have provided evidence that long term episodes of artificially initiated hyperglycaemia induced changes of expression of neuronal isoform of nitric oxide synthase, vasoactive intestinal peptide and galanin in inhibitory neurons inside the stomach ENS. Those changes might be responsible for broad spectrum of gastrointestinal disturbances.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Plaza MA S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (0)] |
| 2. | Thomas CC, Philipson LH. Update on diabetes classification. Med Clin North Am. 2015;99:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1026] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 4. | Pafili K, Maltezos E, Papanas N. NC-stat for the diagnosis of diabetic polyneuropathy. Expert Rev Med Devices. 2017;14:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Gatopoulou A, Papanas N, Maltezos E. Diabetic gastrointestinal autonomic neuropathy: current status and new achievements for everyday clinical practice. Eur J Intern Med. 2012;23:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Demedts I, Masaoka T, Kindt S, De Hertogh G, Geboes K, Farré R, Vanden Berghe P, Tack J. Gastrointestinal motility changes and myenteric plexus alterations in spontaneously diabetic biobreeding rats. J Neurogastroenterol Motil. 2013;19:161-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 8. | Bagyánszki M, Bódi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes. 2012;3:80-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 9. | Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, Driscoll M, Harth J, Larkin M, Christofi M. Delayed Gastric Emptying Is Associated With Early and Long-term Hyperglycemia in Type 1 Diabetes Mellitus. Gastroenterology. 2015;149:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Rodrigues ML, Motta ME. Mechanisms and factors associated with gastrointestinal symptoms in patients with diabetes mellitus. J Pediatr (Rio J). 2012;88:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Camilleri M. Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am. 1996;25:361-378. [PubMed] |
| 12. | Furness JB. The organisation of the autonomic nervous system: peripheral connections. Auton Neurosci. 2006;130:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277:G922-G928. [PubMed] |
| 14. | Bulc M, Gonkowski S, Landowski P, Kamińska B, Całka J. Immunohistochemical evidence of the co-localisation of cocaine and amphetamine regulatory peptide with neuronal isoform of nitric oxide synthase, vasoactive intestinal peptide and galanin within the circular muscle layer of the human caecum. Folia Morphol (Warsz). 2015;74:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Palus K, Rytel L. Co-localisation of cocaine- and amphetamine-regulated transcript peptide and vasoactive intestinal polypeptide in the myenteric plexus of the porcine transverse colon. Folia Morphol (Warsz). 2013;72:328-332. [PubMed] |
| 16. | Whittaker VP. Vasoactive intestinal polypeptide (VIP) as a cholinergic co-transmitter: some recent results. Cell Biol Int Rep. 1989;13:1039-1051. [PubMed] |
| 17. | Arciszewski MB, Sand E, Ekblad E. Vasoactive intestinal peptide rescues cultured rat myenteric neurons from lipopolysaccharide induced cell death. Regul Pept. 2008;146:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Kamińska B, Gonkowski S, Korzon M, Bossowska A, Landowski P, Majewski M. Relations between Leu(5)-enkephalin- (LENK) and VIP-immunoreactive nerve fibres during human drug-resistant colitis. A case study. Folia Morphol (Warsz). 2003;62:509-511. [PubMed] |
| 19. | Arciszewski MB, Barabasz S, Całka J. Immunohistochemical localization of galanin receptors (GAL-R1, GAL-R2, and GAL-R3) on myenteric neurons from the sheep and dog stomach. Ann Anat. 2008;190:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Larsen MO, Rolin B. Use of the Göttingen minipig as a model of diabetes, with special focus on type 1 diabetes research. ILAR J. 2004;45:303-313. [PubMed] |
| 21. | Larsen MO, Wilken M, Gotfredsen CF, Carr RD, Svendsen O, Rolin B. Mild streptozotocin diabetes in the Göttingen minipig. A novel model of moderate insulin deficiency and diabetes. Am J Physiol Endocrinol Metab. 2002;282:E1342-E1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Swindle MM. The development of swine models in drug discovery and development. Future Med Chem. 2012;4:1771-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Brock C, Graversen C, Frøkjaer JB, Søfteland E, Valeriani M, Drewes AM. Peripheral and central nervous contribution to gastrointestinal symptoms in diabetic patients with autonomic neuropathy. Eur J Pain. 2013;17:820-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005;22:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 397] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 25. | Grüssner R, Nakhleh R, Grüssner A, Tomadze G, Diem P, Sutherland D. Streptozotocin-induced diabetes mellitus in pigs. Horm Metab Res. 1993;25:199-203. [PubMed] |
| 26. | Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1222] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 27. | Fricker J. The pig: a new model of diabetic atherosclerosis. Drug Discov Today. 2001;6:921-922. [PubMed] |
| 28. | Juranek JK, Aleshin A, Rattigan EM, Johnson L, Qu W, Song F, Ananthakrishnan R, Quadri N, Yan SD, Ramasamy R. Morphological Changes and Immunohistochemical Expression of RAGE and its Ligands in the Sciatic Nerve of Hyperglycemic Pig (Sus Scrofa). Biochem Insights. 2010;2010:47-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Belai A, Burnstock G. Changes in adrenergic and peptidergic nerves in the submucous plexus of streptozocin-diabetic rat ileum. Gastroenterology. 1990;98:1427-1436. [PubMed] |
| 30. | Belai A, Lincoln J, Milner P, Crowe R, Loesch A, Burnstock G. Enteric nerves in diabetic rats: increase in vasoactive intestinal polypeptide but not substance P. Gastroenterology. 1985;89:967-976. [PubMed] |
| 31. | Belai A, Calcutt NA, Carrington AL, Diemel LT, Tomlinson DR, Burnstock G. Enteric neuropeptides in streptozotocin-diabetic rats; effects of insulin and aldose reductase inhibition. J Auton Nerv Syst. 1996;58:163-169. [PubMed] |
| 32. | Wrzos HF, Cruz A, Polavarapu R, Shearer D, Ouyang A. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig Dis Sci. 1997;42:2106-2110. [PubMed] |
| 33. | Adeghate E, al-Ramadi B, Saleh AM, Vijayarasathy C, Ponery AS, Arafat K, Howarth FC, El-Sharkawy T. Increase in neuronal nitric oxide synthase content of the gastroduodenal tract of diabetic rats. Cell Mol Life Sci. 2003;60:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Ballmann M, Conlon JM. Changes in the somatostatin, substance P and vasoactive intestinal polypeptide content of the gastrointestinal tract following streptozotocin-induced diabetes in the rat. Diabetologia. 1985;28:355-358. [PubMed] |
| 35. | El-Salhy M. Gastrointestinal transit in nonobese diabetic mouse: an animal model of human diabetes type 1. J Diabetes Complications. 2001;15:277-284. [PubMed] |
| 36. | Rytel L, Calka J. Acetylsalicylic acid-induced changes in the chemical coding of extrinsic sensory neurons supplying the prepyloric area of the porcine stomach. Neurosci Lett. 2016;617:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Palus K, Całka J. The Influence of Prolonged Acetylsalicylic Acid Supplementation-Induced Gastritis on the Neurochemistry of the Sympathetic Neurons Supplying Prepyloric Region of the Porcine Stomach. PLoS One. 2015;10:e0143661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Palus K, Całka J. Neurochemical Plasticity of the Coeliac-Superior Mesenteric Ganglion Complex Neurons Projecting to the Prepyloric Area of the Porcine Stomach following Hyperacidity. Neural Plast. 2016;2016:8596214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Burliński PJ, Rychlik A, Całka J. Effects of inflammation and axotomy on expression of acetylcholine transferase and nitric oxide synthetase within the cocaine- and amphetamine-regulated transcript-immunoreactive neurons of the porcine descending colon. J Comp Pathol. 2014;150:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Palus K, Całka J. Alterations of neurochemical expression of the coeliac-superior mesenteric ganglion complex (CSMG) neurons supplying the prepyloric region of the porcine stomach following partial stomach resection. J Chem Neuroanat. 2016;72:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |