Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5977
Peer-review started: February 9, 2017
First decision: April 21, 2017
Revised: May 12, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: August 28, 2017
Processing time: 206 Days and 11.4 Hours
To evaluate the efficacy and safety of HL tablet extracted from magnolia officinalis for treating patients with nonalcoholic fatty liver disease (NAFLD).
Seventy-four patients with NAFLD diagnosed by ultrasonography were randomly assigned to 3 groups given high dose (400 mg) HL tablet, low dose (133.4 mg) HL tablet and placebo, respectively, daily for 12 wk. The primary endpoint was post-treatment change of hepatic fat content (HFC) measured by magnetic resonance spectroscopy. Secondary endpoints included changes of serum aspartate aminotransferase, alanine aminotransferase (ALT), cholesterol, triglyceride, free fatty acid, homeostasis model assessment-estimated insulin resistance, and body mass index (BMI).
The mean HFC of the high dose HL group, but not of the low dose group, declined significantly after 12 wk of treatment (high dose vs placebo, P = 0.033; low dose vs placebo, P = 0.386). The mean changes of HFC from baseline at week 12 were -1.7% ± 3.1% in the high dose group (P = 0.018), -1.21% ± 4.97% in the low dose group (P = 0.254) and 0.61% ± 3.87% in the placebo group (relative changes compared to baseline, high dose were: -12.1% ± 23.5%, low dose: -3.2% ± 32.0%, and placebo: 7.6% ± 44.0%). Serum ALT levels also tended to decrease in the groups receiving HL tablet while other factors were unaffected. There were no moderate or severe treatment-related safety issues during the study.
HL tablet is effective in reducing HFC without any negative lipid profiles, BMI changes and adverse effects.
Core tip: The prevalence of nonalcoholic fatty liver disease (NAFLD) is growing gradually with the increase in population age and obesity, while effective and safe drugs for NAFLD are not yet available. HL tablet, a new botanic drug extracted from Magnolia officinalis, is effective in reducing hepatic fat content without any negative lipid profiles, body mass index changes and adverse effects.
- Citation: Jeong JY, Sohn JH, Baek YH, Cho YK, Kim Y, Kim H. New botanical drug, HL tablet, reduces hepatic fat as measured by magnetic resonance spectroscopy in patients with nonalcoholic fatty liver disease: A placebo-controlled, randomized, phase II trial. World J Gastroenterol 2017; 23(32): 5977-5985
- URL: https://www.wjgnet.com/1007-9327/full/v23/i32/5977.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i32.5977
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease worldwide, and includes a spectrum of diseases from simple steatosis to nonalcoholic steatohepatitis (NASH), and liver cirrhosis[1]. Some patients with NAFLD have NASH and some of these may progress to liver cirrhosis and develop hepatocellular carcinoma[2]. Also, the prevalence of NAFLD is growing gradually with the increase in population age and obesity[3,4]. Life-style change has been the most effective treatment for NAFLD[5-7], while effective and safe drugs for NAFLD are not yet available[1]. Therefore the demand for new drugs for NAFLD is increasing worldwide.
In NAFLD, visceral adipose tissue emits multiple signals that alter lipid and glucose metabolism, causing fat accumulation and a proinflammatory environment in the liver[1]. These effects lead to injury to the liver and other tissues. Oxidative stress, lipotoxicity, and endoplasmic reticulum and mitochondrial apoptotic pathways contribute to liver damage and promote liver fibrosis[1].
Magnolia officinalis (MO) is a traditional medicinal plant that has been used to treat liver diseases and other diseases[8]. Some constituents extracted from MO have been reported to have anti-inflammatory and antioxidant effects[9,10], and MO can be effective against NAFLD. In vitro honokiol, extracted from MO, induced apoptosis of the activated hepatic stellate cells responsible for hepatic fibrosis[11]. Honokiol and magnolol extracted from MO significantly inhibited hepatic toxicity induced by galactosamine, generation of intracellular reactive oxygen species and glutathione depletion[9]. Also, MO inhibited lipid accumulation in free fatty acid (FFA)-exposed hepatocytes[12]. In another study, MO ameliorated body fat accumulation, insulin resistance, and adipose inflammation in high fat-fed mice[13].
We hypothesized that MO would improve steatosis and inflammation in patients with NAFLD. HL tablet is a new botanical drug extracted from MO. The aim of this study was thus to evaluate the efficacy and safety of HL tablet in the treatment of patients with NAFLD.
Our study was a multi-center, randomized allocation, double-blind, placebo controlled, 3 group parallel, phase II trial between November 2013 and May 2015 in 3 hospitals in the Republic of Korea. It was conducted in patients with NAFLD diagnosed by ultrasonographic examination. The inclusion criteria were (1) age 19-75 years; (2) ultrasonographic features of nonalcoholic fatty liver; and (3) serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) above the upper normal limits. Exclusion criteria were (1) serum AST/ALT > 2; (2) type 1 diabetes; (3) other liver diseases (viral hepatitis, autoimmune hepatitis or biliary obstruction, etc.); (4) excessive alcohol consumption ≥ 30 g/d for men and ≥ 20 g/d for women; (5) use of steatogenic medications (amiodarone, methotrexate, systemic glucocorticoids, tetracycline or tamoxifen, etc.) within the past 3 mo; (6) serious underlying diseases (liver cirrhosis, malignancy, severe metabolic disease, serious kidney disease, serious cardiovascular disease or serious lung disease, etc.); (7) history of bariatric surgery within the past 6 mo; (8) contraindication for magnetic resonance spectroscopy (MRS), such as history of pacemaker implantation or shunt operation; and (9) pregnancy, breastfeeding or hypersensitivity to MO. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of each hospital, and was registered at ClinicalTrial.gov (NCT02491905).
After all the included patients had provided written informed consent, they were randomly assigned (1:1:1) to groups given high dose (400 mg) HL tablet, low dose (133.4 mg) HL tablet or placebo daily for 12 wk. Patients visited the clinic at baseline and after 4, 8, and 12 wk of treatment. At each visit, a physical examination was conducted, adverse drug events were recorded, and compliance with medication was assessed by pill counts. MRS was performed at baseline and after 12 wk of treatment. Body mass index (BMI) and blood tests such as serum AST, ALT, lipid profiles [total cholesterol, triglycerides, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, very low density lipoprotein (VLDL) cholesterol and FFA], and homeostasis model assessment-estimated insulin resistance (HOMA-IR) were performed at baseline and after 8 and 12 wk of treatment. A follow-up phone call related to safety was performed at 16 wk.
MRS of the liver was performed to assess HFC at baseline and after 12 wk of treatment. MRS data were collected on 3.0T human scanners (Philips (Philips Medical Systems, Best, Netherlands) at Hanyang University Guri Hospital (site I) and Kangbuk Samsung Hospital (site II), and on a General Electric scanner (GE Healthcare, Waukesha, United States) at Dong-A University Hospital (site III). Patients were placed inside the magnet in a supine position. Prior to data collection, scout images were acquired along the three orthogonal axes in the end-exhalation breath-hold condition. To account for potential liver heterogeneity, two MRS voxels were defined in the liver (one from S5, 6 and the other from S7, 8 for each patient) avoiding major blood vessels. The edges of the voxels were at least 1 cm from the borders of the liver. A point-resolved spectroscopy (PRESS) sequence was used[14]. The sequence parameters for all three sites were: repetition time (TR) = 2000 ms, spectral bandwidth = 2500 Hz, 2048 data points, and voxel size = 2.5 cm × 2.5 cm × 2.5 cm. At sites I and II, data were collected under the end-exhalation breath-hold condition using echo times (TEs) = 36 and 100 ms, number of receiver channels (NRC) = 32, and number of signal averages (NSA) = 4. At site III, data were collected under the shallow breathing condition using TE = 30 and 100 ms, NRC = 20, and NSA = 8. The data acquired at the two different TEs were used for T2 collection. Immediately after each scan, the quality of the spectrum was examined based on the signal intensities and line shapes (including line widths) of the water (about 4.7 ppm) and fat (about 1.3 ppm) signals. In cases where a spectrum was considered contaminated (e.g., due to patient movement), the scan was repeated with the consent of the patient. After the MRS scan, the raw data were stored. The voxels in the post-treatment MRS scan were carefully co-registered with those in the pre-treatment, baseline scan by referring to the previous data (voxel locations superimposed on the scout images in the three orthogonal directions).
The MRS data were processed with a jMRUI (v5.0)[15]. First, they were Fourier-transformed, line-broadened and phase-corrected, and the peak areas of the water (about 4.7 ppm) and lipid (methylene at about 1.3 ppm) resonances were obtained using AMARES for the individual spectra acquired at two different TEs[16]. Then, the T2’s of water and lipid were corrected by assuming single exponential decay. From the T2-corrected water and lipid signal intensities, a hepatic fat fraction (HFF) was calculated as lipid/(water + lipid) × 100 for each voxel. Finally, the mean HFF value over the two voxels was used as the HFC value for each patient.
The primary endpoint was the post-treatment change in HFC measured by MRS. Secondary endpoints included post-treatment changes of serum AST, ALT, cholesterol, triglycerides, FFA, HOMA-IR, and BMI. Safety assessments included adverse events, laboratory findings, vital signs and electrocardiograms. Adverse events were classified as mild, moderate or severe, and as certainly, probably/likely, possibly, not likely, and not due treatment, or not known.
The 18 subjects in each group provided a power of 90% for identifying a difference of 4.6% in liver fat decrease between placebo and the low dose HL group. In previous studies, pioglitazone was shown to produce a 10% reduction in liver fat compared to baseline and 0% in the placebo[17]. Also, the 8% weight loss group in a weight loss program was shown to have a 4.6% reduction in liver fat compared to baseline[18]. Therefore, we considered that the high dose HL group would have a 10% reduction in liver fat compared to baseline and the low dose HL group a 4.6% reduction. To detect these levels of change, a sample size of 23 subjects in each group was needed, assuming a 20% drop-out rate.
Analyses were conducted principally by full analysis set and were supplemented by per protocol set. Continuous variables are given as mean values with SD or median values with inter-quartile ranges. Categorical variables are given as frequencies or percentages. Baseline characteristics of the placebo group and each HL group (high dose vs placebo, low dose vs placebo) were evaluated using a two sample t-test or Wilcoxon’s rank sum test for continuous variables and Pearson’s χ2 test or Fisher’s exact test for categorical variables. Differences between variables were evaluated using two-sample t-tests or Wilcoxon’s rank sum tests. Changes of HFC from baseline after 12 wk of treatment within each group were evaluated using paired t tests or Wilcoxon signed rank tests. P values < 0.05 in 2-sided tests were considered statistically significant. SPSS 19.0 for Windows (SPSS Inc, Chicago, IL, United States) was used for all statistical analyses.
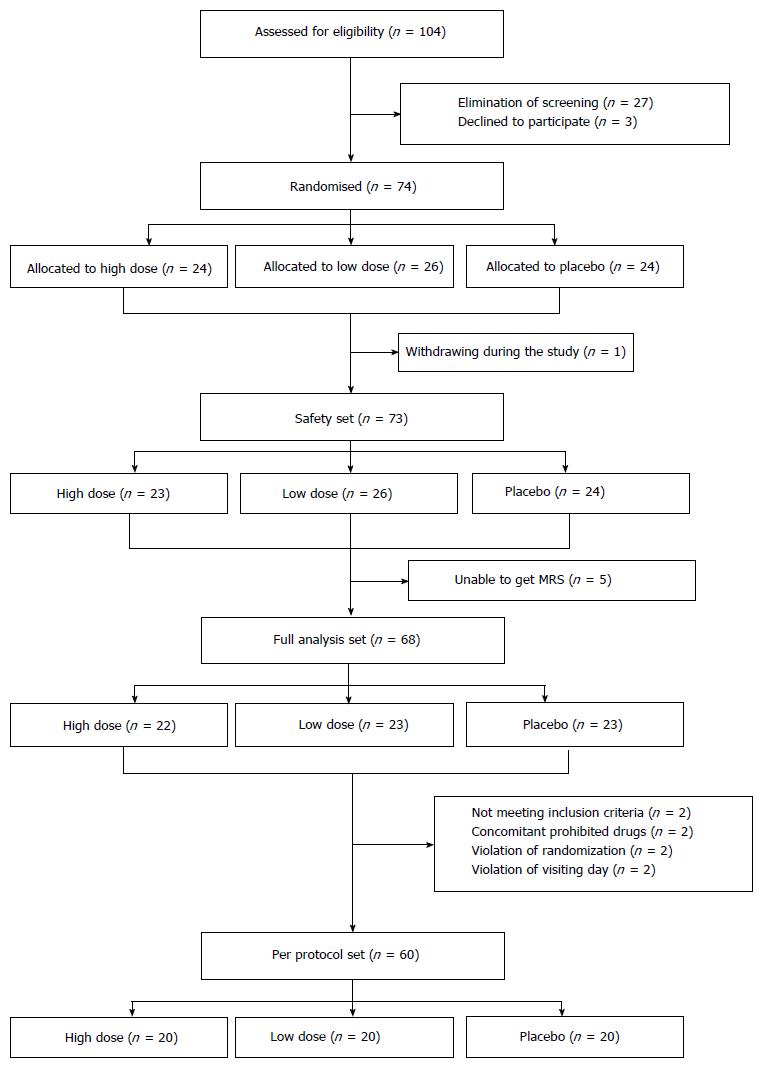
A total of 104 patients were screened in this study. Of these, 74 patients with NAFLD diagnosed by ultrasonographic examination were randomly assigned to groups receiving high dose HL (n = 24), low dose HL (n = 26), and placebo (n = 24), respectively. Finally, a safety analysis set, full analysis set and per protocol set were analyzed in 73, 68, and 60 patients, respectively (Figure 1). Most of the enrolled patients completed the study (60/74; 81.1%).
Baseline characteristics were mostly similar in the 3 groups, as shown in Table 1. Patients in this study had a mean age of 39.1 ± 9.5 years in the high dose HL group, 45.5 ± 11.5 years in the low dose HL group and 42.7 ± 11.2 years in the placebo group. The low dose HL group was significantly low in males compared to the placebo group (P = 0.044). Hepatic fat by MRS showed a tendency to be higher in the high dose HL group than the placebo group (high dose vs placebo: 16.1% ± 7.1% vs 12.0% ± 7.5%, P = 0.065).
| Characteristic | High dose (n = 22) | Low dose (n = 23) | Placebo (n = 23) | P value | |
| High dose vs placebo | Low dose vs placebo | ||||
| Age, yr | 39.1 ± 9.5 | 45.5 ± 11.5 | 42.7 ± 11.2 | 0.474 | 0.355 |
| Males, n (%) | 20 (90.9) | 14 (60.9) | 20 (87.0) | 1.0001 | 0.0441 |
| Height, cm | 170.1 ± 7.1 | 166.7 ± 10.3 | 170.4 ± 8.4 | 0.916 | 0.184 |
| Weight, kg | 82.4 ± 12.1 | 78.4 ± 16.0 | 82.9 ± 13.1 | 0.891 | 0.301 |
| BMI, kg/m2 | 28.2 ± 3.4 | 28.2 ± 4.0 | 28.4 ± 3.7 | 0.805 | 0.855 |
| Above moderate steatosis in US, n (%) | 18 (81.8) | 16 (69.6) | 16 (69.6) | 0.4911 | 1.000 |
| MRS liver fat, % | 16.1 ± 7.1 | 13.3 ± 7.1 | 12.0 ± 7.5 | 0.065 | 0.526 |
| AST, IU/L | 52.0 ± 19.1 | 58.4 ± 24.9 | 46.3 ± 20.5 | 0.461 | 0.077 |
| ALT, IU/L | 89.8 ± 34.8 | 90.3 ± 50.9 | 67.4 ± 32.7 | 0.109 | 0.078 |
| Total cholesterol, mg/dL | 205.0 ± 40.3 | 208.1 ± 31.2 | 194.5 ± 36.2 | 0.155 | 0.179 |
| Triglyceride, mg/dL | 190.3 ± 80.6 | 210.8 ± 137.9 | 286.7 ± 216.1 | 0.308 | 0.162 |
| HDL cholesterol, mg/dL | 46.3 ± 7.1 | 49.3 ± 10.9 | 43.6 ± 9.9 | 0.337 | 0.071 |
| LDL cholesterol, mg/dL | 136.0 ± 36.3 | 133.6 ± 29.7 | 118.6 ± 30.7 | 0.053 | 0.099 |
| VLDL cholesterol, mg/dL | 22.7 ± 11.2 | 25.3 ± 16.3 | 32.4 ± 21.5 | 0.263 | 0.214 |
| Free fatty acid, μEq/L | 526.2 ± 209.5 | 523.7 ± 341.6 | 581.9 ± 634.7 | 0.135 | 0.700 |
| HOMA-IR | 2.6 ± 1.6 | 2.5 ± 1.0 | 3.5 ± 2.4 | 0.167 | 0.3262 |
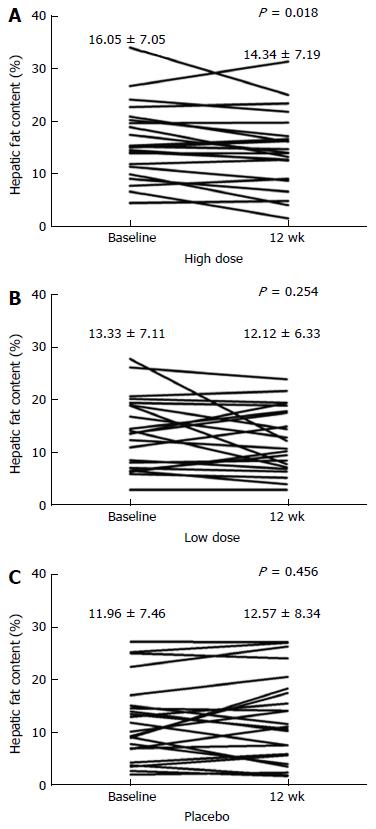
The mean HFC of the high dose HL group, but not of the low dose group, declined significantly after 12 wk of treatment (high dose vs placebo, P = 0.033; low dose vs placebo, P = 0.386) (Table 2). The mean changes of HFC from baseline at week 12 were -1.7% ± 3.1% in the high dose group (P = 0.018), -1.21% ± 4.97% in the low dose group (P = 0.254) and 0.61% ± 3.87% in the placebo group (relative changes compared to baseline, high dose were: -12.1% ± 23.5%, low dose: -3.2% ± 32.0%, and placebo: 7.6% ± 44.0%) (Table 2 and Figure 2). The per protocol analysis yielded similar results for the primary end points in the HL groups.
| Treatment group | Full analysis set | Per protocol set | ||||||
| n | Baseline | 12 wk | P value | n | Baseline | 12 wk | P value | |
| Placebo, % | 23 | 11.96 ± 7.46 | 12.57 ± 8.34 | 20 | 12.59 ± 7.70 | 13.59 ± 8.47 | ||
| Low dose, % | 23 | 13.33 ± 7.11 | 12.12 ± 6.33 | 0.3861 | 20 | 12.99 ± 6.86 | 11.37 ± 6.02 | 0.1641 |
| High dose, % | 22 | 16.05 ± 7.05 | 14.34 ± 7.19 | 0.0332 | 20 | 16.12 ± 7.19 | 14.77 ± 7.15 | 0.0392 |
Secondary end points are shown in Table 3. There was a modest but non-significant decrease in serum ALT level in both HL groups (vs high dose: -12.73 ± 29.30 IU/L, P = 0.097; vs low dose: -13.65 ± 39.56 IU/L, P = 0.153). Serum AST levels were similar in all three groups.
| Variable | High dose (n = 22) | Low dose (n = 23) | Placebo (n = 23) | P value | |
| High dose vs placebo | Low dose vs placebo | ||||
| AST, IU/L | -7.82 ± 20.59 | -2.74 ± 33.79 | -2.96 ± 21.19 | 0.865 | 0.397 |
| ALT, IU/L | -12.73 ± 29.30 | -13.65 ± 39.56 | -0.17 ± 19.58 | 0.097 | 0.153 |
| Total cholesterol, mg/dL | -7.86 ± 27.92 | 2.22 ± 32.89 | -0.61 ± 22.47 | 0.532 | 0.613 |
| Triglyceride, mg/dL | -16.50 ± 52.47 | 29.57 ± 186.19 | -86.91 ± 157.20 | 0.041 | 0.031 |
| HDL cholesterol, mg/dL | 1.32 ± 14.98 | -1.78 ± 8.71 | 1.83 ± 8.62 | 0.289 | 0.567 |
| LDL cholesterol, mg/dL | 9.05 ± 27.78 | -5.35 ± 23.63 | 5.74 ± 19.98 | 0.088 | 0.253 |
| VLDL cholesterol, mg/dL | 0.43 ± 12.05 | 9.52 ± 27.85 | -9.77 ± 17.47 | 0.047 | 0.007 |
| Free fatty acid, μEq/L | -73.23 ± 283.52 | 15.70 ± 304.68 | -73.87 ± 666.93 | 0.146 | 0.921 |
| HOMA IR | -0.01 ± 1.41 | 0.18 ± 0.77 | -1.32 ± 2.44 | 0.174 | 0.019 |
| BMI, kg/m2 | 0.01 ± 0.81 | -0.06 ± 0.97 | -0.20 ± 0.80 | 0.608 | 0.904 |
Levels of triglycerides decreased significantly in the placebo group (-86.91 ± 157.20 mg/dL) compared with each HL group (vs high dose: -16.50 ± 52.47 mg/dL, P = 0.041; vs low dose: 29.57 ± 186.19 mg/dL, P = 0.031). And levels of VLDL cholesterol also decreased significantly in the placebo group (-9.77 ± 17.47 mg/dL) compared with the HL groups (vs high dose: 0.43 ± 12.05 mg/dL, P = 0.047; vs low dose: 9.52 ± 27.85 mg/dL, P = 0.007). Other lipid profiles (total cholesterol, HDL cholesterol, LDL cholesterol, and FFA) were similar in the three groups, as was BMI. A small decrease in HOMA-IR, was observed in the placebo group whereas no change was seen in the low HL group (P = 0.019). However, there was no difference in HOMA-IR between placebo and the high HL group. Per protocol analysis yielded a similar pattern for secondary end points (Supplement Table 1).
Four patients (17.4%) treated with high dose HL, 10 patients (38.5%) treated with low dose HL and 9 placebo patients (37.5%) experienced at least one adverse event (Supplementary Table 2). Treatment-related adverse events are shown in Table 4. All the treatment-related adverse events were mild, and no patient was withdrawn because of a treatment-related events.
| System organ class preferred term | High dose (n = 23) | Low dose (n = 26) | Placebo (n = 24) |
| Gastrointestinal disorders | |||
| Abdominal pain upper | 0 | 1 (3.85) | 0 (0) |
| Nausea | 0 | 0 (0) | 1 (4.17) |
| Nervous system disorders | |||
| Dizziness | 0 | 1 (3.85) | 0 (0) |
In this randomized, double-blind and placebo-controlled study, we evaluated the efficacy and safety of HL tablet extracted from MO for hepatic fat reduction in patients with NAFLD. Treatment with HL tablet for 12 wk lowered hepatic fat measured by MRS and did not result in any negative lipid profiles or changes of BMI. The HL tablet was well-tolerated.
At the present time, no effective and safe pharmacologic therapy for patients of NAFLD is available[1]. Weight loss due to life style modifications such as diet and excise has been the most effective therapy for NAFLD, leading to reduction of intrahepatic fat content; however, maintaining such benefits is difficult[1,19]. Pioglitazone, a PPAR-γ agonist, significantly decreased HFC, FFA levels and insulin resistance in patients with NASH, and improved histologic findings such as hepatic steatosis and inflammation[17,20,21]. However long-term administration of pioglitazone carries risks of weight gain, postmenopausal bone loss or malignancies such as bladder cancer[20-24]. Vitamin E is an antioxidant, and high dose RRR-α-tocopherol (800 IU/d) improves serum AST levels, serum ALT levels and histologic findings[20], but safety concerns have also been raised about long-term administration of vitamin E[1]. Obeticholic acid (OCA, 6α-ethyl-chenodeoxycholic acid), a farnesoid X receptor agonist, is receiving attention for patients with NAFLD[25]. In phase IIb randomized trial (the FLINT study), OCA led to histological improvement, including reduced hepatic fibrosis in non-cirrhotic patients with NASH[26]. However, histological improvement was seen in only 45% of those treated with OCA, and pruritus, the main side effect, occurred in 23% of the patients[26].
The reason for progression from simple steatosis to NASH is not clear. However, the most likely idea is that triglycerides accumulate in hepatocytes due to increased peripheral insulin resistance (“first hit” theory), and then oxidative stress causes fat peroxidation, liver cell damage and inflammatory cytokine activation ("second hit" theory)[27]. This implies that fat accumulation in hepatocytes is the initial cause of NASH. Given that fat accumulation in hepatocytes can lead to NASH, reducing intrahepatic fat by treatment with HL tablet would be a key to preventing the "first hit" leading to NAFLD and this would prevent the transition to NASH. Furthermore, despite treatment for only 12 wk, there was a significant reduction of mean HFC in the high HL group, and we anticipate that treatment for 24 wk would have a greater effect.
In patients with NAFLD, elevation in serum ALT may correlate with HFC and insulin resistance[28]. HOMA-IR has been extensively used as a marker of insulin resistance in large population epidemiology studies. In the present study there was no improvement of HOMA-IR in the high HL group but serum ALT levels showed a modest though non-significant decrease in the HL groups. Though we did not carry out any liver biopsies in this study and HOMA-IR is not the gold standard for measuring insulin resistance[29], our results suggest that HL tablet may improve not only insulin resistance but also NASH. Longer-term studies of insulin resistance with use of higher doses of HL tablet are needed.
We observed no significant difference in most lipid profiles between the placebo group and the HL groups. However, surprisingly, levels of triglyceride and of VLDL cholesterol were significantly lower in the placebo group than either HL group. Because this study was a randomized, double-blind and placebo-controlled study and routine treatment was simultaneously performed, we recommended life style modifications to the patients as NAFLD treatment. Also, the enrolled patients knew that they suffered from NAFLD, and had the good sense to undertake life style modifications. Although this is not certain, we presume that these unexpected results occurred because the placebo group complied better with the suggested life style modifications than either HL tablet group.
HL tablet was well-tolerated by the patients. All treatment-related adverse events were mild. Because HL tablet does not cause weight gain, unlike pioglitazone, this will lead to few harmful effects in patients with metabolic syndrome such as diabetes and dyslipidemia[30].
This study had several limitations. First, it involved a relatively small number of patients and a short duration of treatment, because it was a phase II clinical trial. Second, since liver biopsies were not performed, we do not know how many patients with NASH were included in the study and cannot discuss any histologic findings. However, since patients with NAFLD in this study were excluded those with serum AST/ALT ratio > 2 and had higher baseline serum ALT level than serum AST level (AST/ALT ratio < 1), we think that more patients with simple steatosis than with advanced liver fibrosis were included[31].
In conclusion, HL tablet is effective in reducing HFC in short-term treatment (12 wk) without causing any negative lipid profiles, BMI changes and serious adverse effects. It may be a new and effective drug for treating NAFLD. Larger trials are warranted to assess the long-term efficacy of HL tablet in patients with NAFLD.
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease worldwide and the prevalence of NAFLD is growing gradually with the increase in population age and obesity. But, effective and safe drugs for NAFLD are not yet available.
The demand for new drugs for NAFLD is increasing worldwide. Magnolia officinalis (MO) is a traditional medicinal plant that has been used to treat liver diseases and other diseases. Some constituents extracted from MO are reported to have anti-inflammatory and antioxidant effects and MO can be effective against NAFLD. So, the authors hypothesized that MO would improve steatosis and inflammation in patients with NAFLD.
The authors evaluated the efficacy and safety of HL tablet, a new botanical drug extracted from MO, in the treatment of patients with NAFLD. The high dose (daily 400 mg) HL tablet is effective in reducing hepatic fat content (HFC) in short-term treatment (12 wk) without causing any negative lipid profiles, body mass index (BMI) changes and adverse effects.
HL tablet is effective in reducing HFC without any negative lipid profiles, BMI changes and adverse effects. Larger trials are warranted to assess the long-term efficacy of HL tablet in patients with NAFLD.
HL tablet is a new botanical drug extracted from MO. Magnetic resonance spectroscopy data were collected on 3.0T human scanners.
This is a study of phase II trial of HL tablet as a therapy for NAFLD. HL tablet is effective in reducing HFC without any negative lipid profiles, BMI changes and adverse effects. The results of this study are particularly of interest for the reader of the journal.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ikura Y, Thompson JR S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1758] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 2. | Ahmed A, Wong RJ, Harrison SA. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin Gastroenterol Hepatol. 2015;13:2062-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (2)] |
| 3. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1620] [Article Influence: 115.7] [Reference Citation Analysis (1)] |
| 4. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2294] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 5. | Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 6. | Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut. 2007;56:1760-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Wang RT, Koretz RL, Yee HF Jr. Is weight reduction an effective therapy for nonalcoholic fatty liver? A systematic review. Am J Med. 2003;115:554-559. [PubMed] |
| 8. | Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 2011;130:157-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 342] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 9. | Park EJ, Zhao YZ, Na M, Bae K, Kim YH, Lee BH, Sohn DH. Protective effects of honokiol and magnolol on tertiary butyl hydroperoxide- or D-galactosamine-induced toxicity in rat primary hepatocytes. Planta Med. 2003;69:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Munroe ME, Arbiser JL, Bishop GA. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J Immunol. 2007;179:753-763. [PubMed] |
| 11. | Park EJ, Zhao YZ, Kim YH, Lee BH, Sohn DH. Honokiol induces apoptosis via cytochrome c release and caspase activation in activated rat hepatic stellate cells in vitro. Planta Med. 2005;71:82-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Seo MS, Hong SW, Yeon SH, Kim YM, Um KA, Kim JH, Kim HJ, Chang KC, Park SW. Magnolia officinalis attenuates free fatty acid-induced lipogenesis via AMPK phosphorylation in hepatocytes. J Ethnopharmacol. 2014;157:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Kim YJ, Choi MS, Cha BY, Woo JT, Park YB, Kim SR, Jung UJ. Long-term supplementation of honokiol and magnolol ameliorates body fat accumulation, insulin resistance, and adipose inflammation in high-fat fed mice. Mol Nutr Food Res. 2013;57:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333-348. [PubMed] |
| 15. | Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141-152. [PubMed] |
| 16. | Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35-43. [PubMed] |
| 17. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 18. | Tiikkainen M, Bergholm R, Vehkavaara S, Rissanen A, Häkkinen AM, Tamminen M, Teramo K, Yki-Järvinen H. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701-707. [PubMed] |
| 19. | Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 20. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2473] [Article Influence: 164.9] [Reference Citation Analysis (2)] |
| 21. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 22. | Billington EO, Grey A, Bolland MJ. The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis. Diabetologia. 2015;58:2238-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Turner RM, Kwok CS, Chen-Turner C, Maduakor CA, Singh S, Loke YK. Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78:258-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, Ehrlich SF, Mamtani R, Bilker W, Vaughn DJ. Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. JAMA. 2015;314:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 25. | Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574-582.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 738] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 26. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1800] [Article Influence: 180.0] [Reference Citation Analysis (3)] |
| 27. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] |
| 28. | Maximos M, Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Biernacki D, Suman A, Weber M, Cusi K. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology. 2015;61:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 29. | Buchanan TA, Watanabe RM, Xiang AH. Limitations in surrogate measures of insulin resistance. J Clin Endocrinol Metab. 2010;95:4874-4876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 31. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1091] [Article Influence: 42.0] [Reference Citation Analysis (1)] |