Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5904
Peer-review started: February 4, 2017
First decision: April 21, 2017
Revised: June 19, 2017
Accepted: July 22, 2017
Article in press: July 24, 2017
Published online: August 28, 2017
Processing time: 213 Days and 8.5 Hours
To evaluate the effects of phosphatase and tension homologue deleted on chromosome ten (PTEN) gene on collagen metabolism in hepatic fibrosis and the underlying mechanisms.
Rat primary hepatic stellate cells (HSCs) and human LX-2 cells were transfected with adenovirus containing cDNA constructs encoding wild-type PTEN (Ad-PTEN), PTEN mutant G129E gene (Ad-G129E), and RNA interference constructs targeting the PTEN sequence PTEN short hairpin RNA to up-regulate and down-regulate the expression of PTEN. HSCs were assayed using fluorescent microscopy, real-time polymerase chain reaction, and western blotting. Moreover, a CCl4-induced rat hepatic fibrosis model was established to investigate the in vivo effects. Hematoxylin and eosin, and Masson’s trichrome were used to assess the histological changes. The expression of collagen I and III was assessed using immunohistochemistry and western blot analysis.
Elevated expression of PTEN gene reduced serum levels of alanine transaminase and aspartate transaminase, decreased collagen deposition in the liver, and reduced hepatocyte necrosis. In contrast, knockdown of PTEN expression had an opposite effect, such as increased collagen deposition in the liver, and was molecularly characterized by the increased expression of matrix metalloproteinase (MMP)-13 (P < 0.01) and MMP-2 (P < 0.01), as well as decreased expression of the tissue inhibitor of metalloproteinase (TIMP)-1 (P < 0.01) and TIMP-2 (P < 0.01).
These data indicated that gene therapy using recombinant adenovirus encoding PTEN might be a novel way of treating hepatic fibrosis.
Core tip: Phosphatase and tension homologue deleted on chromosome ten (PTEN) has a negative relation with the activation and proliferation of hepatic stellate cells (HSCs), which is the central event in liver fibrogenesis as HSCs are the major source of collagens and matrix metalloproteinases in fibrotic liver. In this study, adenoviruses containing cDNA constructs encoding wild-type PTEN (Ad-PTEN) and PTEN mutant G129E gene (Ad-G129E) were constructed to over-express the PTEN gene in both rat primary HSCs and human LX-2 cells as well as in the CCl4-induced rat liver fibrosis model. The adenovirus-mediated over-expression of the PTEN gene attenuated extracellular matrix (ECM) synthesis (collagens I and III) and promoted ECM degradation, representing a possible novel anti-fibrosis therapy.
- Citation: Xie SR, An JY, Zheng LB, Huo XX, Guo J, Shih D, Zhang XL. Effects and mechanism of adenovirus-mediated phosphatase and tension homologue deleted on chromosome ten gene on collagen deposition in rat liver fibrosis. World J Gastroenterol 2017; 23(32): 5904-5912
- URL: https://www.wjgnet.com/1007-9327/full/v23/i32/5904.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i32.5904
Cirrhosis, with its manifestation of liver fibrosis, represents a major medical problem worldwide[1,2]. Hepatic stellate cells (HSCs) are one of the cell types that play a critical role in the development and maintenance of liver fibrosis. Under fibrogenic conditions, HSCs undergo a complex activation process with morphological and phenotypic changes from quiescent vitamin A-storing cells to activated myofibroblast-like cells under fibrogenic conditions, resulting in increased synthesis and deposition of extracellular matrix (ECM) components, such as collagen I[3,4].
Phosphatase and tension homologue deleted on chromosome ten (PTEN) is the first tumor-suppressing gene found to inhibit the proliferation and promote the apoptosis of tumor cells[5,6]. PTEN has pleiotropic effects including pulmonary fibrosis, renal fibrosis, and cardiac interstitial fibrosis[7-10]. The absence of PTEN in specific hepatic cells leads to not only hepatocellular carcinoma but also nonalcoholic steatohepatitis, which has been found to be associated with hepatic fibrosis[11].
A previous study showed that the expression of PTEN was decreased in rat fibrotic liver tissues and HSCs induced by bile duct ligation in vivo[12]. During the reversal of liver fibrosis, pretreated PTEN mRNA and protein expression normalized, showing the relationship between PTEN and the severity of rat hepatic fibrosis[13]. The study presented herein investigated the in vitro and in vivo effects of PTEN on liver fibrosis using adenoviral transduction of wild-type PTEN (Ad-PTEN), mutant PTEN (Ad-G129E), and PTEN short hairpin RNA (PTEN shRNA) to better characterize the molecular mechanisms of PTEN in liver fibrosis.
Adult male Wistar rats weighing 350-400 g were obtained from the Experimental Animal Center of Hebei Medical University, Hebei Province, China. The study was performed in compliance with the national ethical guidelines for the care and use of laboratory animals, following the internationally accepted principles for laboratory animal use and care as found in the United States’ guidelines (National Institutes of Health publication #85-23, revised in 1985).
Rat primary HSCs were isolated from normal healthy male Wistar rats using in situ recirculating perfusion technology, as described in a previous study[14]. Then, Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum was used for cell culture; passages 2-3 were used in this study. Human LX-2 cells were obtained from Mount Sinai School of Medicine, authorized by Dr. Friedman.
Adenovirus containing cDNA constructs encoding wild-type PTEN (Ad-PTEN) with green fluorescent protein (GFP), PTEN mutant G129E gene (Ad-G129E) with GFP, and the empty virus control (Ad-GFP) were kindly provided by Prof. Junshan Zhu from the Third Military Medical University in China. RNA interference targeting PTEN sequence shRNA with enhanced GFP was established by Wuhan Genesil Biotechnology Co., Ltd (Wuhan, China). The transfection was performed as described in a previous study[15].
The rat primary HSCs and human LX-2 cells were divided into five groups: (1) control group, with serum-free antibiotic-free DMEM; (2) Ad-GFP group, with Ad-GFP transfection; (3) Ad-PTEN group, with Ad-PTEN transfection; (4) Ad-G129E group, with Ad-G129E transfection; and (5) PTEN shRNA group, with PTEN shRNA transfection.
A real-time polymerase chain reaction assay was performed using a previously established protocol[13,16]. Primer Express 5.0 was used to design the following primers: PTEN (rat), forward 5’-GGAAAGGACGGACTGG TGTA-3’ and reverse 5’-GGAAAGGACGGACTGGT GTA-3’; PTEN (human), forward 5’-ACCGCCAAATTTAAT TGCAG-3’ and reverse 5’-GGGTCCTGAATTGGAGGAA T-3’; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (rat), forward 5’-GGCAAGTTCAACGGCA CAG-3’ and reverse 5’-CGCCAGTAGACTCCACGACAT-3’; and GAPDH (human), forward 5’-ACTTTGGTATCGTGG AAGGACT-3’ and reverse 5’-GTAGAGGCAGGGATGAT GTTCT-3’. The primers were synthesized by SBS Genetech Co., Ltd (Beijing, China). The mRNA expression of genes was normalized to GAPDH.
Western blotting was performed as described in a previous study[12]. Anti-PTEN, anti-α-smooth muscle actin, anti-collagen I, anti-collagen III, anti-matrix metalloproteinase (anti-MMP)-13, anti-MMP-2, anti-tissue inhibitor of metalloproteinase (anti-TIMP)-1 and anti-TIMP-2 antibodies (1:200), and anti-GAPDH antibody (1:500) were used as primary antibodies.
The CCl4-induced rat hepatic fibrosis model was established as described in a previous study[13]. Rats were randomly divided into pretreatment and treatment groups. Pretreatment with recombinant adenovirus (2 × 109 pfu/100 μL/rat) through tail vein injection was conducted on rats once a week by administering CCl4 for 7 wk. Treatment with adenovirus (2 × 109 pfu/100 μL/rat) through tail vein injection was performed on rats once a week starting in the fourth week postadministration of CCl4 for 4 wk. Recombinant adenoviruses used were Ad-GFP, Ad-PTEN, Ad-G129E, and PTEN shRNA.
Hematoxylin and eosin (H&E) staining and Masson’s trichrome (MT) staining were performed to assess the histological changes and fibrosis in liver tissues. Immunohistochemical staining was used to further check the deposition of collagens I and III in the fibrotic liver; the procedure was performed as described in a previous study[12]. Immunofluorescent staining was also performed on frozen liver sections as described in a previous study to check the changes in the expression of PTEN in liver tissue[13].
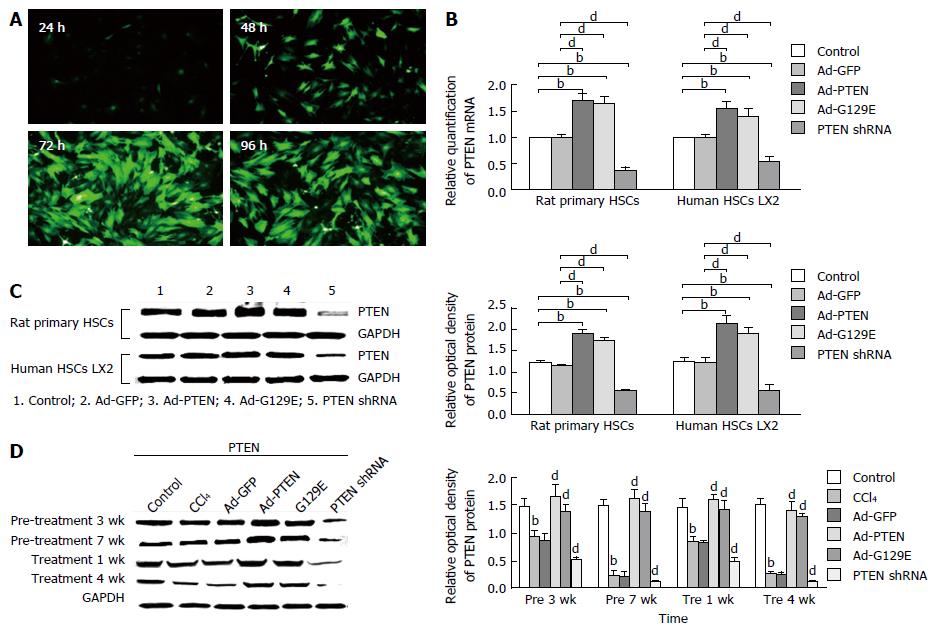
Adenoviral transfection using Ad-GFP was performed initially to establish the feasibility to modulate the expression of PTEN in rat primary HSCs and human LX-2 cells. This study showed that an adenovirus multiplicity of infection of 50 for 72 h gave the best transfection in rat primary HSCs (94.46% efficiency; Figure 1A) and human LX-2 cells (89.89% efficiency). At 72 h posttransfection, the mRNA and protein expression of PTEN in both rat primary HSCs and human LX-2 cells significantly increased in the Ad-PTEN group (mRNA: 1.698, 1.547 and protein: 1.91 ± 0.09, 2.13 ± 0.01, respectively) and Ad-G129E group (mRNA: 1.624, 1.479 and protein: 1.74 ± 0.08, 1.98 ± 0.12, respectively) compared with the Ad-GFP group (mRNA: 0.994, 0.998 and protein: 1.15 ± 0.04, 1.21 ± 0.14, respectively)(P < 0.01), and significantly decreased in the PTEN shRNA group (mRNA: 0.357, 0.548 and protein: 0.56 ± 0.04, 0.58 ± 0.13, respectively) (Figure 1B and C).
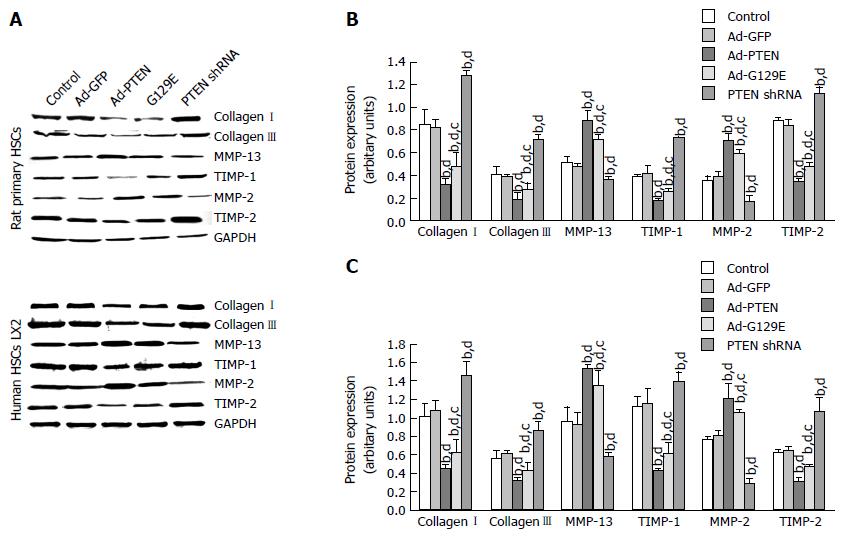
Collagen deposition in fibrotic liver tissues mainly comprised collagens I and III. The protein expression of collagens I and III in rat primary HSCs was found to be significantly decreased in the Ad-PTEN (0.32 ± 0.05 and 0.18 ± 0.02, respectively) and Ad-G129E groups compared with the CCl4 control and Ad-GFP groups (Figure 2A and B). In contrast, the expression of collagens I and III significantly increased in the PTEN shRNA group compared with the CCl4 control and Ad-GFP groups. A similar tendency was also found in experiments using human LX-2 cells (Figure 2A and C).
MMP-13 and MMP-2 play a critical role in the metabolism of collagens I and III. In rat primary HSCs, a significantly higher expression of MMP-13 and MMP-2 was found in the Ad-PTEN and Ad-G129E groups compared with the PTEN shRNA and CCl4 control groups. As expected, the expression of MMP-13 and MMP-2 was significantly reduced in the PTEN shRNA group compared with the control and Ad-GFP groups (Figure 2A and B). Similar regulation of MMP-13 and MMP-2 was also found using human LX-2 cells (Figure 2A and C).
The degree of collagen deposition was due to the balance between collagenases and their inhibitors. The inhibitors of MMP-13 and MMP-2 were additionally measured by western blotting at 72 h posttransfection in both rat primary HSCs and human LX2 HSCs. The expression of TIMP-1 and TIMP-2 was significantly down-regulated by Ad-PTEN and Ad-G129E compared with the CCl4 control and Ad-GFP groups (Figure 2A and B). Transfection of PTEN shRNA up-regulated these inhibitors of collagenases compared with the control and Ad-GFP groups (Figure 2).
The increased expression of PTEN gene in the Ad-PTEN and Ad-G129E groups was significantly reduced, whereas the reduced PTEN gene expression in the PTEN shRNA group was increased, along with the serum levels of alanine transaminase and aspartate transaminase compared with the CCl4 control and Ad-GFP groups, indicating improved liver function (Tables 1 and 2).
| Group | ALT (U/L) | AST (U/L) | ALB (g/L) | TBIL (μmol/L) | DBIL (μmol/L) |
| Pre 1 wk | |||||
| Control | 58.09 ± 6.54 | 147.23 ± 10.25 | 28.95 ± 2.14 | 2.15 ± 0.54 | 1.12 ± 0.32 |
| CCl4 | 414.45 ± 29.37 | 339.12 ± 45.37 | 27.20 ± 2.35 | 2.12 ± 0.38 | 1.70 ± 0.21 |
| Ad-GFP | 426.24 ± 30.76 | 289.37 ± 20.76 | 27.92 ± 3.12 | 2.13 ± 0.31 | 1.56 ± 0.12 |
| Ad-PTEN | 404.23 ± 26.78 | 283.76 ± 23.45a | 29.12 ± 4.25 | 1.98 ± 0.34 | 1.42 ± 0.31 |
| Ad-G129E | 409.98 ± 43.24 | 289.76 ± 35.43a | 28.82 ± 3.23 | 2.00 ± 0.34 | 1.50 ± 0.21 |
| PTEN shRNA | 2970.11 ± 267.34b | 1690.25 ± 200.34b | 27.10 ± 3.23 | 4.03 ± 0.78b | 2.67 ± 0.56b |
| Pre 3 wk | |||||
| Control | 60.71 ± 5.34 | 150.54 ± 12.34 | 27.65 ± 3.28 | 2.34 ± 0.23 | 1.28 ± 0.77 |
| CCl4 | 532.21 ± 34.02 | 804.12 ± 67.34 | 25.31 ± 4.32 | 17.31 ± 1.21 | 7.78 ± 2.01 |
| Ad-GFP | 589.34 ± 26.45 | 787.45 ± 43.21 | 26.70 ± 3.15 | 15.04 ± 2.12 | 7.34 ± 1.29 |
| Ad-PTEN | 422.37 ± 34.23a | 478.43 ± 50.34a | 27.31 ± 2.43 | 5.78 ± 1.01a | 3.24 ± 0.78a |
| Ad-G129E | 506.45 ± 57.32 | 526.43 ± 32.98a | 26.59 ± 1.37 | 10.54 ± 2.76a | 6.21 ± 2.37 |
| PTEN shRNA | 3440.32 ± 342.32b | 3090.39 ± 228.23b | 24.41 ± 2.34b | 28.37 ± 4.78b | 14.56 ± 2.42b |
| Pre 5 wk | |||||
| Control | 59.22 ± 7.34 | 145.38 ± 11.32 | 28.37 ± 2.56 | 2.34 ± 0.78 | 1.43 ± 0.22 |
| CCl4 | 672.34 ± 17.37 | 817.56 ± 46.32 | 26.70 ± 3.02 | 15.23 ± 2.78 | 7.65 ± 1.22 |
| Ad-GFP | 597.45 ± 33.21 | 753.24 ± 32.12 | 26.30 ± 1.76 | 16.30 ± 3.56 | 8.72 ± 2.11 |
| Ad-PTEN | 456.43 ± 32.12a | 566.76 ± 53.21a | 28.54 ± 2.12a | 9.31 ± 2.12a | 4.50 ± 0.89a |
| Ad-G129E | 492.37 ± 40.65a | 705.43 ± 63.21a | 27.21 ± 1.35 | 10.56 ± 1.23a | 5.61 ± 1.23a |
| PTEN shRNA | 2060.21 ± 325.34b | 2490.34 ± 532.12b | 24.23 ± 1.23b | 17.31 ± 2.67b | 9.45 ± 2.12b |
| Pre 7 wk | |||||
| Control | 60.21 ± 3.19 | 152.12 ± 15.34 | 28.34 ± 1.15 | 2.38 ± 0.59 | 1.48 ± 0.26 |
| CCl4 | 605.23 ± 85.37 | 1110.32 ± 215.32 | 22.76 ± 3.21 | 15.26 ± 1.23 | 8.65 ± 0.32 |
| Ad-GFP | 623.45 ± 56.34 | 1134.23 ± 121.24 | 23.43 ± 1.21 | 14.87 ± 1.02 | 9.34 ± 0.98 |
| Ad-PTEN | 456.45 ± 32.12a | 183.23 ± 34.23a | 26.73 ± 2.23a | 4.32 ± 0.97 | 2.62 ± 0.42 |
| Ad-G129E | 545.87 ± 43.21a | 694.32 ± 25.34a | 25.63 ± 1.23a | 4.56 ± 0.32 | 2.54 ± 0.23 |
| PTEN shRNA | 1825.23 ± 28.23b | 1878.45 ± 56.87b | 21.90 ± 3.23b | 17.23 ± 3.56b | 9.08 ± 1.32b |
| Group | ALT (U/L) | AST (U/L) | ALB (g/L) | TBIL (μmol/L) | DBIL (μmol/L) |
| Tre 1 wk | |||||
| Control | 57.78 ± 8.23 | 146.78 ± 12.23 | 27.99 ± 1.18 | 2.49 ± 0.55 | 1.65 ± 0.36 |
| CCl4 | 684.32 ± 78.34 | 840.32 ± 32.25 | 25.26 ± 3.25 | 15.45 ± 3.23 | 6.56 ± 1.12 |
| Ad-GFP | 645.65 ± 34.26 | 832.12 ± 40.32 | 24.54 ± 1.21 | 14.31 ± 1.21 | 7.21 ± 2.12 |
| Ad-PTEN | 384.98 ± 36.23b | 398.43 ± 43.23b | 27.60 ± 2.34b | 11.21 ± 1.21b | 5.32 ± 1.46b |
| Ad-G129E | 495.34 ± 45.12b | 546.32 ± 32.12b | 26.12 ± 2.43 | 13.23 ± 2.12 | 7.86 ± 1.23b |
| PTEN shRNA | 2030.31 ± 112.34a | 1730.54 ± 87.34a | 24.12 ± 2.34a | 34.21 ± 5.34a | 17.23 ± 2.12a |
| Tre 2 wk | |||||
| Control | 57.46 ± 3.58 | 142.34 ± 10.24 | 29.15 ± 3.21 | 2.68 ± 0.74 | 1.55 ± 0.46 |
| CCl4 | 712.34 ± 34.54 | 843.23 ± 54.32 | 26.32 ± 1.34 | 14.32 ± 5.32 | 8.32 ± 2.12 |
| Ad-GFP | 697.45 ± 45.76 | 876.34 ± 33.32 | 25.79 ± 2.32 | 13.34 ± 2.13 | 7.86 ± 1.23 |
| Ad-PTEN | 338.12 ± 12.34b | 407.34 ± 32.12b | 27.56 ± 3.23b | 6.56 ± 1.21b | 3.72 ± 0.78b |
| Ad-G129E | 364.32 ± 34.56b | 408.67 ± 23.78b | 27.21 ± 1.22b | 10.32 ± 1.21b | 5.34 ± 1.09b |
| PTEN shRNA | 2040.21 ± 126.32a | 1860.32 ± 210.32a | 25.60 ± 3.12a | 27.12 ± 4.21a | 14.78 ± 2.12a |
| Tre 3 wk | |||||
| Control | 58.54 ± 6.58 | 148.45 ± 13.27 | 29.12 ± 3.15 | 2.12 ± 0.21 | 1.48 ± 0.24 |
| CCl4 | 1892.32 ± 113.12 | 2018.32 ± 123.45 | 22.14 ± 1.23 | 8.18 ± 1.10 | 5.21 ± 0.65 |
| Ad-GFP | 1746.32 ± 98.23 | 1879.23 ± 119.34 | 23.10 ± 2.13 | 9.12 ± 1.23 | 5.72 ± 0.54 |
| Ad-PTEN | 524.35 ± 34.12b | 690.35 ± 54.23b | 27.30 ± 3.21b | 6.45 ± 0.32b | 4.12 ± 0.54b |
| Ad-G129E | 576.23 ± 43.21b | 820.43 ± 67.32b | 25.20 ± 1.21b | 8.12 ± 0.87b | 4.89 ± 0.81b |
| PTEN shRNA | 1937.56 ± 32.21a | 2390.32 ± 112.23a | 21.34 ± 2.34a | 10.76 ± 1.23a | 6.10 ± 0.54a |
| Tre 4 wk | |||||
| Control | 62.31 ± 8.12 | 155.37 ± 13.27 | 29.91 ± 3.12 | 2.49 ± 0.32 | 1.65 ± 0.21 |
| CCl4 | 594.39 ± 32.18 | 1110.32 ± 89.34 | 23.45 ± 2.12 | 17.32 ± 2.32 | 9.28 ± 1.23 |
| Ad-GFP | 576.45 ± 23.43 | 886.43 ± 45.67 | 22.79 ± 1.21 | 13.45 ± 2.45 | 7.12 ± 1.34 |
| Ad-PTEN | 490.32 ± 32.23b | 528.34 ± 32.12b | 25.31 ± 3.21b | 7.34 ± 1.21b | 4.65 ± 0.98b |
| Ad-G129E | 530.23 ± 22.56b | 628.43 ± 45.34b | 25.12 ± 1.23b | 10.54 ± 2.32b | 6.54 ± 1.21b |
| PTEN shRNA | 1148.32 ± 54.32a | 1270.34 ± 121.28a | 20.37 ± 3.12a | 26.32 ± 3.56a | 14.56 ± 2.13a |
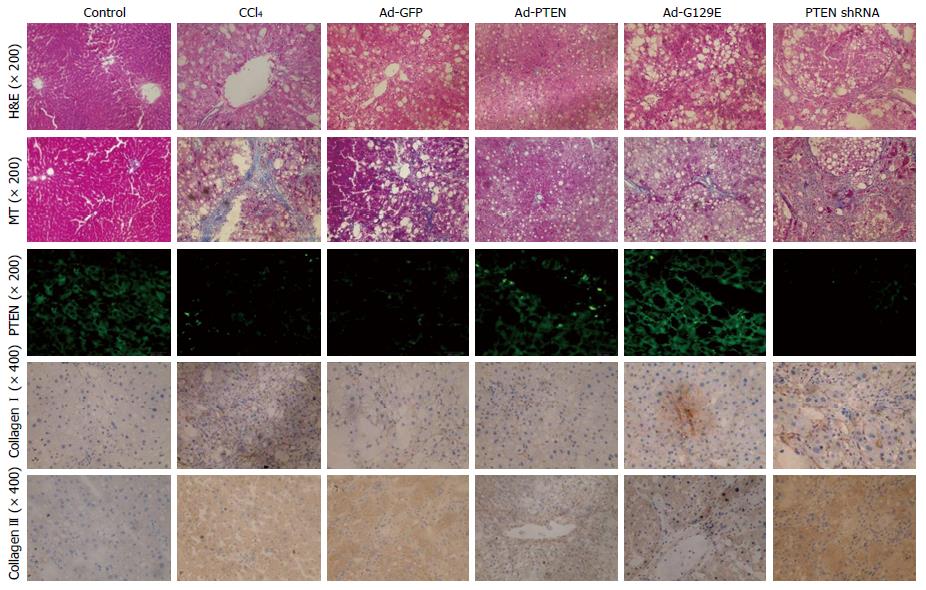
H&E and MT staining confirmed that the increased expression of PTEN in the Ad-PTEN and Ad-G129E groups led to reduced hepatocyte necrosis and collagen deposition in liver tissue compared with that in the control groups (Figure 3). Immunofluorescent staining for PTEN was performed to see whether the improved pathology was associated with changes in the expression of the PTEN gene. It was found that the expression of the PTEN gene significantly increased with Ad-PTEN and Ad-G129E recombinant adenovirus in both the prevention and treatment groups (Figure 3).
Moreover, the total protein was isolated from liver tissues in each group, and western blot analysis of the expression of PTEN was performed. Consistent with the findings from previous studies[13], the protein expression of PTEN was significantly decreased in rats treated with CCl4. The protein expression of PTEN was significantly increased in rats treated with Ad-PTEN in the pretreatment and treatment groups compared with the control group (Figure 1D).
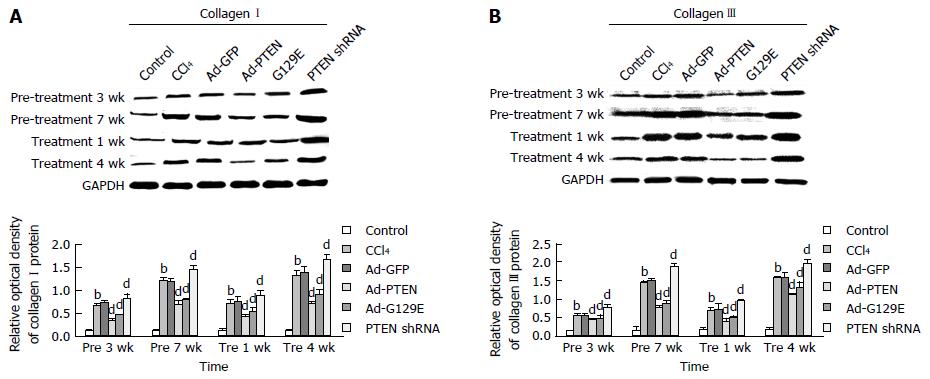
Then, western blot analysis was performed to check the protein expression of collagens I and III in the rat liver tissue at each time point. Compared with the Ad-GFP group, the expression of collagens I and III significantly decreased with the enhanced expression of PTEN in the Ad-PTEN group at pre 3 wk, pre 7 wk, pre 1 wk, and pre 4 wk (Figure 4). In contrast, the reduced expression of PTEN with PTEN shRNA significantly increased the expression of collagens I and III (Figure 4).
Hepatic fibrosis is the accumulation of ECM in response to chronic liver injury that ultimately leads to cirrhosis[1,2]. Cirrhosis is associated with increased morbidity and mortality and results in substantial economic and social costs. At present, no effective therapy is available to treat or reverse hepatic fibrosis.
Under chronic injury, HSCs are activated to produce more ECM, mainly comprised of collagens I and III in the liver tissue. Moreover, HSCs also regulate the balance of MMPs and TIMPs, which determines the degree of collagen deposition in the liver[1,17-19]. PTEN has been found to be involved in myocardial fibrosis, renal fibrosis, and lung fibrosis[8-10]. A previous study found that higher expression of PTEN reduced the number of activated HSCs to negatively regulate fibrogenesis in vivo[12,13]. This suggested that PTEN may also regulate the accumulation of ECM components in liver fibrosis because ECM is mainly produced from activated HSCs[1].
The expression of PTEN was modulated in this study using recombinant adenovirus that either increased or reduced the expression of PTEN. This study showed that reducing the expression of PTEN conferred worsened liver fibrosis through its modulation of collagens I and III, MMP-13 and MMP-2, and TIMP-1 and TIMP-2. These PTEN-dependent changes in collagen, collagenases, and collagenase inhibitors reduced collagen deposition that was associated with CCl4-induced liver fibrosis.
The human homologue of rat MMP-13 is MMP-1 and is expressed by HSCs, fibroblasts, Kupffer cells, and so forth[20]. MMP-13 remodels the surrounding tissue to clear room for deposition of newly synthesized ECM. The activity of MMP-13 can be inhibited by TIMP-1[21]. MMP-2 mainly serves to degrade the collagen in the basement membrane[22]. TIMP-2 is essential for MMP-2 activation, as it can bind to pro-MMP-2 and then combine with MT1-MMP to activate pro-MMP-2. However, over-expressed TIMP-2 can inhibit MMP-2 activity, causing excessive collagen deposition[20,23]. In this study, MMP-13 and MMP-2, the major collagenases involved in collagen degradation[24,25], increased with a high expression level of the PTEN gene, which also down-regulated the expression of TIMP-1 and TIMP-2. The lower expression of PTEN could invert the ratio of MMP-13/TIMP-1 and MMP-2/TIMP-2 and cause severe collagen deposition. These data suggested that PTEN might regulate hepatic collagen metabolism through regulation of MMP-13, MMP-2, TIMP-1, and TIMP-2.
Gene therapy using adenovirus vector has been shown to be effective in modulating the expression of a gene of interest[26-28]. In this study, treatment with recombinant adenovirus carrying a highly expressed PTEN gene in rats with CCl4-induced hepatic fibrosis improved the liver function and reduced the collagen deposition in liver tissue.
In conclusion, these data demonstrated that PTEN might affect collagen deposition in the liver through MMP-13, MMP-2, TIMP-1, and TIMP-2. Gene therapy using recombinant adenovirus encoding wild-type PTEN may represent a novel way for treating hepatic fibrosis.
Phosphatase and tension homologue deleted on chromosome ten (PTEN) plays an essential role in the activation of hepatic stellate cells (HSCs), which are the major source of collagens and matrix metalloproteinases in the fibrotic liver. Liver fibrosis results from the excessive deposition of extracellular matrix (ECM) components, mainly comprising collagens I and III, which are produced by HSCs.
Previous studies have shown that PTEN had a negative relation with the activation and proliferation of HSCs, which is the central event in liver fibrogenesis. The collagen synthesis could be inhibited by over-expression of the PTEN gene. Adenoviruses containing cDNA constructs encoding wild-type PTEN (Ad-PTEN) and PTEN mutant G129E gene (Ad-G129E) were used in both rat primary HSCs and human LX-2 cells, as well as in the CCl4-induced rat liver fibrosis model.
Recent reports highlighted the importance of collagen metabolism in HSCs. This novel study reported that the adenovirus-mediated over-expression of the PTEN gene could attenuate ECM synthesis and promote ECM degradation, which represents a potential tool for novel anti-fibrosis therapies.
The results of this study indicated that the over-expressed PTEN gene might represent a novel tool for the treatment and reversal of hepatic fibrosis.
It’s an interesting topic about effects of the PTEN gene on collagen metabolism in hepatic fibrosis and the underlying mechanisms.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Yuan YF S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 2. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2164] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 3. | Wasmuth HE, Weiskirchen R. [Pathogenesis of liver fibrosis: modulation of stellate cells by chemokines]. Z Gastroenterol. 2010;48:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Atzori L, Poli G, Perra A. Hepatic stellate cell: a star cell in the liver. Int J Biochem Cell Biol. 2009;41:1639-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Kim HA, Kim KJ, Seo KH, Lee HK, Im SY. PTEN/MAPK pathways play a key role in platelet-activating factor-induced experimental pulmonary tumor metastasis. FEBS Lett. 2012;586:4296-4302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Shi Y, Paluch BE, Wang X, Jiang X. PTEN at a glance. J Cell Sci. 2012;125:4687-4692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Nho RS, Hergert P. IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PLoS One. 2014;9:e94616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, Hogaboam CM, Flaherty KR, Martinez FJ, Kontos CD. Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10). Am J Respir Crit Care Med. 2006;173:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Lan R, Geng H, Polichnowski AJ, Singha PK, Saikumar P, McEwen DG, Griffin KA, Koesters R, Weinberg JM, Bidani AK. PTEN loss defines a TGF-β-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol Renal Physiol. 2012;302:F1210-F1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Singla DK. Akt-mTOR Pathway Inhibits Apoptosis and Fibrosis in Doxorubicin-Induced Cardiotoxicity Following Embryonic Stem Cell Transplantation. Cell Transplant. 2015;24:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Vinciguerra M, Veyrat-Durebex C, Moukil MA, Rubbia-Brandt L, Rohner-Jeanrenaud F, Foti M. PTEN down-regulation by unsaturated fatty acids triggers hepatic steatosis via an NF-kappaBp65/mTOR-dependent mechanism. Gastroenterology. 2008;134:268-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Hao LS, Zhang XL, An JY, Karlin J, Tian XP, Dun ZN, Xie SR, Chen S. PTEN expression is down-regulated in liver tissues of rats with hepatic fibrosis induced by biliary stenosis. APMIS. 2009;117:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Zheng L, Chen X, Guo J, Sun H, Liu L, Shih DQ, Zhang X. Differential expression of PTEN in hepatic tissue and hepatic stellate cells during rat liver fibrosis and its reversal. Int J Mol Med. 2012;30:1424-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Ma J, Li F, Liu L, Cui D, Wu X, Jiang X, Jiang H. Raf kinase inhibitor protein inhibits cell proliferation but promotes cell migration in rat hepatic stellate cells. Liver Int. 2009;29:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Hao LS, Zhang XL, An JY, Yao DM, Karlin J, Fang SM, Jiang HQ, Bai WY, Chen S. Adenoviral transduction of PTEN induces apoptosis of cultured hepatic stellate cells. Chin Med J (Engl). 2009;122:2907-2911. [PubMed] |
| 16. | An J, Zheng L, Xie S, Dun Z, Hao L, Yao D, Shih DQ, Zhang X. Down-regulation of focal adhesion kinase by short hairpin RNA increased apoptosis of rat hepatic stellate cells. APMIS. 2011;119:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Takashima M, Parsons CJ, Ikejima K, Watanabe S, White ES, Rippe RA. The tumor suppressor protein PTEN inhibits rat hepatic stellate cell activation. J Gastroenterol. 2009;44:847-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Povero D, Busletta C, Novo E, di Bonzo LV, Cannito S, Paternostro C, Parola M. Liver fibrosis: a dynamic and potentially reversible process. Histol Histopathol. 2010;25:1075-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 19. | Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1324-G1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46:955-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Okazaki I, Noro T, Tsutsui N, Yamanouchi E, Kuroda H, Nakano M, Yokomori H, Inagaki Y. Fibrogenesis and Carcinogenesis in Nonalcoholic Steatohepatitis (NASH): Involvement of Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of Metalloproteinase (TIMPs). Cancers (Basel). 2014;6:1220-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, Vogt A, Dienes HP, Lammert F, Reichen J. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 23. | Ngu JM, Teng G, Meijndert HC, Mewhort HE, Turnbull JD, Stetler-Stevenson WG, Fedak PW. Human cardiac fibroblast extracellular matrix remodeling: dual effects of tissue inhibitor of metalloproteinase-2. Cardiovasc Pathol. 2014;23:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Radbill BD, Gupta R, Ramirez MC, DiFeo A, Martignetti JA, Alvarez CE, Friedman SL, Narla G, Vrabie R, Bowles R. Loss of matrix metalloproteinase-2 amplifies murine toxin-induced liver fibrosis by upregulating collagen I expression. Dig Dis Sci. 2011;56:406-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Matono T, Koda M, Tokunaga S, Sugihara T, Ueki M, Murawaki Y. The effects of the selective mineralocorticoid receptor antagonist eplerenone on hepatic fibrosis induced by bile duct ligation in rat. Int J Mol Med. 2010;25:875-882. [PubMed] |
| 26. | Podolska K, Stachurska A, Hajdukiewicz K, Małecki M. Gene therapy prospects--intranasal delivery of therapeutic genes. Adv Clin Exp Med. 2012;21:525-534. [PubMed] |
| 27. | Vicente T, Peixoto C, Carrondo MJ, Alves PM. Virus production for clinical gene therapy. Methods Mol Biol. 2009;542:447-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Cai XG, Xia JR, Li WD, Lu FL, Liu J, Lu Q, Zhi H. Anti-fibrotic effects of specific-siRNA targeting of the receptor for advanced glycation end products in a rat model of experimental hepatic fibrosis. Mol Med Rep. 2014;10:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |