Published online Aug 14, 2017. doi: 10.3748/wjg.v23.i30.5538
Peer-review started: April 25, 2017
First decision: June 1, 2017
Revised: June 20, 2017
Accepted: July 4, 2017
Article in press: July 4, 2017
Published online: August 14, 2017
Processing time: 113 Days and 21.8 Hours
To explore the let-7a-mediated anti-cancer effect of Yangzheng Sanjie decoction (YZSJD) in gastric cancer (GC) cells.
YZSJD-containing serum (YCS) was prepared using traditional Chinese medicine serum pharmacology methods. After YCS treatment, cell proliferation and apoptosis were assessed by cell counting kit-8 assay and flow cytometry, respectively, and miRNA expression profiles were determined using qPCR arrays. Let-7a expression was examined by in situ hybridization in GC tissues and by qPCR in GC cells. c-Myc protein expression was detected by immunohistochemistry in GC tissues, and by Western blot in cell lines.
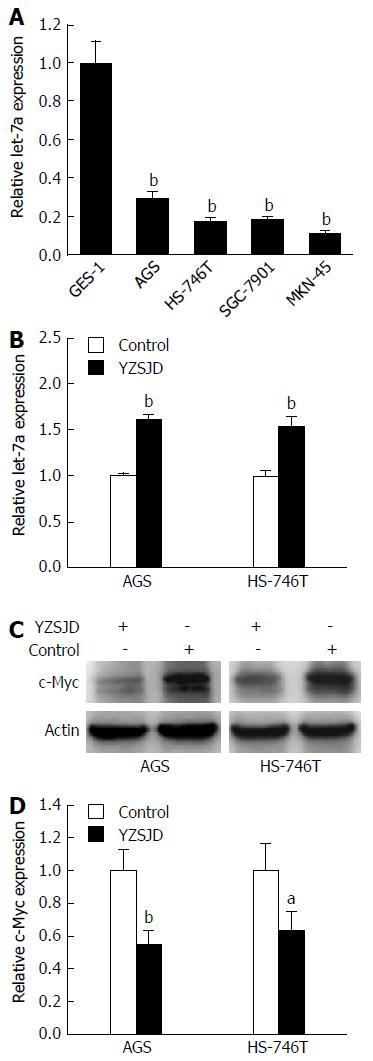
YZSJD significantly inhibited proliferation and induced apoptosis in AGS and HS-746T GC cells. After treatment with YCS, the miRNA expression profiles were altered and the reduced let-7a levels in both cell lines were up-regulated, accompanied by a decrease in c-Myc expression. Moreover, decreased let-7a expression and increased c-Myc expression were observed during the progression of gastric mucosa cancerization.
YZSJD inhibits proliferation and induces apoptosis of GC cells by restoring the aberrant expression of let-7a and c-Myc.
Core tip: Let-7a reduction plays an important role in gastric tumourigenesis through the derepression of c-Myc expression. Our data demonstrate that Yangzheng Sanjie decoction (YZSJD) inhibits proliferation and induces apoptosis in gastric cancer (GC) cells by regulating the aberrant expression of let-7a and c-Myc. These findings provide new evidence that YZSJD has therapeutic potential in GC treatment and that miRNA regulation may be a novel molecular mechanism through which Chinese herbal medicine exhibits anti-cancer activity.
- Citation: Deng HX, Yu YY, Zhou AQ, Zhu JL, Luo LN, Chen WQ, Hu L, Chen GX. Yangzheng Sanjie decoction regulates proliferation and apoptosis of gastric cancer cells by enhancing let-7a expression. World J Gastroenterol 2017; 23(30): 5538-5548
- URL: https://www.wjgnet.com/1007-9327/full/v23/i30/5538.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i30.5538
Gastric cancer (GC) is one of the leading causes of cancer-related deaths in the world[1]. The low early diagnosis rate of GC and the limited treatment options for advanced GC are the major reasons for the high mortality rate in GC patients. Considering the narrow therapeutic window, long-term drug resistance and toxic side effects of chemotherapeutic agents, safer and more effective therapies should be developed to improve the prognosis of GC.
As an important source of novel agents with pharmaceutical potential, Chinese herbal medicines have become increasingly popular in cancer treatment as alternative and complementary therapy modalities[2-6]. One Chinese medicine formula, Yangzheng Sanjie decoction (YZSJD), which contains the ingredients Astragali Radix, Scutellariae Barbatae Herba, Arisaematis Rhizoma Preparatum, Citri Sarcodactylis Fructus, Cremastrae Pseudobulbus and Curcumae Longae Rhizoma, has shown good clinical effects in the treatment of chronic atrophic gastritis with precancerous lesions[7]. Several components of YZSJD have recently been reported to exert antiproliferative effects in several cancer cell lines[8-10]. However, the potential role of YZSJD in the treatment of GC and the precise mechanisms that may be involved in the proliferation and apoptosis of GC cells have not yet been clearly addressed.
Increasing evidence has revealed that microRNAs (miRNAs) play critical roles in the initiation, progression and aggressiveness of human cancers and may be potential therapeutic targets for malignancies. MiRNAs are a class of small noncoding RNAs of 21-23 nucleotides that negatively regulate gene expression by base-pairing with the 3′-untranslated regions of their target messenger RNAs[11,12]. The miRNA let-7 is down-regulated in many cancer types compared with normal tissue, indicating that the let-7 family serves as tumour suppressors[13,14]. Involved in the complex regulation of c-Myc, let-7a participates in the genesis and maintenance of c-Myc-dysregulated cancers[15]. Kim et al[16] demonstrated that let-7 inhibits c-Myc expression by targeting the c-Myc 3’-UTR in a HuR-let-7 interdependent manner. Let-7b increased drug sensitivity in chemotherapy-resistant GC cells by targeting c-Myc[17]. A more recent study revealed that let-7a down-regulates the expression of PKM2 by regulating the expression of c-Myc and hnRNPA1 and therefore inhibits the proliferation, migration and invasion of GC cells[18].
In a previous clinical study, we found that YZSJD can effectively slow, block or reverse the progression of GC precancerous lesions[19]. In this study, we aimed to explore the miRNA-mediated anticancer effect of YZSJD in vitro. The present study was therefore designed to evaluate cell proliferation and apoptosis in GC cells treated with YZSJD-containing serum (YCS) and to screen and verify the potential miRNA targets. Furthermore, we detected the expression of let-7a and its target gene c-Myc in GC tissues, matched gastric precancerous tissues and normal gastric mucosa tissues to investigate the role of let-7a in the progression of gastric mucosa cancerization.
YZSJD, a Chinese herbal compound prescribed by Dr. Geng-Xin Chen at the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, is composed of Astragali Radix 15 g, Codonopsis Radix 15 g, Scutellariae Barbatae Herba 15 g, Arisaematis Rhizoma Preparatum 10 g, Citri Sarcodactylis Fructus 10 g, Cremastrae Pseudobulbus 10 g, Curcumae Longae Rhizoma 10 g and Curcumae Rhizoma 15 g. All the medicinal materials used to prepare formulae were purchased from Kangmei Pharmaceutical Co., Ltd. (Guangzhou, China) and were identified by two pharmacognosy experts. The herbs (100 g) were soaked in distilled water (1000 mL) and boiled for 30 min twice, and then the extracts were filtered, mixed and centrifuged. The upper layer was concentrated to 0.9 g crude extract per millilitre in a rotary evaporator (SENCO, China) and stored at -20 °C for future use. To establish quality control standards for YZSJD, the crude extract preparation and subsequent high-performance liquid chromatography (HPLC) determination were repeated ten times. The similarity of the HPLC fingerprints of 10 batches of YZSJD samples was assessed using the Computer-Aided Similarity Evaluation System for Chromatographic Fingerprint of TCM (Chinese Pharmacopoeia Commission, version 2004A).
Forty male SD rats (SPF grade, weighing 250 ± 20 g) were purchased from the laboratory animal centre of Southern Medical University. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation. Subsequently, the rats were randomly and equally divided into a YZSJD group and a Control group. Animals in the YZSJD group were gavaged with an equivalent dose of YZSJD (9 g/kg), while those in the Control group were administered the same volume of normal saline once daily. On the seventh day, blood was drawn from the abdominal aorta 1 h after feeding, and the serum was isolated. Each group of sera was mixed, sterilized by filtration and inactivated at 56 °C before being stored at -20 °C. These experiments were approved by the Institutional Animal Care and Use Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, and efforts were made to minimize animal suffering.
The human GC cell lines AGS and HS-746T were purchased from the American Type Culture Collection (Manassas, VA, United States), the cell lines MKN-45 and SGC-7901 were obtained from the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China), and the human immortalized gastric mucosa cell line GES-1 was provided by the Beijing Institute for Cancer Research. The cells were cultured in RPMI-1640 medium (HyClone, United States) supplemented with 10% foetal bovine serum (HyClone, United States) and maintained in a humidified incubator with 5% CO2 at 37 °C.
AGS and HS-746T cells were suspended and seeded in 96-well plates at a density of 6000 cells/well or in 6-well plates at a density of 20000 cells/well. The cells were divided into a YZSJD group and a Control group. After 12 h of culture, the cells in the YZSJD group were treated with 10% YCS, while those in the Control group were treated with 10% normal rat serum.
The effects of YZSJD on AGS and HS-746T cell proliferation were estimated using the Cell Counting Kit-8 (CCK-8) assay (Jingxin, China). After 24, 48 or 72 h of incubation with YCS or normal rat serum, 10 μL of CCK-8 solution was added to each well of a 96-well plate, followed by a 2-h incubation in the dark. Cell proliferation was evaluated by the absorbance of each well at 450 nm, which was measured with a VICTOR X5 Multilabel Plate Reader (PerkinElmer, United States).
The effects of YZSJD on apoptosis were determined by flow cytometry using an Annexin V-FITC Apoptosis Detection Kit (BD Pharmingen, United States). After 48 h of incubation with YCS or normal rat serum, cells in 6-well plates were harvested and resuspended in 1 × binding buffer at a concentration of 1 × 106 cells/mL. Then, 5 μL of Annexin V-FITC and 10 μL of propidium iodide were added to 100 μL of the cell suspension. The cells were incubated for 15 min in the dark before 400 μL of 1 × binding buffer was added. The samples were analysed by flow cytometry within 1 h.
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, United States) following the manufacturer’s instructions. Contaminating DNA in the RNA preparations was removed with DNase I, and the RNA was purified using an RNeasy MinElute Cleanup Kit (Qiagen, Germany). The RNA quantity and purity were assessed using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, United States), and RNA integrity was examined by denaturing agarose gel electrophoresis.
The expression of mature miRNAs was detected using ExiLENT SYBR Green master mix (Exiqon, Denmark) and the microRNA Ready-to-Use PCR, Human panel I + II (V4.M) (Exiqon, Denmark), according to the manufacturer’s instructions. Briefly, the template RNA was reverse transcribed using a Universal cDNA Synthesis Kit (Exiqon, Denmark), and the reverse transcription products were then amplified and detected on a 7900 Real-Time PCR System (Applied Biosystems, United States) using the following thermocycler conditions: denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 1 min.
Data analysis was performed with the GenEx qPCR analysis software (http://www.exiqon.com/mirna-pcranalysis). U6 snRNA was used as an endogenous control. The fold change for each miRNA was calculated as 2-ΔΔCt. We filtered out raw data for which the cycle threshold values were greater than 30 from the 372 total human miRNAs, and the differentially expressed miRNAs with a fold-change ≥ 1.5 in at least one cell line were included in the further analyses.
Total RNA was isolated from AGS and HS-746T cells with Trizol reagent (Invitrogen, CA, United States). The RNA concentration and purity were assessed using a NanoDrop 2000C spectrophotometer (Thermo Fisher Scientific, United States). Mature let-7a was reverse transcribed to cDNA using a PrimeScript RT reagent kit (TaKaRa, Japan), and the specific cDNA was amplified using a SYBR Premix Ex Taq II kit (TaKaRa, Japan) according to the manufacturer’s instructions. The Bulge-Loop hsa-let-7a-5p qRT-PCR Primer Set for reverse transcription and quantitative PCR was purchased from Ribobio Biotechnology (Guangzhou, China). The reverse transcription conditions consisted of an initial incubation at 42 °C for 15 min followed by 85 °C for 5 s in a T100 PCR instrument (Bio-Rad, United States). The qPCR amplification was performed as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s on a 7500 Real-Time PCR System (Applied Biosystems, United States). U6 was used as an endogenous control, and the ΔΔCt method was used for let-7a quantification.
Cells were lysed in RIPA buffer (Beyotime, China) to extract total cellular proteins. Equal amounts of protein for each sample were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, United States). After being blocked with WB blocking solution (Beyotime, China) for 1 h, the membranes were incubated with a rabbit c-Myc monoclonal antibody (Cell Signaling Technology, United States) or a mouse β-actin monoclonal antibody (Boster, China) at 4 °C overnight. The next day, the membranes were incubated with either a goat anti-rabbit IgG or a goat anti-mouse IgG horseradish peroxidase-conjugated secondary antibody (Boster, China) at 37 °C for 1 h. Immunoreactivity was visualized using an enhanced chemiluminescence reagent (Beyotime, China).
Tissue from 11 patients with pathologically diagnosed GC who underwent radical resection without radiotherapy and chemotherapy at the First Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangzhou, Guangdong province, China) from May 2014 to November 2014 was collected. The diagnosis and histopathologic type of GC were determined according to the rules of the Japanese Gastric Cancer Association[20]. The clinical and pathological characteristics of the patients are shown in Table 1. All study participants provided written informed consent and donated a piece of GC tissue, matched tissue adjacent to the carcinoma and distal normal gastric tissue. The samples were fixed in 4% paraformaldehyde immediately after surgery and then embedded in paraffin. Histopathology was confirmed independently by two experienced pathologists unaware of the patients’ clinical history. The present study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine.
| Characteristic | n (%) |
| Age (yr) | |
| < 60 | 6 (54.5) |
| ≥ 60 | 5 (45.5) |
| Sex | |
| Male | 8 (72.7) |
| Female | 3 (27.3) |
| Lymph node metastasis | |
| Negative | 6 (54.5) |
| Positive | 5 (45.5) |
| Histopathology | |
| Adenocarcinoma | 11 (100) |
| Others | 0 (0) |
| Grade | |
| Well/moderately differentiated | 5 (45.5) |
| Poorly differentiated | 6 (54.5) |
| Stage | |
| Early | 2 (18.2) |
| Advanced | 9 (81.8) |
| Surgical therapy | |
| Negative | 0 (0) |
| Positive | 11 (100) |
In situ hybridization was performed to detect let-7a in paraffin-embedded tissue sections using the Enhanced Sensitive ISH Detection Kit (Boster, China) and DIG-labelled hsa-let-7a miRCURY LNA Detection probe (Exiqon, Denmark). After pretreatment and enzymatic digestion, slides were incubated with let-7a hybridization solution overnight at 4 °C. After a 30 min blocking step, the slides were treated with an anti-digoxigenin antibody for 1 h. Then, SABC-POD solution was added to the slides for 20 min at 37 °C. Next, the slides were treated with biotin peroxidase for 20 min and diaminobenzidine (DAB) chromogenic reagent (Boster, China) for 20 min. Counterstaining was performed with haematoxylin. Images were taken on an Olympus BX53 microscope equipped with a digital camera.
Signals were semi-quantitatively evaluated based on the cytoplasmic staining intensity and the percentage of positive cells. Staining intensity was divided into four levels: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong); the percentage of positive cells was graded as 0 (none), 1 (< 10%), 2 (10%-50%), 3 (50%-80%) and 4 (> 80%). A total in situ hybridization (ISH) score was calculated by multiplying the scores of intensity and percentage. According to the scores, signals were assessed as follows: - (≤ 1), + (2 to 4), ++ (4 to 8) and +++ (≥ 9).
Immunohistochemistry (IHC) was performed on paraffin sections using a ready-to-use rabbit anti-human c-Myc monoclonal antibody (MXB, China). The sections were first deparaffinized in xylene and rehydrated through a graded alcohol series. Endogenous peroxidase was blocked with 3% H2O2 for 10 min, and antigen retrieval was carried out with sodium citrate buffer at 0.1 MPa and 121 °C for 5 min. After a blocking step using 5% BSA, slides were separately incubated with 50 μL of primary antibody at 4 °C overnight. Then, they were visualized by incubation with a biotin-conjugated secondary antibody followed by streptavidin and DAB (Boster, China). Counterstaining was performed with haematoxylin. The c-Myc score of each sample was based on the nuclear staining. The data were evaluated as described above.
All reactions were performed in triplicate. Measurement data are expressed as the mean ± SD, and statistical analyses were performed using one-way analysis of variance. Ordinal data were analysed by Radit analysis. P < 0.05 was considered statistically significant.
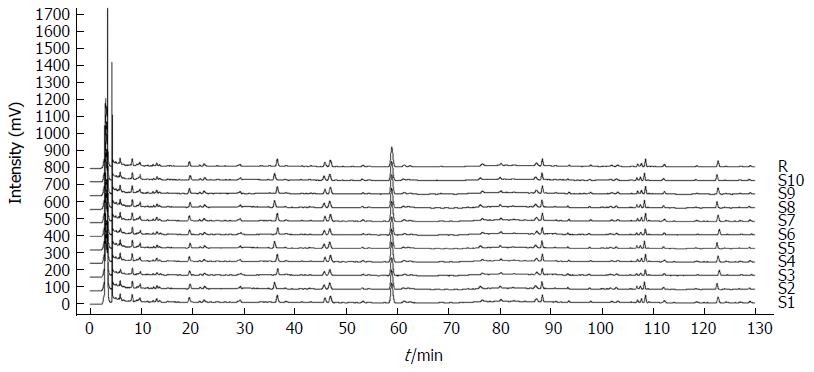
To establish quality control standards for YZSJD, HPLC was used to analyse 10 batches of YZSJD samples. As illustrated in Figure 1, the YZSJD HPLC fingerprints consisted of 22 characteristic peaks. The similarity scores of the fingerprinting profiles of the 10 batches of samples were above 0.95, which indicated the consistency and stability of the extracts and the preparation procedure.
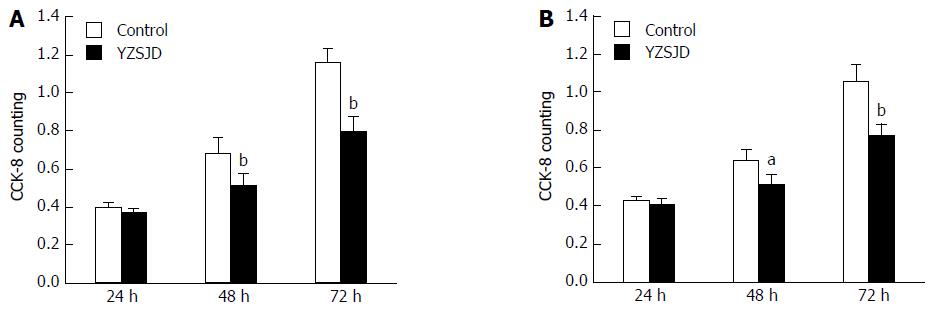
To investigate the biological effect of YZSJD on GC progression in vitro, AGS and HS-746T GC cells were treated with YCS. We evaluated cell proliferation using the CCK-8 assay. As shown in Figure 2, YCS significantly decreased the viability of AGS and HS-746T cells in a time-dependent manner.
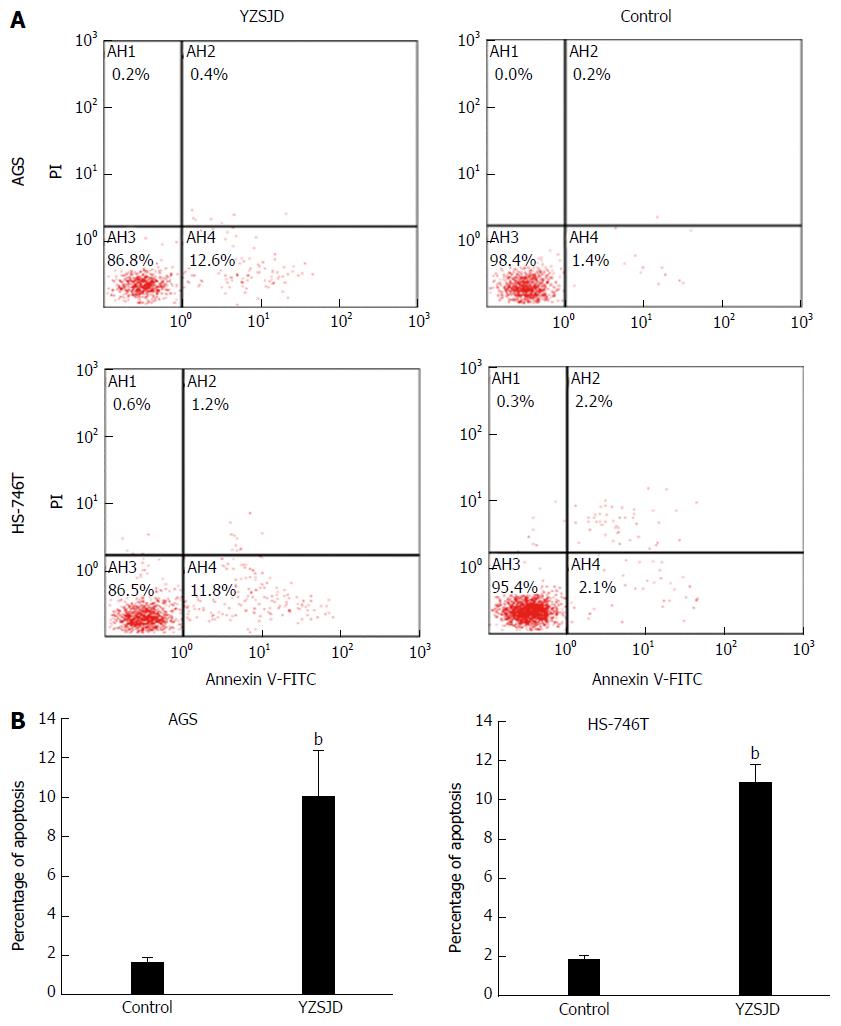
Next, we assessed cell apoptosis using flow cytometry. The early apoptosis rates of the two cell lines were significantly increased after treatment with YCS (P < 0.01). After 48 h treatment with YCS, the early apoptosis rates reached 9.97% ± 2.35% (AGS) and 10.9% ± 0.85% (HS-746T), while in the Control groups, the proportions of Annexin V+/PI- cells were only 1.67% ± 0.23% (AGS) and 1.8% ± 0.27% (HS-746T) (Figure 3).
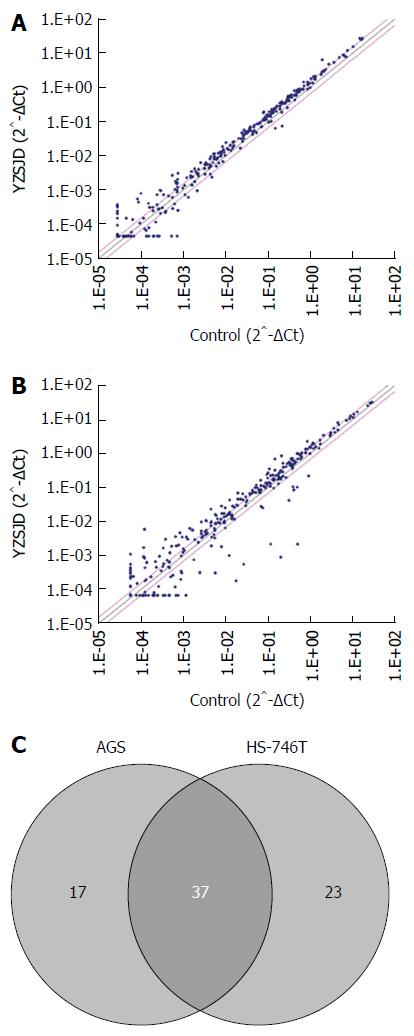
To explore the molecular mechanism involved in YZSJD-induced anticancer activity, we employed qPCR arrays to determine the changes in the miRNA expression profile in AGS and HS-746T GC cells treated with YCS. As shown in Figure 4A and B, the expression levels of a large number of miRNAs were altered. Using a recommended cut-off of 30 Ct, we identified 54 differentially expressed miRNAs in AGS cells and 60 in HS-746S cells. In both cell lines, 37 miRNAs showed the same expression alteration trend, 35 of which were up-regulated and 2 of which were down-regulated (Figure 4C and Table 2). Notably, almost all of the members of the let-7 family were altered: hsa-let-7a-5p, hsa-let-7d-3p, hsa-let-7f-5p and hsa-miR-98-5p were consistently up-regulated in both cell lines (Table 2), hsa-let-7g-5p was up-regulated only in AGS cells, and hsa-let-7b-5p, hsa-let-7c-5p, hsa-let-7d-5p, hsa-let-7e-5p and hsa-let-7i-5p were up-regulated only in HS-746T cells (data not shown).
| miRNA ID | Fold change (YZSJD/control) | |
| AGS | HS-746T | |
| hsa-let-7a-5p | 1.54 | 1.80 |
| hsa-let-7d-3p | 1.66 | 1.53 |
| hsa-let-7f-5p | 1.68 | 2.70 |
| hsa-miR-7-5p | 1.56 | 2.03 |
| hsa-miR-15a-5p | 1.66 | 2.04 |
| hsa-miR-15b-5p | 1.62 | 3.26 |
| hsa-miR-21-3p | 1.86 | 1.75 |
| hsa-miR-22-3p | 1.58 | 1.78 |
| hsa-miR-22-5p | 1.74 | 1.60 |
| hsa-miR-25-3p | 1.74 | 1.76 |
| hsa-miR-26b-5p | 1.64 | 1.80 |
| hsa-miR-27a-3p | 2.07 | 1.59 |
| hsa-miR-27b-3p | 1.55 | 1.66 |
| hsa-miR-30a-5p | 1.71 | 4.65 |
| hsa-miR-30b-5p | 1.55 | 1.73 |
| hsa-miR-30d-5p | 1.67 | 1.98 |
| hsa-miR-30e-3p | 1.52 | 1.77 |
| hsa-miR-32-5p | 1.60 | 1.83 |
| hsa-miR-92b-3p | 1.58 | 1.95 |
| hsa-miR-98-5p | 1.51 | 1.55 |
| hsa-miR-99a-5p | 1.73 | 1.56 |
| hsa-miR-105-5p | 1.57 | 2.59 |
| hsa-miR-125a-5p | 1.67 | 1.72 |
| hsa-miR-139-5p | 2.35 | 1.91 |
| hsa-miR-181b-5p | 1.78 | 2.68 |
| hsa-miR-186-5p | 1.65 | 1.71 |
| hsa-miR-192-5p | -2.27 | -4.17 |
| hsa-miR-193b-3p | 3.33 | 1.53 |
| hsa-miR-212-3p | 1.81 | 1.67 |
| hsa-miR-215-5p | -2.94 | -4.76 |
| hsa-miR-361-5p | 1.83 | 1.84 |
| hsa-miR-374a-5p | 1.83 | 2.06 |
| hsa-miR-450a-5p | 1.59 | 1.65 |
| hsa-miR-452-5p | 1.61 | 2.58 |
| hsa-miR-454-3p | 1.59 | 2.44 |
| hsa-miR-484 | 1.65 | 2.18 |
| hsa-miR-514a-3p | 1.60 | 3.34 |
We performed qRT-PCR to detect let-7a expression in GC cell lines with different genetic backgrounds. Significant down-regulation of let-7a expression was found in AGS, HS-746T, MKN-45 and SGC-7901 cells (P < 0.01) (Figure 5A). Next, the let-7a expression in AGS and HS-746T cells treated with YCS was further verified. As shown in Figure 5B, the levels of let-7a were significantly up-regulated (P < 0.01). We then conducted Western blot analysis to determine the c-Myc expression. The c-Myc protein expression was lower in the YZSJD groups than in the Control groups (Figure 5C and D). These results indicate that YZSJD increased the expression of let-7a and decreased c-Myc protein expression in AGS and HS-746T cells.
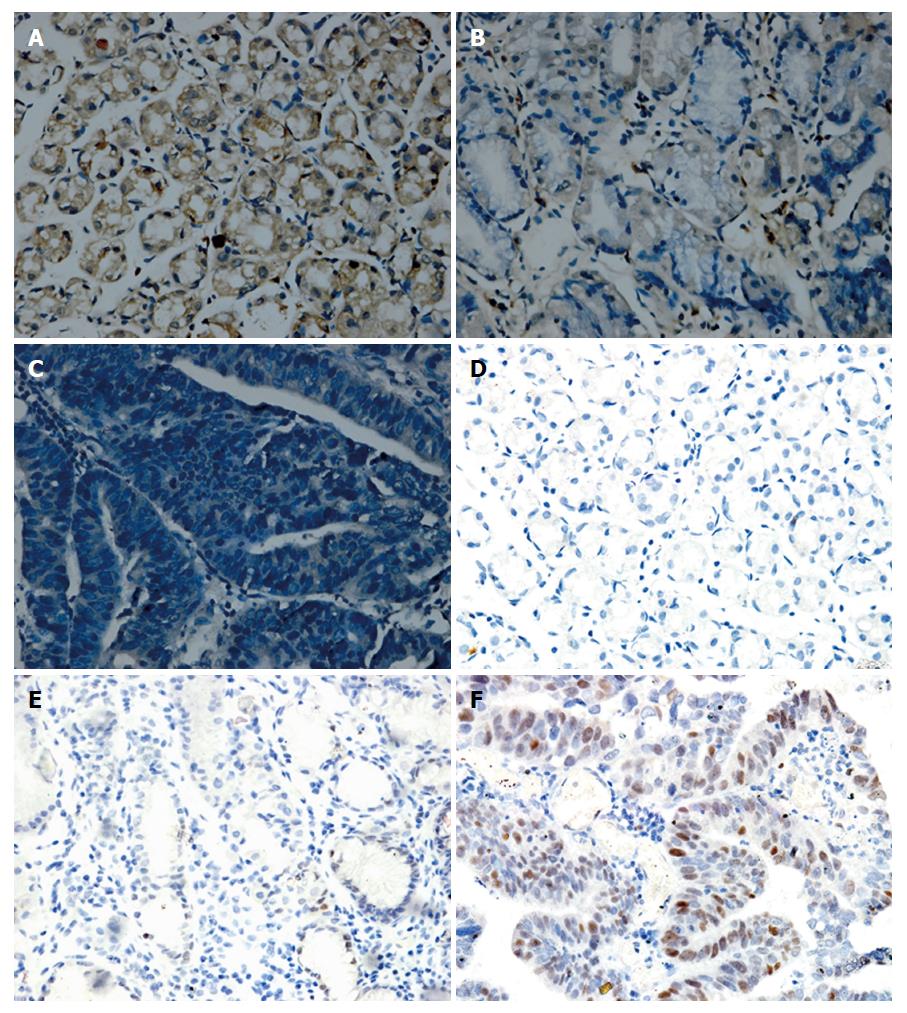
We examined let-7a expression using ISH and detected c-Myc expression by IHC in GC tissues, matched para-carcinoma tissues and distal normal gastric mucosal tissues. A significant reduction of let-7a expression was observed in GC tissues, while let-7a was predominantly expressed in distal normal gastric mucosa tissues. Conversely, the IHC-based assessment of c-Myc expression demonstrated a decreasing trend from GC tissues and adjacent para-cancerous tissues to distal normal gastric tissues (Figure 6). There were significant differences in the expression of let-7a and c-Myc among the three different grades of gastric mucosa tissues (P < 0.05) (Table 3).
| Tissue | Let-7a | c-Myc | ||||||||
| (-) | (+) | (++) | (+++) | P value | (-) | (+) | (++) | (+++) | P value | |
| Ca | 3 | 7 | 1 | 0 | < 0.05 | 0 | 2 | 4 | 5 | < 0.05 |
| Pc | 0 | 8 | 3 | 0 | 1 | 6 | 3 | 1 | ||
| N | 0 | 1 | 6 | 4 | 4 | 6 | 1 | 0 | ||
Our results show that let-7a expression was significantly lower in GC tissues than in the matched precancerous tissues and normal gastric mucosa epithelium, while c-Myc expression exhibited an opposite trend. YZSJD significantly inhibited proliferation and induced apoptosis in AGS and HS-746T cells, and YCS treatment resulted in up-regulation of let-7a and down-regulation of c-Myc in both cell types.
Currently, chemotherapy, endoscopic and surgical treatment are the major treatment modalities for GC. Since chemotherapeutics have the insurmountable problems of multi-drug resistance and negative side effects, and endoscopic and surgical treatments rely to a large extent on early detection of GC, the quality of life and 5-year overall survival rate for GC patients remain low. However, due to clear curative effects and low toxicity, traditional Chinese medicine has been recognized to have health and well-being benefits in cancer treatment[21,22].
YZSJD, a Chinese herbal compound prescribed by Dr. Geng-Xin Chen, has been used to treat gastric precancerous lesions at the Second Affiliated Hospital of Guangzhou University of Chinese Medicine for more than a decade. In our previous clinical study, we found that YZSJD has a definite therapeutic effect on the precancerous lesions of patients with chronic atrophic gastritis by targeting EGF and EGFR[7,19]. In this study, we explored the anti-cancer effects of YZSJD in two GC cell lines and investigated the underlying mechanism. YZSJD significantly inhibited proliferation and induced apoptosis in AGS and HS-746T cells.
To explore the molecular mechanism involved in YZSJD-induced anticancer activity, we employed qPCR arrays to determine the changes that occurred in the miRNA expression profile in AGS and HS-746T GC cells upon YCS treatment. The expression levels of a large number of miRNAs were altered. Notably, almost all of the members of the let-7 family were up-regulated by YZSJD. Of these, hsa-let-7a-5p, hsa-let-7d-3p, hsa-let-7f-5p and hsa-miR-98-5p were consistently up-regulated in both cell lines. Because of its high abundance, we focused on let-7a in this study. Subsequent verification experiments confirmed the influence of YZSJD on let-7a expression. To monitor the downstream effectors induced by the differential expression of let-7a, we analysed the expression of c-Myc, a classical target gene of the let-7 family, and found obvious down-regulation of the protein.
Let-7 was the first human miRNA discovered and is known as a classical anti-oncomiR[23]. Pairing between let-7 and its target genes, such as Myc, RAS or HMGA2, has been shown to be pivotal in tumourigenesis[15,24,25]. Although some conflicting data have emerged indicating that let-7 may have diverse functions in different forms of cancer[26], there is evidence showing the significance of let-7 loss in GC oncogenesis and metastasis[27-30]. In this study, we further investigated the association between let-7 and the risk of GC in matched tissue samples. Consistent with previous studies, our results indicate that let-7a expression diminished progressively during the progression of gastric mucosa cancerization.
The c-Myc oncogene is highly amplified in many cancer types and contributes to tumourigenesis[31,32]. Functioning as an important transcription factor, c-Myc protein also plays a crucial role in gastric carcinogenesis[33]. Chen et al[34] found that targeting c-Myc strongly inhibited cell growth and induced apoptosis in SGC7901 GC cells. Our data showed that c-Myc expression was increased markedly in GC tissues compared with matched precancerous tissues and normal gastric mucosae. Our data suggest that the reduction of let-7a may play an important role in GC occurrence and development through the derepression of c-Myc protein expression.
Several studies have revealed that let-7 inhibits the proliferation, migration, invasion and tumour metastasis of GC cells both in vitro and in vivo[28-30,35]. Let-7 miRNAs target the c-Myc 3’-UTR and negatively regulate its protein expression[15,16]. More recently, it was reported that let-7a inhibits the proliferation, migration and invasion of GC cells by suppressing the c-Myc/hnRNPA1/PKM2 pathway[18]. Given that reduced let-7a is involved in GC oncogenesis and that its expression increased in both AGS and HS-746T cells treated with YCS, we speculate that YZSJD suppressed proliferation and induced apoptosis in GC cells possibly by regulating the let-7a-c-Myc pathway.
In conclusion, the reduction of let-7a expression may play an important role in gastric tumourigenesis through c-Myc derepression. YZSJD inhibits proliferation and induces apoptosis by enhancing let-7a expression in GC cells. These findings provide new evidence that YZSJD has therapeutic potential in the treatment of GC and that miRNA expression regulation may be a novel molecular mechanism through which Chinese herbal medicine exhibits anti-cancer activity.
We thank the First Affiliated Hospital of Guangzhou University of Chinese Medicine for providing human GC samples.
Chinese herbal medicine has become an increasingly popular cancer therapy modality. Yangzheng Sanjie decoction (YZSJD) showed beneficial effects in the treatment of precancerous lesions of gastric cancer (GC) patients according to our published data. However, its therapeutic effect and underlying mechanism associated with GC remain unknown.
Let-7 miRNAs have been shown to act as tumour suppressors in GC by targeting c-Myc and are promising therapeutic targets for cancer treatment. In this study, the authors explored the let-7a-mediated anticancer effect of YZSJD in vitro. These data demonstrate that YZSJD inhibits proliferation and induces apoptosis by regulating the aberrant expression of let-7a and c-Myc in GC cells.
In the present manuscript, the authors present the novel findings that reduction of let-7a expression could play an important role in gastric tumourigenesis through the derepression of c-Myc expression and that YZSJD inhibits proliferation and induces apoptosis in GC cells by regulating the let-7a-c-Myc pathway.
These findings provide new evidence that YZSJD has therapeutic potential in the treatment of GC and that miRNA expression regulation may be a novel molecular mechanism through which Chinese herbal medicine exhibits anti-cancer activity, laying a new foundation for further research into the molecular mechanisms of GC treatment with Chinese herbal medicine.
The let-7 family (let-7a/b/c/d/e/f/g/i and miR-98) is a cluster of broadly conserved miRNAs. By targeting numerous oncogenes and signalling pathways (c-Myc, Ras, HMGA2, cyclin D, cyclin A, CDK4/6, Lin28, etc.), let-7 blocks tumour formation, progression and metastasis and induces cell apoptosis through post-transcriptional regulation.
This is an interesting basic study on new traditional Chinese medicine in regulation of the proliferation and apoptosis of GC cells. The authors present the novel findings that reduction of let-7a expression could play an important role in gastric tumourigenesis through the derepression of c-Myc expression and that YZSJD inhibits proliferation and induces apoptosis in GC cells by regulating the let-7a-c-Myc pathway. These findings provide new evidence that YZSJD has therapeutic potential in the treatment of GC and that miRNA expression regulation may be a novel molecular mechanism through which Chinese herbal medicine exhibits anti-cancer activity.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bernal G, Eleftheriadis NP S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21366] [Article Influence: 2136.6] [Reference Citation Analysis (3)] |
| 2. | Sun Y. The role of Chinese medicine in clinical oncology. Chin J Integr Med. 2014;20:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Guo Z, Jia X, Liu JP, Liao J, Yang Y. Herbal medicines for advanced colorectal cancer. Cochrane Database Syst Rev. 2012;CD004653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Li SG, Chen HY, Ou-Yang CS, Wang XX, Yang ZJ, Tong Y, Cho WC. The efficacy of Chinese herbal medicine as an adjunctive therapy for advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e57604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Hu Y, Wang S, Wu X, Zhang J, Chen R, Chen M, Wang Y. Chinese herbal medicine-derived compounds for cancer therapy: a focus on hepatocellular carcinoma. J Ethnopharmacol. 2013;149:601-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Li B, Gan R, Yang Q, Huang J, Chen P, Wan L, Guo C. Chinese Herbal Medicines as an Adjunctive Therapy for Unresectable Pancreatic Cancer: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2015;2015:350730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Chen G, Mou T, Li H. Clinical observation on treatment of chronic atrophic gastritis before canceration with Yangzheng Sanjie Tang in 55 cases. Jilin Zhongyiyao. 2007;27:17-18. |
| 8. | Cho WC, Leung KN. In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 2007;252:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Dai Z, Liu X, Ji Z, Liu L, Kang H, Wang X, Diao Y. The effect-enhancing and toxicity-reducing action of the extract of herba Scutellariae barbatae for chemotherapy in hepatoma H22 tumor-bearing mice. J Tradit Chin Med. 2008;28:205-210. [PubMed] |
| 10. | Wang T, Xuan X, Li M, Gao P, Zheng Y, Zang W, Zhao G. Astragalus saponins affect proliferation, invasion and apoptosis of gastric cancer BGC-823 cells. Diagn Pathol. 2013;8:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5384] [Cited by in RCA: 5607] [Article Influence: 295.1] [Reference Citation Analysis (0)] |
| 12. | Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5705] [Cited by in RCA: 6030] [Article Influence: 317.4] [Reference Citation Analysis (0)] |
| 13. | Thomson JM, Parker J, Perou CM, Hammond SM. A custom microarray platform for analysis of microRNA gene expression. Nat Methods. 2004;1:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 590] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 14. | Park SM, Shell S, Radjabi AR, Schickel R, Feig C, Boyerinas B, Dinulescu DM, Lengyel E, Peter ME. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell Cycle. 2007;6:2585-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762-9770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 590] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 16. | Kim HH, Kuwano Y, Srikantan S, Lee EK, Martindale JL, Gorospe M. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 2009;23:1743-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 450] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 17. | Yang X, Cai H, Liang Y, Chen L, Wang X, Si R, Qu K, Jiang Z, Ma B, Miao C. Inhibition of c-Myc by let-7b mimic reverses mutidrug resistance in gastric cancer cells. Oncol Rep. 2015;33:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Tang R, Yang C, Ma X, Wang Y, Luo D, Huang C, Xu Z, Liu P, Yang L. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in gastric cancer. Oncotarget. 2016;7:5972-5984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Chen G, Li H. Influences of Yangzheng Sanjie decoction on EGF and EGFR of gastric mucosa at precancerosis of chronic atrophic gastritis. Guiding Journal of TCM. 2007;13:20-22. |
| 20. | Santiago JM, Sasako M, Osorio J. [TNM-7th edition 2009 (UICC/AJCC) and Japanese Classification 2010 in Gastric Cancer. Towards simplicity and standardisation in the management of gastric cancer]. Cir Esp. 2011;89:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Feng Y, Wu C, Li J. Promising Role and Probable Molecular Mechanisms of TCM in Gastric Cancer Treatment. Liaoning Zhongyi Zazhi. 2017;44:200-203. [DOI] [Full Text] |
| 22. | He P, Shen K, Hu B. Progress of Traditional Chinese Medicine in Treating Gastric Cancer. Zhongguo Zhongyiyao Xuekan. 2012;30:280-282. [DOI] [Full Text] |
| 23. | Sun X, Liu J, Xu C, Tang SC, Ren H. The insights of Let-7 miRNAs in oncogenesis and stem cell potency. J Cell Mol Med. 2016;20:1779-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2664] [Cited by in RCA: 2707] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 25. | Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 919] [Cited by in RCA: 898] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 26. | Tsang WP, Kwok TT. Let-7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis. 2008;13:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Zhu YM, Zhong ZX, Liu ZM. Relationship between let-7a and gastric mucosa cancerization and its significance. World J Gastroenterol. 2010;16:3325-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Yang Q, Jie Z, Cao H, Greenlee AR, Yang C, Zou F, Jiang Y. Low-level expression of let-7a in gastric cancer and its involvement in tumorigenesis by targeting RAB40C. Carcinogenesis. 2011;32:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Han X, Chen Y, Yao N, Liu H, Wang Z. MicroRNA let-7b suppresses human gastric cancer malignancy by targeting ING1. Cancer Gene Ther. 2015;22:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Yu J, Feng J, Zhi X, Tang J, Li Z, Xu Y, Yang L, Hu Z, Xu Z. Let-7b inhibits cell proliferation, migration, and invasion through targeting Cthrc1 in gastric cancer. Tumour Biol. 2015;36:3221-3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Dang CV. MYC on the path to cancer. Cell. 2012;149:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1984] [Cited by in RCA: 2588] [Article Influence: 199.1] [Reference Citation Analysis (0)] |
| 32. | Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med. 2014;4:a014241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 627] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 33. | Calcagno DQ, Leal MF, Assumpcao PP, Smith MA, Burbano RR. MYC and gastric adenocarcinoma carcinogenesis. World J Gastroenterol. 2008;14:5962-5968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Chen JP, Lin C, Xu CP, Zhang XY, Fu M, Deng YP, Wei Y, Wu M. Molecular therapy with recombinant antisense c-myc adenovirus for human gastric carcinoma cells in vitro and in vivo. J Gastroenterol Hepatol. 2001;16:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Liang S, He L, Zhao X, Miao Y, Gu Y, Guo C, Xue Z, Dou W, Hu F, Wu K. MicroRNA let-7f inhibits tumor invasion and metastasis by targeting MYH9 in human gastric cancer. PLoS One. 2011;6:e18409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |