Published online Aug 14, 2017. doi: 10.3748/wjg.v23.i30.5499
Peer-review started: March 2, 2017
First decision: April 5, 2017
Revised: May 16, 2017
Accepted: June 1, 2017
Article in press: June 1, 2017
Published online: August 14, 2017
Processing time: 167 Days and 10.6 Hours
To develop a MRI-based method for accurate determination of liver volume (LV) and to explore the effect of long-term everolimus (EVR) treatment on LV in PCK rats with hepatomegaly.
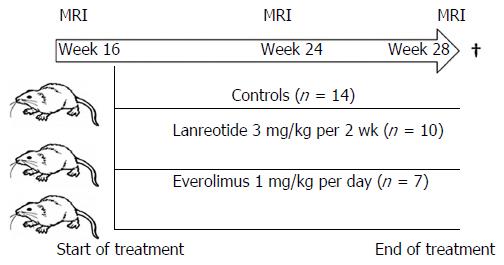
Thirty-one female PCK rats (model for polycystic-liver-disease: PCLD) were randomized into 3 groups and treatment was started at 16 wk, at the moment of extensive hepatomegaly (comparable to what is done in the human disease). Animals received: controls (n = 14), lanreotide (LAN: 3 mg/kg per 2 wk) (n = 10) or everolimus (EVR: 1 mg/kg per day) (n = 7). LV was measured at week 16, 24, 28. At week 28, all rats were sacrificed and liver tissue was harvested. Fibrosis was evaluated using quantitative image analysis. In addition, gene (quantitative RT-PCR) and protein expression (by Western blot) of the PI3K/AkT/mTOR signaling pathway was investigated.
LV determination by MRI correlated excellent with the ex vivo measurements (r = 0.99, P < 0.001). The relative changes in LV at the end of treatment were: (controls) +31.8%; (LAN) +5.1% and (EVR) +8.8%, indicating a significantly halt of LV progression compared with controls (respectively, P = 0.01 and P = 0.04). Furthermore, EVR significantly reduced the amount of liver fibrosis (P = 0.004) thus might also prevent the development of portal hypertension. There was no difference in phosphorylation of Akt (Threonine 308) between LAN-treated PCK rats control PCK rats, whereas S6 was significantly more phosphorylated in the LAN group. Phosphorylation of Akt was not different between controls and EVR treated rats, however, for S6 there was significantly less phosphorylation in the EVR treated rats. Thus, both drugs interact with the PI3K/AkT/mTOR signaling cascade but acting at different molecular levels.
Everolimus halts cyst growth comparable to lanreotide and reduces the development of fibrosis. mTOR-inhibition should be further explored in PCLD patients especially those that need immunosuppression.
Core tip: The continuous increase of liver cysts volume in polycystic-liver-disease (PCLD) leads to extensive hepatomegaly and portal hypertension, an indication for liver transplantation. The effect of mTOR-inhibition on liver volume (LV) in PCLD is unclear. We developed an accurate, non-invasive, MRI-based method to determine LV in a PCLD rat model. When treatment is started at the moment of extensive hepatomegaly (as in humans), the mTOR inhibitor everolimus halt disease progression and also of the development of fibrosis in this model. We speculate that everolimus, given after kidney transplantation in patients with PCLD, can prevent the development of symptomatic hepatomegaly.
- Citation: Temmerman F, Chen F, Libbrecht L, Vander Elst I, Windmolders P, Feng Y, Ni Y, De Smedt H, Nevens F, van Pelt J. Everolimus halts hepatic cystogenesis in a rodent model of polycystic-liver-disease. World J Gastroenterol 2017; 23(30): 5499-5507
- URL: https://www.wjgnet.com/1007-9327/full/v23/i30/5499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i30.5499
Polycystic liver disease (PCLD) is a fibrocystic liver diseases, a group of genetic disorders in which cysts occur either only in the liver, like in autosomal dominant PCLD, or in liver and the kidneys as in autosomal dominant polycystic kidney disease (ADPKD)[1,2]. In these patients due to the continuous increase in volume and number of cysts, the liver enlarges and may become disabling and in advanced stages, the patients develop portal hypertension[3].
The rat Pck gene is orthologous to the human PKHD1 gene and responsible for Autosomal Recessive Polycystic Kidney Disease. The animals have a splicing mutation in the Pkhd1 gene encoding fibrocystin/polyductin (FPC). FPC and polycystin-1 and -2, -proteins mutated in human ADPKD-, are co-localized to the primary cilium of the cholangiocytes. The PCK rat is used worldwide as model to study PCLD[4-7].
Two key signaling pathways have been implicated in the increased proliferation of PCK cholangiocytes leading to cyst formation. First, the defective ciliary structure in the cholangiocytes and integrated sensory/transducing functions result in a decreased intracellular Ca2+ and increased cytosolic cyclic adenosine monophosphate [cAMP]cyt, causing cholangiocyte proliferation, abnormal cell-matrix interactions, and altered fluid secretion. Basal levels of cAMP are maintained by the orchestration of multiple factors in which somatostatin receptors (SSTRs) play an important role. Octreotide, a somatostatin analogue, with high binding affinity for SSTR2 and 5, decreases [cAMP]cyt and reduces liver-cyst volume in the PCK rat. Both lanreotide (LAN) and octreotide reduce liver volume (LV) in patients with PCLD[8-13].
The other important pathway involves the mammalian target of Rapamycin (mTOR). mTOR is a serine/threonine kinase present in two distinct complexes. The first is mTOR complex 1 (mTORC1), composed of mTOR, Rptor (Raptor: Regulatory Associated Protein of mTOR, Complex 1), GβL, and DEPTOR. It is a master growth-regulator that senses and integrates diverse nutritional and environmental factors. The second complex, mTOR complex 2 (mTORC2), is composed of mTOR, Rictor, GβL, Sin1, PRR5/Protor-1, and DEPTOR. mTORC2 promotes cellular survival by activating Akt, regulates cytoskeletal dynamics and controls ion transport and growth[14,15]. Increased activation of the Phosphatidylinositol-4,5-bisphosphate 3-kinase/AkT/ mTOR (PI3K/Akt/mTOR) pathway has also been shown to be involved in the cystic proliferation of cholangiocytes of the PCK rat. Sirolimus, an mTOR inhibitor, delayed cyst growth in the Han:SPRD rat model and in a mouse model of ADPKD[16,17]. In human ADPKD patients, sirolimus reduced LV in patients who underwent kidney transplantation in one study[18]. However, the evidence of a beneficial effect of mTOR inhibitors to reduce LV in the PCK rat and in prospective studies in humans is not robust[19,20].
From preclinical studies performed in the PCK rat and from human PCLD data, is it clear that the hepatic cystic disease progression shows large inter-individual variability. To overcome this we explored the value of non-invasive repeated measurements of LV by MRI in the PCK rat.
The aims of the present study were therefor: (1) to develop an MRI-based method to evaluate accurately changes in LV in a rat model of PCLD; and (2) to investigate the long-term efficacy of everolimus (EVR) on liver disease when administered starting at the moment of already marked hepatomegaly, a situation mimicking the clinical situation.
Thirty-one 10-wk-old female PCK rats (Charles River, France) were used for this study. This rat model is derived from a Crj:CD (Sprague Dawley) rat strain, originating in Japan[4-7]. The animals were housed in an environment with normal humidity and a 12/12 daylight cycle receiving a normal diet. They were randomly assigned to three groups: (1) controls; (2) LAN as positive control; or (3) EVR. At week 16, when the animals had developed extensive hepatomegaly, baseline MRI was performed. The next day, therapy was started and continued for a period of 3 mo. No animals died or were excluded for other reasons over the entire study period. At indicated times (week 16, 24 and 28), serial MRI were performed (Figure 1). All rats were sacrificed the day after their last MRI at week 28 using Nembutal anesthesia. After vertical laparotomy, the hepatic hilum and hepatic veins were clipped simultaneously to avoid change in LV by loss of blood. Livers were removed, weighted and the ex vivo LV was determined using a graduated glass cylinder filled with saline 0.9% at 37 °C (accuracy of 1 mL). Tissue samples were stored for molecular analysis in Trizol (Invitrogen/Life Technologies, United States), snap-frozen for protein analysis and fixed with formalin for histology.
All animal experiments were approved by the Ethical Committee for animal welfare (KU Leuven, P164/2010).
Lanreotide 3 mg/kg was administered every 2 wk intramuscular (somatuline, gift from Ipsen Pharma; Merelbeke, Belgium). Everolimus oral solution 1 mg/kg per day (Certican®; gift from Novartis Pharma; Basel, Switzerland) was administered via the drinking water without further additives. Dosages were chosen based on previous published data[10,12,20]. We used black drinking bottles to ensure light protection in the EVR group. Drug solutions were freshly prepared every morning and adjusted to body weight and fluid intake once weekly.
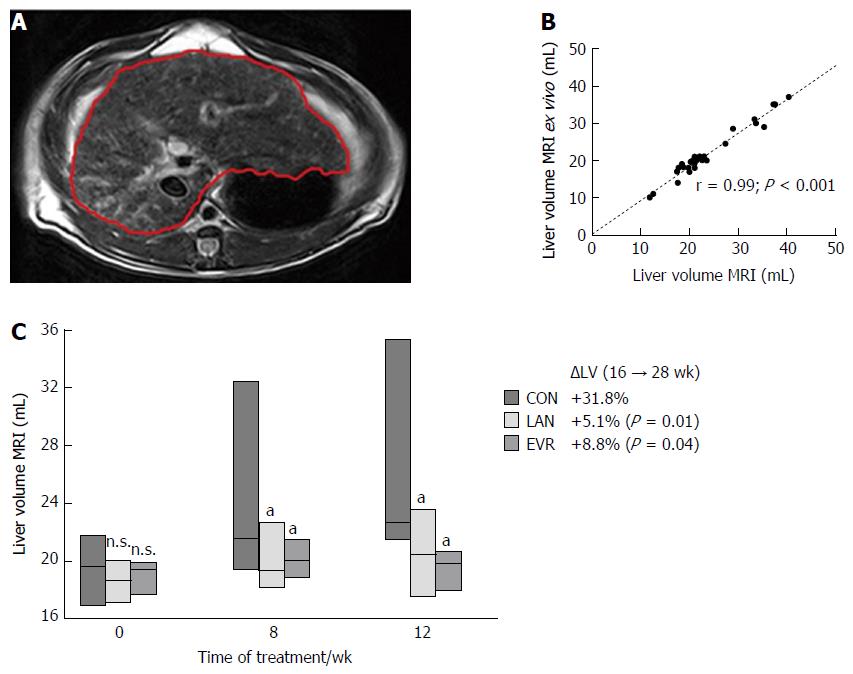
The PCK rats were anaesthetized with Isoflurane 2% and placed in a human wrist coil. MRI scanning (1.5T MRI scanner; slice thickness 0.3 cm; inter-slice gap: 0.03 cm) for liver volumetry was performed by 3 radiologists, blinded for the study groups; on the day before the start of treatment (week 16), at week 24 and at week 28 (i.e., 8 and 12 wk after the start of therapies). T1 and T2 weighted MR images were acquired. Liver area was measured and summed for all slices of T2 weighted images by using a built-in freehand region of interest (Figure 2A). Then, the LV was calculated using the following formula: LV = Σ liver area on each slice × (slice thickness + gap).
Liver samples were fixed in buffered formalin (4%), washed in PBS and embedded in paraffin. Picrosirius red collagen staining was performed to measure fibrosis. All liver biopsies were analyzed by an expert liver pathologist, blinded for the groups. Fibrosis was assessed using Olympus Stream image analysis software 1.9 Software following image acquisition using a light microscope and color digital camera (Olympus CMOS camera SC30 Münster, Germany). Fibrosis was scored by 2 independent researchers and expressed as percentage of total liver parenchyma from 4 random selected samples per animal.
Liver samples stored at -80 °C were homogenized using Tissue Lyzer LT (Qiagen) in RIPA buffer at 4 °C (50 mmol/L Tris pH 8.0, 150 mmol/L NaCl, 0.01% sodium dodecyl sulfate, 1% NP-40 (nonionic polyoxyethylene surfactant), 0.5% sodium desoxycholaat, 1 mmol/L 1-phenylmethylsulfonyl fluoride) containing protease inhibitor mix (Complete Protease Inhibitor Cocktail, Roche Applied Science, Penzberg, Germany). Protein concentrations were assessed with the BCA-kit (Abcam, Cambridge United Kingdom) and the protein in the lysates were adjusted to the same concentration (40 μg/20 μL). SDS sample buffer (62.5 mmol/L Tris, 10% glycerol, 2% sodium dodecyl sulfate, 0.05% Bromophenol blue and β-mercaptoethanol) was added, samples were boiled for 5 min and separated on miniprotean TGX anykD gel from Biorad (Biorad, Hercules, CA, United States). After electrotransfer to nitrocellulose membrane and blocking in Phosphate-buffered saline (PBS) containing 0.1% Tween and 5% non-fat dried milk, the membranes were overnight incubated with the primary antibody in PBS supplemented with 0.1% Tween and 5% non-fat dry milk powder. Antibodies used were: anti-P-S6Rp (Ser235/236, #4858), anti-Tot S6Rp (#2217), anti-P-Akt (Thr308,#) anti-Tot Akt (#2967), all purchased from Cell Signaling Technology (Beverly, Massachusetts, United States), and β-actin (Sigma-Aldrich, St. Louis, MO, United States) as loading control. Thereafter, the corresponding secondary horseradish peroxidase-coupled antibodies were applied to the membranes for one hour (Dako, Heverlee, Belgium). After addition of enhanced chemiluminescence reagent (Pierce ECL Western Blotting Substrate, #32106, Thermo Scientific/Life Technologies, Carlsbad, CA, United States), digital detection was performed using ChemiDoc™ imaging system with Image Lab™ image acquisition software (Biorad). Expression of P-S6Rp, total S6Rp, P-Akt, and total Akt was normalized to β-actin levels. For comparison of different blots, a pool of liver homogenates of 28-wk-old Sprague Dawley rat livers was placed on each gel as internal control.
Gene expression was assessed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). RNA was isolated from tissue stored in Trizol with the RNeasy Kit (Qiagen, Chatsworth, CA, United States) according to the manufacturer’s instructions. One microgram of cellular RNA was transcribed into cDNA using SuperScript II reverse transcriptase and random hexamer primers (Invitrogen/Life Technologies, United States). The PCR reaction was carried out in a mixture that contained appropriate sense- and anti-sense primers and a probe in Taqman Univeral PCR Master Mixture (Applied Biosystems, Foster City, United States) (Table 1). β-2 microglobulin (B2M) was used as house-keeping gene. Each sample was assayed in duplicate on an A7500 Fast Real-Time PCR System (Applied Biosystems). The mean ∆Ct value (with SE) vs the reference (B2M) was calculated.
| Gene | Full name | Assay ID applied biosystems |
| SSTR2 | somatostatin receptor 2 | Rn01464950_g1 |
| SSTR5 | somatostatin receptor 5 | Rn02535169_s1 |
| mTOR | mechanistic target of rapamycin (serine/threonine kinase) | Rn00693900_m1 |
| Rptor | regulatory associated protein of mTOR, complex 1 | Rn01464431_m1 |
| B2M | beta-2 microglobulin | Rn00560865_m1 |
Data were analyzed using MedCalc version 14.12.0 (Medcalc, Ostend, Belgium: http://www.medcalc.org). Descriptive statistics including mean and SE for continuous variables were computed or median with IQR (interquartile range) (25%-75%) as appropriate. Differences in continuous variables between treated and non-treated rats were investigated using, One Way ANOVA, t-test for independent samples or the Mann Whitney U test, as appropriate. Repeated measurements ANOVA and serial measurements were used to compare paired observations between groups, as appropriate. To assess correlations, non-parametric testing Pearson correlation coefficient was determined. Bland-Altman plots assessed agreement in accuracy of the techniques. To compare percentages between groups, χ2 was used. All P values resulted from two-sided statistical tests, and P < 0.05 is considered significant.
To validate MRI as a tool, we determined LV by two methods. (1) In vivo LV was determined by MRI (Figure 2A); and (2) To measure the LV ex vivo, we removed the liver at the end of the experiment (week 28, corresponding with 3 mo of treatment). Pearson correlation between in vivo LV (MRI) and ex vivo LV was excellent (r = 0.99, P < 0.001) (Figure 2B) and that allows us to use MRI as a reliable method to explore LV. At baseline rat body weight (gram) in the 3 groups: controls, LAN and EVR were not different, respectively: 316 (SE: 3.2); 318 (SE: 5.6) and 299 (SE: 2.7). Also at baseline, the median LV’s (range) for controls, LAN and EVR, were not different, respectively: 19.6 mL (17.0; 21.7); 18.7 mL (17.0; 20.0); 19.4 mL (17.8; 20.6) (P = 0.754). The mean of the relative increase in LV (95%CI) in the 3 groups after 12 wk of treatment (from week 16 to week 28) was respectively: +31.8% (19.0;44.0); +5.1% (-12.0; 23.0) and +8.8% (-1.7; 19.0). Both treatment groups (LAN, EVR) significantly halted LV progression compared with controls (P = 0.01 and P = 0.04). The absolute LVs at different times are given in Figure 2C. There was no significant difference in effect on LV between LAN and EVR after 8 wk or 12 wk of treatment.
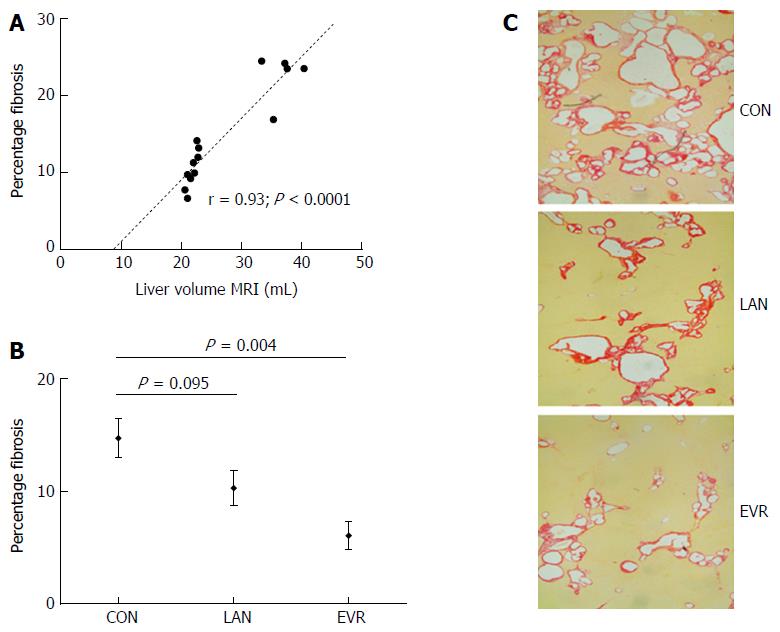
Fibrosis, scored independently by 2 researchers, showed excellent agreement with a difference of 0.5% (95%CI: -0.2-1.3). In the 14 control PCK rats [mean percentage fibrosis: 14.7% (SE: 1.8)], we found a strong Pearson correlation (r) between LV (MRI) and the relative amount of fibrosis (r = 0.93; P < 0.0001) (Figure 3A). EVR significantly suppressed the development of fibrosis in PCK rats (P = 0.004) whereas LAN showed a trend to reduction (P = 0.095) (Figure 3B). Representative histological images of picrosirius red staining are shown in Figure 3C.
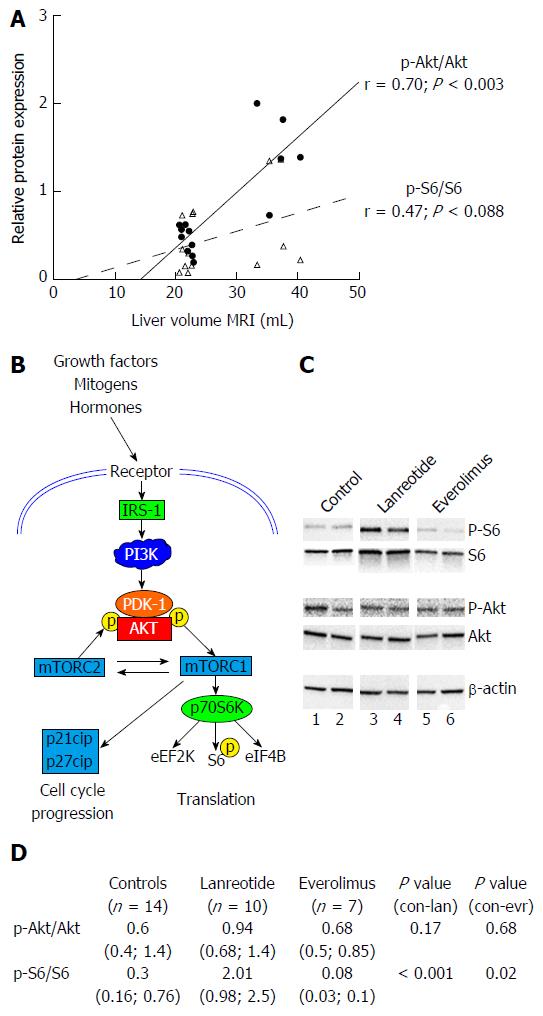
Expression of components of the PI3K/Akt/mTOR pathway was investigated. In the control animals, we observed a very strong correlation between the p-Akt/Akt phosphorylation ratio and the LV (r = 0.7, P < 0.003) while the p-S6/S6 ratio correlated only moderately (r = 0.47, P = 0.088) (Figure 4A).
There was no difference in phosphorylation of Akt (Threonine 308) between LAN-treated PCK rats control PCK rats, whereas S6 was significantly more phosphorylated in the LAN group. The p-S6/S6 ratios for LAN and controls were respectively: 2.01 (0.98; 2.53) and 0.33 (0.16; 0.76) (P < 0.001). The level of phosphorylation of Akt at Threonine 308 was not different between controls and EVR treated rats. However, S6 was significantly less phosphorylated in the EVR treated rats (Figure 4C and D).
These observations indicate that both drugs interact with the PI3K/AkT/mTOR signaling cascade but acting at different molecular levels (Figure 4B), both mechanisms leading to halting of the disease in this model of PCLD.
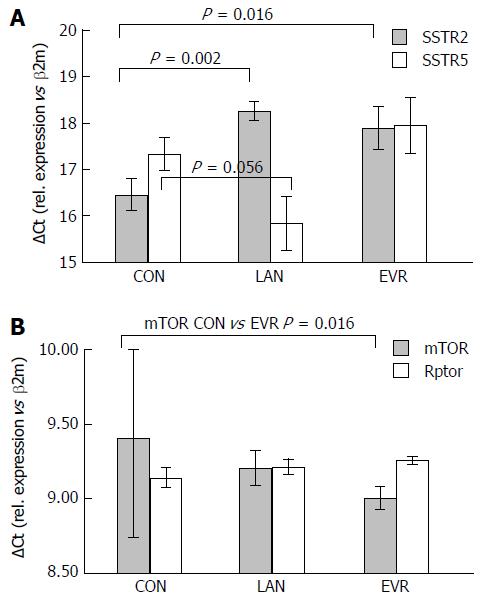
LAN and EVR treated rats showed a significant lower gene expression of SSTR2 while expression of SSTR5 was increased (borderline significant) in the LAN treated rats (Figure 5). EVR treatment resulted in a decreased SSTR5 gene expression compared to LAN treated animals. After 12 wk of treatment, we observed an increased gene expression of mTOR but not of Rptor in the EVR treated rats vs controls. LAN treatment did not affect gene expression of mTOR. Demonstrating again that both drugs have different mechanisms of action on cyst growth.
Similar to what is observed in human PCLD patients; we found a large inter- and intra-litter variation of the severity of hepatic polycystic disease in the PCK model, which had previously also been found by Mason et al[21]. Therefore, there is a great need for a reliable technique that can accurately assess LV in this animal model and further that allows repeated measurements during drug treatment. High Resolution Ultrasonography has recently been used to assess renal cysts in the PCK rats[22]. Also, as an indirect assessment method, T1 relaxation time was proposed as an imaging marker of liver disease for the PCK model[23] but neither of these was used to determined LV. For the current study, we explored MRI T2 weighted images to longitudinally measure LV and to investigate individual responses. We validated this method in the PCK model. We showed that it is accurate and reproducible, with an excellent correlation between LV determined by MRI and ex vivo LV determination. The assessment with MRI allowed us to detect changes in the same animal in time accurately. Treatment with LAN was used as positive treatment control and as expected, we observed an increase in LV with placebo and a stabilization with LAN, an observation similar to what is seen in patients with PCLD[10-13].
Important to note is that no animals died or were excluded for other reasons over the entire study period. Using this non-invasive technique, we could drastically reduce the number of animals necessary for this study.
In the present study, we investigated the effect of the mTOR inhibitor EVR on cyst growth since its effect in humans or in the corresponding animal model, is still unclear and controversial due to the limited number of available studies[20,24]. We found that EVR prevented cyst enlargement in the PCK rat model, using a clinical relevant experimental design in which we started the treatment at the moment of severe hepatomegaly, mimicking the clinical situation in humans. The study was restricted to female rats since symptomatic PCLD mainly affects mostly women and because it has been demonstrated that female PCK rats display a more progressive liver enlargement compared to male PCK rats.
Treatment with LAN induced a reduction of SSTR2 gene expression (in agreement with the expected molecular effect of LAN). In line with previous findings of Masyuk et al[8] with octreotide and pasireotide, we also observed upon treatment with LAN a mild reduction in fibrosis compared to untreated animals that might be an indirect effect of lower growth of the liver cysts. With EVR however, we observed a significant reduction of the amount of fibrosis, which may be explained by the well-known direct effect of mTOR inhibition on fibrosis progression[25]. Since PCLD, belonging to the fibrocystic liver diseases, is complicated in a later stage by portal hypertension, the present observations further support an additional potential clinical benefit of this drug on portal hypertension by reducing the amount of fibrosis.
The molecular observations made in this model (the combination of protein and gene expression) indicate a dysregulation of the PI3K/AkT/mTOR signaling cascade by both LAN and EVR, each acting at a different level and both mechanisms leading to halting of the disease. mTOR is a serine/threonine kinase and can form two protein complexes: mTORC1 (mTOR, Rptor), which is inhibited EVR, and mTORC2 (mTOR, Rictor). Ribosomal protein S6 kinase (S6K) is the direct downstream target of mTORC1 and regulates the downstream translational initiation machinery to control cell growth, proliferation and autophagy. mTORC 2 controls actin cytoskeleton and resistance to apoptosis. In animals treated with EVR, we observed an increased mTOR gene expression which is probably due to the prolonged administration of EVR in an attempt of the cell to compensate for the inhibitory effects of EVR on mTOR. S6-protein, downstream of mTOR, was less phosphorylated in EVR-treated animals compared to control PCK rats. Renken and colleagues investigated sirolimus in PCK rats, the time-dependent effect was assessed by using different groups sacrificed at different time points. No longitudinal follow-up of LV in the same animal was performed their study. They could only observe subtle effects of sirolimus on mTOR-specific S6 kinase in the liver[26]. The large inter- and intra-litter variability of hepatic disease progression may explain why they in their study could not observe a beneficial effect on liver disease progression. Further studies on the molecular mechanisms involved in PCLD in (pre)clinical models are needed.
In conclusion, our method of LV measurement with MRI is shown to be highly sensitive and allowed us to detect accurately changes in time in a non-invasive way. Long-term everolimus treatment halts liver cyst growth and reduces the development of fibrosis in this rat model. Our observations support the rational to explore further everolimus for the prevention of the development of symptomatic liver disease, such as in patients with ADPKD after kidney transplantation who are in need of an immunosuppressive drug.
The polycystic liver diseases (PCLD) represent a group of genetic disorders, in which cysts occur in the liver (ADPLD), or occur as well in the liver as in the kidneys (ADPKD). In PCLD, the liver becomes polycystic at a late stage, e.g., in ADPKD patients, the prevalence is 85%, and 94% in subgroups of age (resp. 25 to 34, and 35 to 46 year). Most of the patients with PCLD are asymptomatic; however, in a subpopulation of 1%-3%, expansion of liver cysts cause invalidating abdominal symptoms furthermore, symptomatic ADPLD patients are mainly females. The most common complication in patients with PCLD is extensive hepatomegaly, which may lead to malnutrition and can be lethal and cyst-related complications include hemorrhage, infections, and rupture. There is no medical treatment approved for PCLD and to date, the only definitive treatment in those patients with large liver volumes (LV) is liver transplantation (LT).
Octreotides and lanreotides have been shown to reduce LV in patients with PCLD, beneficial effects of mTOR-inhibition in patients is still a matter of debate. This was investigated in these experiments in the PCK-rat model of PCLD as preclinical evaluation. The authors designed the study representative for the hospital situation and started treatment at the moment of marked hepatomegaly (representing the symptomatic female patient). They developed and validated a MRI-based method to determine LV that can be used for longitudinal follow-up of disease progression and supported the observations with molecular analysis.
The authors developed a unique MRI-based method that was shown to be sensitive and statistically reliable. Repeat measurements allowed for monitoring individual responses and reducing the number of animals required for this type of study. This method is a great step forward for it allows preclinical testing of drugs for PCLD under controlled conditions. Everolimus looks beneficial to reduce the cyst volume in PCLD with a secondary benefit on fibrosis.
The imaging technique that they describe can be used to study drugs or drug combinations in preclinical setting in the representative animal model of PCK rats that for logistic reasons (number of symptomatic patients, slow progress of the disease, inter-individual variation of progression) is difficult to organize in patients. Ultimately, this approach can lead to a reduction of patients that require a transplantation. Polycistic liver and kidney diseases are closely related. Kidney transplantation is a frequent treatment for autosomal dominant polycystic kidney disease (ADPKD, autosomal dominant PKD or adult-onset PKD), and this study gives arguments to use everolimus in patients that need immunosuppression as it can have beneficial effects for the livers at risk in ADPKD patients and complications due to progressive fibrosis.
In this study, authors have shown that everolimus halts hepatic cystogenesis in a rodent model of PCLD. They have developed a MRI-based method for accurate determination of LV and investigated that everolimus halted cyst growth comparable to lanreotide and reduced the development of fibrosis, mTOR-inhibition should be further explored in PCLD patients especially those that need immunosuppression. The study has been well performed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dang SS S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Desmet VJ. Ludwig symposium on biliary disorders--part I. Pathogenesis of ductal plate abnormalities. Mayo Clin Proc. 1998;73:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 125] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Temmerman F, Missiaen L, Bammens B, Laleman W, Cassiman D, Verslype C, van Pelt J, Nevens F. Systematic review: the pathophysiology and management of polycystic liver disease. Aliment Pharmacol Ther. 2011;34:702-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Van Keimpema L, De Koning DB, Van Hoek B, Van Den Berg AP, Van Oijen MG, De Man RA, Nevens F, Drenth JP. Patients with isolated polycystic liver disease referred to liver centres: clinical characterization of 137 cases. Liver Int. 2011;31:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Katsuyama M, Masuyama T, Komura I, Hibino T, Takahashi H. Characterization of a novel polycystic kidney rat model with accompanying polycystic liver. Exp Anim. 2000;49:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int. 2001;59:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 520] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 7. | Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD). Hepatology. 2009;49:160-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3’,5’-cyclic monophosphate. Gastroenterology. 2007;132:1104-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, Kim B, King BF, Glockner J, Holmes DR. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, de Man RA, Drenth JP. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137:1661-1668.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Gevers TJ, Inthout J, Caroli A, Ruggenenti P, Hogan MC, Torres VE, Nevens F, Drenth JP. Young women with polycystic liver disease respond best to somatostatin analogues: a pooled analysis of individual patient data. Gastroenterology. 2013;145:357-365.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Temmerman F, Gevers T, Ho TA, Vanslembrouck R, Coudyzer W, van Pelt J, Bammens B, Pirson Y, Drenth JP, Nevens F. Safety and efficacy of different lanreotide doses in the treatment of polycystic liver disease: pooled analysis of individual patient data. Aliment Pharmacol Ther. 2013;38:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Temmerman F, Ho TA, Vanslembrouck R, Coudyzer W, Billen J, Dobbels F, van Pelt J, Bammens B, Pirson Y, Nevens F. Lanreotide Reduces Liver Volume, But Might Not Improve Muscle Wasting or Weight Loss, in Patients With Symptomatic Polycystic Liver Disease. Clin Gastroenterol Hepatol. 2015;13:2353-2359.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5794] [Cited by in RCA: 6598] [Article Influence: 507.5] [Reference Citation Analysis (1)] |
| 15. | Ren XS, Sato Y, Harada K, Sasaki M, Furubo S, Song JY, Nakanuma Y. Activation of the PI3K/mTOR pathway is involved in cystic proliferation of cholangiocytes of the PCK rat. PLoS One. 2014;9:e87660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wüthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han: SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant. 2006;21:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Zafar I, Ravichandran K, Belibi FA, Doctor RB, Edelstein CL. Sirolimus attenuates disease progression in an orthologous mouse model of human autosomal dominant polycystic kidney disease. Kidney Int. 2010;78:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, Torres VE. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol. 2008;19:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 432] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 20. | Chrispijn M, Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Drenth JP. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: results from a randomized controlled trial. J Hepatol. 2013;59:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Mason SB, Liang Y, Sinders RM, Miller CA, Eggleston-Gulyas T, Crisler-Roberts R, Harris PC, Gattone VH. Disease stage characterization of hepatorenal fibrocystic pathology in the PCK rat model of ARPKD. Anat Rec (Hoboken). 2010;293:1279-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Kapoor S, Rodriguez D, Mitchell K, Wüthrich RP. High Resolution Ultrasonography for Assessment of Renal Cysts in the PCK Rat Model of Autosomal Recessive Polycystic Kidney Disease. Kidney Blood Press Res. 2016;41:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Gao Y, Erokwu BO, DeSantis DA, Croniger CM, Schur RM, Lu L, Mariappuram J, Dell KM, Flask CA. Initial evaluation of hepatic T1 relaxation time as an imaging marker of liver disease associated with autosomal recessive polycystic kidney disease (ARPKD). NMR Biomed. 2016;29:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Khan S, Dennison A, Garcea G. Medical therapy for polycystic liver disease. Ann R Coll Surg Engl. 2016;98:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Patsenker E, Schneider V, Ledermann M, Saegesser H, Dorn C, Hellerbrand C, Stickel F. Potent antifibrotic activity of mTOR inhibitors sirolimus and everolimus but not of cyclosporine A and tacrolimus in experimental liver fibrosis. J Hepatol. 2011;55:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Renken C, Fischer DC, Kundt G, Gretz N, Haffner D. Inhibition of mTOR with sirolimus does not attenuate progression of liver and kidney disease in PCK rats. Nephrol Dial Transplant. 2011;26:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |