Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5379
Peer-review started: January 19, 2017
First decision: February 23, 2017
Revised: March 10, 2017
Accepted: April 21, 2017
Article in press: April 21, 2017
Published online: August 7, 2017
Processing time: 199 Days and 23.6 Hours
To investigate the factors influenced the efficacy of tolvaptan (TLV) in liver cirrhosis.
We retrospectively enrolled 61 consecutive patients with refractory hepatic ascites. All of them had been treated with furosemide and spironolactone before admission, and treated with TLV for 7 d in our hospital. The effect of TLV was defined by the rate of body weight loss, and the factors that influenced TLV efficacy were analyzed using multiple regression.
Coexistent hepatocellular carcinoma (HCC) was the only significant predictive variable that attenuated the efficacy of TLV. In stratified analysis, high doses of furosemide decreased the efficacy of TLV in patients with HCC, and increased efficacy in those without HCC. In the latter, a high Child-Pugh-Turcotte score had a positive influence and a high concentration of lactate dehydrogenase had a negative influence on the effectiveness of TLV.
Development of ascites may differ between patients with liver failure and those with HCC progression. A sufficient preceding dose of furosemide decreases diuretic effect of TLV.
Core tip: Efficacy of tolvaptan (TLV) for hepatic ascites has been confirmed but some patients are resistant to the drug. We investigate influencing factors of the efficacy of TLV. As a result, coexistent hepatocellular carcinoma (HCC) was the only significant predictive variable that attenuated the efficacy of TLV. In stratified analysis, high doses of furosemide decreased the efficacy of TLV in patients with HCC, and increased efficacy in those without HCC. This study suggests that development of ascites may differ between patients with liver failure and those with HCC progression. A sufficient preceding dose of furosemide decreases diuretic effect of TLV.
- Citation: Miyazaki M, Yada M, Tanaka K, Senjyu T, Goya T, Motomura K, Kohjima M, Kato M, Masumoto A, Kotoh K. Efficacy of tolvaptan for the patients with advanced hepatocellular carcinoma. World J Gastroenterol 2017; 23(29): 5379-5385
- URL: https://www.wjgnet.com/1007-9327/full/v23/i29/5379.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i29.5379
Fluid retention such as ascites, edema or pleural effusion is a major complication of advanced liver cirrhosis, which results from distortion of hepatic architecture and increased hepatic vascular tone, and a subsequent derangement in the extracellular fluid volume regulatory mechanisms [1,2].
Tolvaptan (TLV) is a vasopressin V2 receptor antagonist, which has been accepted as a useful diuretic for patients with cirrhosis. Hypersecretion of antidiuretic hormone (ADH) is involved in the development of hepatic ascites, and inhibition of ADH causes free water excretion[3]. Cumulated reports investigating the influence of TLV on patients with cirrhosis have demonstrated its effectiveness, although a few patients are resistant to TLV[4-9]. The renin-angiotensin-aldosterone (RAA) system, another hormonal regulator of hepatic ascites, is thought to be one of the main causes of hepatic ascites. Spironolactone, an inhibitor of aldosterone, combined with furosemide, is recommended as the first-line treatment[10,11]. However, there are also non-responders as well as responders to TLV[12,13]. Those findings indicate that the pathogenesis of cirrhotic ascites includes multiple hormonal pathways and the dominant pathway varies among individuals.
Portal hypertension is also involved in the development of cirrhotic ascites. Overt ascites is rarely seen if the post-sinusoidal pressure gradient is < 12 mmHg[14]. Tumor thrombus in the main portal vein trunk can cause an abrupt development of ascites. Although many patients with cirrhosis are complicated by hepatocellular carcinoma (HCC), studies investigating the correlation between ascites and HCC have been rare. Furthermore, scarce investigators payed attention to the influence of HCC on the effect of TLV. Suzuki et al[15] recently reported the efficacy and safety of TLV for the patients with advanced HCC, but the number of the enrolled patients was only 9. A recent study analyzing the efficacy of TLV has shown that uncontrolled neoplasm is an independent factor that negatively affects TLV treatment, but the mechanism remains to be clarified[5].
Excess excretion of free water with TLV carries a risk of hypernatremia[16]. The use of concomitant natriuretic diuretics such as furosemide or spironolactone is required to avoid such an adverse reaction. However, the best plan for the combination of TLV and other diuretics relating to efficacy and safety has been rarely examined. It is possible that appropriate use of concomitant diuretics might reduce the number of non-responders to TLV.
We have treated patients suffering from refractory hepatic ascites with TLV regardless of being complicated with HCC for 5 years. Compared with earlier studies, the population included a high number of patients with advanced HCC. In the present study, we retrospectively investigated the factors that influenced the efficacy of TLV with multiple regression analysis, to clarify the best combination of TLV and other diuretics. Richness of variety of our treated patients might contribute to draw some useful information.
From July 2011 to September 2015, 84 consecutive patients with refractory hepatic ascites who were treated with TLV were referred to our hospital. All of them were hospitalized for ≥ 1 wk to monitor their serum sodium concentration and body weight (BW). Although they had been treated with furosemide and/or spironolactone before admission, only 61 patients who had received both drugs were enrolled. They consisted of 36 men and 25 women, ranging in age from 43 to 91 years. They all underwent computed tomography on admission, which showed liver cirrhosis and massive ascites, and 32 had HCC (Table 1).
| HCC (+) | HCC (-) | P vaule | |
| n | 32 | 29 | |
| Age | 71.0 ± 11.2 | 61.8 ± 11.3 | 0.0064 |
| Sex (M/F) | 19/13 | 17/12 | 0.9523 |
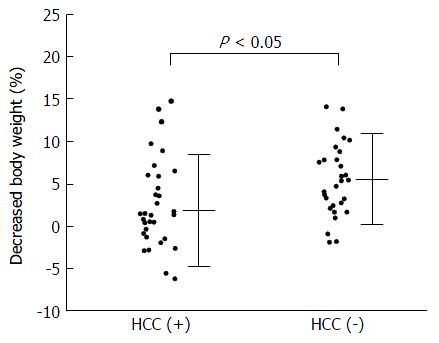
| Decreased BW (kg) | 1.68 ± 3.81 | 3.43 ± 3.00 | 0.0130 |
| Decreased BW (%) | 2.56 ± 5.23 | 5.36 ± 4.27 | 0.0127 |
| TLV (3.75/7.5/15 mg/d) | 3/24/5 | 2/22/5 | 0.9325 |
| Furosemide (≤ 20/20 <, ≤ 40/> 40 mg/d) | 8/13/11 | 8/11/10 | 0.9671 |
| Spironolactone (≤ 25/25 <, ≤ 50/> 50 mg/d) | 2/8/22 | 2/10/17 | 0.6986 |
| Portal thrombus (+/-) | 8/24 | 0/29 | 0.0039 |
| Albumin (g/dL) | 2.64 ± 0.38 | 2.31 ± 0.48 | 0.0045 |
| CPT score | 10.7 ± 2.0 | 11.1 ± 1.7 | 0.2357 |
| BUN (mg/dL) | 21.3 ± 8.2 | 18.6 ± 10.2 | 0.1584 |
| Na (mEq/L) | 134.1 ± 5.7 | 134.6 ± 5.4 | 0.5620 |
| K (mEq/L) | 4.02 ± 0.67 | 3.85 ± 0.55 | 0.4827 |
| eGFR (mL/min) | 58.66 ± 21.78 | 69.52 ± 40.39 | 0.8285 |
| LDH (IU/L) | 293.8 ± 164.1 | 259.6 ± 107.2 | 0.4228 |
| Platelet count (× 104/μL) | 10.87 ± 5.38 | 12.40 ± 11.35 | 0.8852 |
| T factor of TNM classification (T1/T2/T3a and T3b) | 6/13/13 | - | - |
The daily dose of TLV was determined to be 3.75, 7.5, or 15 mg roughly according to the patient’s body size. TLV was continuously administered for 7 d from the time of admission, while the preceding furosemide and spironolactone were concomitantly given without changing the doses.
This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committees at our institutions.
The efficacy of TLV was determined using the following formula: Decreased BW% = (BW before TLV - BW at day 7)/BW before TLV × 100%.
The individual correlation between the decreased BW% and the factors that might influence TLV efficacy was tested by linear regression analysis for consecutive variables, or by post hoc analysis for categorical ones. To confirm the predictive factors for the decreased BW%, those variables were analyzed using stepwise multiple regression analysis. The evaluated factors were the daily dose of TLV, furosemide and spironolactone, complicating HCC, portal thrombosis in the main trunks, T factor of TNM classification of Union for International Cancer Control, Child-Pugh-Turcotte (CPT) score, serum levels of blood urea nitrogen, sodium, potassium, and lactate dehydrogenase (LDH), estimated glomerular filtration rate, and platelet count. All laboratory data were derived upon admission. If the samples of consecutive variables did not conform to a normal distribution, they were transformed into logistic or Johnson’s SU distribution before application to regression analysis. In comparison between two groups, averages of consecutive variables were tested by Wilcoxon’s test, and the categorical variables were tested by Pearson’s χ2 test. All statistical analyses were performed using JMP version 13.0.0 software (SAS Institute Inc., Cary, NC, United States). In all analysis, a P value < 0.05 was considered statistically significant.
In evaluating the correlation between decreased BW% and individual variables, there was no significant correlation, except for the coexistence of HCC (Figure 1). Stepwise multiple regression analysis showed that a model with the coexistence of HCC and serum LDH concentration was significant for predicting the decrease of BW% (Table 2), but the coefficient of the latter was not significant (P = 0.0669). Coexistence of HCC was the only significant predictive factor, which means that the patients with HCC would be more resistant to TLV treatment than those without HCC.
| Variables | Coefficient | 95%CI | F | P value |
| For all patients | ||||
| HCC (-/+) | -1.2734 | (-2.4886, -0.0581) | 4.3995 | 0.0403 |
| Ln (LDH) | -2.7773 | (-5.7547, 0.2000) | 3.4866 | 0.0669 |
| For the patients with HCC | ||||
| Furosemide (≤ 40 mg vs > 40 mg/d) | -2.0574 | (-0.2775, -3.8373) | 5.5889 | 0.0250 |
| T-factor (T1 and T2 vs T3) | -1.6036 | (-0.0932, 3.3494) | 3.7494 | 0.0629 |
| For the patients without HCC | ||||
| Furosemide (≤ 20 mg vs > 20 mg/d) | 2.0854 | (3.8667, 0.3040) | 5.8379 | 0.0237 |
| CPT score | 1.4320 | (0.3801, 2.4839) | 7.8941 | 0.0097 |
| Ln (LDH) | -6.2691 | (-11.0598, -1.4786) | 7.2950 | 0.0125 |
The coefficient of determination of this model was small (R2 = 0.1334), and the result indicated the possibility that the ascites of the patients with HCC had a different development mechanism from that of the patients without HCC. Therefore, a stratified analysis was subsequently performed dividing the patients into those with and without HCC.
Patients with HCC were younger by about 10 years than those without HCC, but the other variables did not differ significantly, except that serum albumin concentration was higher in the former (Table 1).
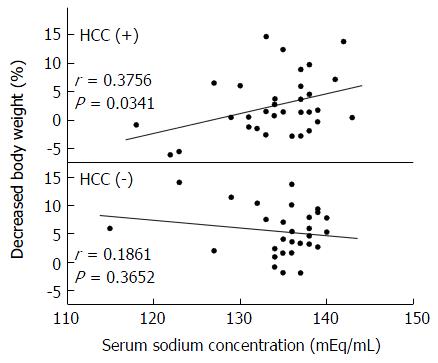
The correlations between decreased BW% and every variable in Table 1 were examined by linear regression analysis or by post hoc analysis for the patients with and without HCC. Only the correlation between sodium concentration and decreased BW% in the patients with HCC was significant (Figure 2), and patients with lower sodium concentration were resistant to TLV.
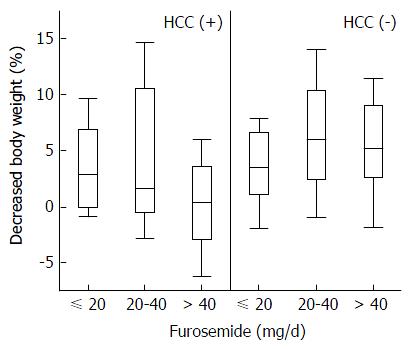
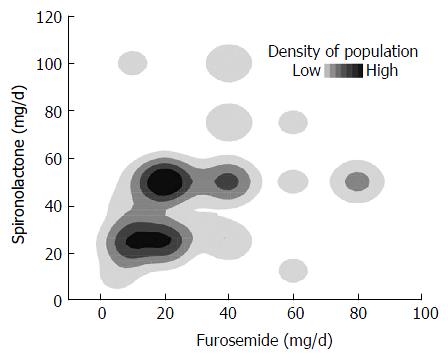
Stepwise multiple regression analysis selected the dose of furosemide and T factor as predictive factors in patients with HCC, but the coefficient of the T factor was not significant (P = 0.0629). In the patients without HCC, the dose of furosemide, CPT score, and serum LDH concentration were independent significant predictive factors (Table 2). Although the dose of furosemide was selected as a significant predictive factor in both groups, the effect was opposite. The high dose of furosemide decreased TLV efficacy in patients with HCC, whereas it improved efficacy in those without HCC (Figure 3). In contrast, spironolactone, the other natriuretic diuretic, was not a predictive factor of TLV efficacy. In comparing the doses of both natriuretic diuretics, a large amount of total diuretics was mainly regulated by increasing the dose of furosemide (Figure 4).
The diuretic efficacy of TLV in patients with cirrhosis has been confirmed in earlier studies[4-9]. The reported effective ratio, however, was 36%-63%, and the prediction of the effectiveness before treatment is still difficult. The diuretic effect of TLV appears as an antagonist of ADH, and the heterogeneous efficacy is thought to be based on various degrees of contribution of ADH to development of ascites. In this study, we showed that the response to TLV differed between patients with and without HCC, which indicates that ascites develops differently in patients with and without HCC.
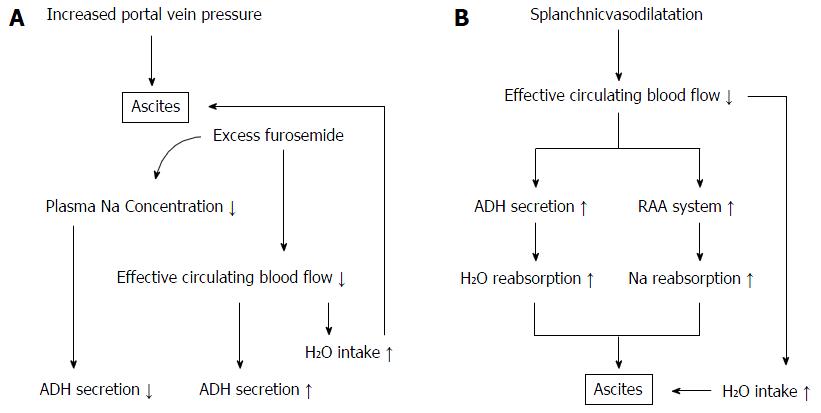
Although the pathogenic factors and signaling pathways that cause cirrhotic ascites are complex, it is commonly accepted that two main events trigger the development of ascites: splanchnic vascular dilatation caused by vasodilators relating to the progression of liver failure, and portal hypertension[17,18]. The former leads to a decrease of systemic circulating blood flow volume and subsequent reabsorption of sodium by the RAA system and water by the ADH system (Figure 5B). If patients with HCC have a unique mechanism for development of ascites, it might be a dominant role of portal hypertension because it has been established that HCC progresses with microvascular invasion, even in its early stage. Pawlik et al[19] analyzed patients with HCC who underwent liver transplantation, and revealed that HCC < 3 cm in diameter was already accompanied by 25% minor vascular invasion, which reached 63% in HCC > 6.5 cm. Recently, Chen et al[20] showed that macro- and microvascular invasion were independent factors relating to the development of ascites in patients with HCC caused by hepatitis B virus. These findings indicate that the development of ascites following HCC progression is mainly caused by portal hypertension subsequent to vascular invasion.
In our study, stepwise multivariate regression analysis of patients with HCC selected a high T factor and a large dose of furosemide as independent factors that could attenuate the diuretic effect of TLV. Patients with HCC had a significantly higher level of serum albumin and were younger by about 10 years than those without HCC, which indicated that ascites in the former appeared at an earlier stage of liver cirrhosis. In many of the patients with HCC, therefore, vascular invasion and increased portal hypertension might have triggered the development of ascites. Administration of a sufficient dose of furosemide in such conditions should have decreased plasma osmotic pressure and systemic circulating blood flow volume. As a decline in plasma osmotic pressure suppresses secretion of ADH, it is possible that the efficacy of TLV in patients with such a background would have been limited (Figure 5A). The positive significant correlation between sodium concentration before treatment with TLV and decreased BW% seems to support this hypothesis. Pose et al[21] recently reported that TLV had a limited effect in patients with cirrhosis and severe hyponatremia. Their result has a similar explanation. Nakagawa et al[22] reported the analysis of 40 cirrhotic patients treated with TLV including 12 with HCC. They showed that the progression of portal hypertension attenuated the effect of TLV, but the mechanism was unclear. A multivariate analysis including the dose of concomitant natriuretic diuretics and serum sodium concentration might have been helpful to clarify the process.
In patients without HCC, a high CPT score and a large dose of furosemide amplified the efficacy of TLV, while high serum LDH concentration attenuated efficacy. High CPT score indicates progression of liver failure involving an increase in vasodilators, therefore, the RAA system and ADH should play a greater role than portal hypertension in the development of ascites. A sufficient dose of furosemide in that condition should offset the effect of the RAA system, leading to an increase in ADH signaling. This speculation might explain the positive correlation of CPT score and dose of furosemide with TLV response. The increase of serum LDH concentration is thought to reflect the excessive decrease of systemic circulating blood flow volume, because transcription of LDH increases in hypoxic circumstances[23]. The significant LDH increase indicates an excessive decrease in renal blood flow, which should reduce the effect of ADH.
The dose of spironolactone was not selected as a significant parameter in the multiple regression analysis in contrast to that of furosemide. The difference could have arisen from the imbalanced use of the drugs. As shown in Figure 4, the total dose of diuretic drugs seems to have been controlled mainly by the dose of furosemide, which was probably based on the desire to avoid hyperkalemia. In discussing the mechanism of the combined use of furosemide and spironolactone with TLV, the important thing is the natriuretic potency of them. Therefore, at least in this study, the dose of furosemide should roughly represent the total natriuretic potency of furosemide and spironolactone.
We believe that the opposite influence of the concomitant use of furosemide on the efficacy of TLV is clinically important. Generalizing our results to patients with hepatic ascites, furosemide should be given in sufficient dose with TLV to patients with ascites that is predominantly induced by liver failure. However, furosemide should be withheld from patients with ascites that is predominantly caused by portal hypertension, which includes many of the patients with advanced HCC-induced ascites. However, the number of patients in our study was too small to make definite recommendations, and an additional, larger study is required.
In conclusion, our results suggest that ascites in patients with HCC responds less well to TLV compared with ascites in patients without HCC. The difference arises from the variant mechanisms of the development of ascites. Excess use of preceding natriuretic diuretics might attenuate the diuretic efficacy of TLV. Further studies including hormonal parameters are required to establish appropriate combined treatment with TLV and natriuretic diuretics.
The efficacy of tolvaptan (TLV) for hepatic ascites has been confirmed but some patients are resistant to the drug. The mechanism of the development of hepatic ascites is complex, and the influence of concomitant natriuretic diuretics or complicated hepatocellular carcinoma (HCC) on the efficacy of TLV remains to be clarified.
The authors investigated the factors that influenced the efficacy of TLV with multiple regression analysis, to clarify the best combination of TLV and other diuretics.
In this study, coexistent HCC was the only significant predictive variable that attenuated the efficacy of TLV. In stratified analysis, high doses of furosemide decreased the efficacy of TLV in patients with HCC, and increased efficacy in those without HCC.
This study suggests that development of ascites may differ between patients with liver failure and those with HCC progression. A sufficient preceding dose of furosemide decreases diuretic effect of TLV.
TLV is a vasopressin V2 receptor antagonist, which has been accepted as a useful diuretic for patients with cirrhosis.
The manuscript presents a good idea in the project of experimentation but the background, the introduction as well as the conclusions are very deficient of adequate and recent bibliography in the field and of a well discussed comment on results.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Ohira M, Pelagalli A, Yuan YS S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Kashani A, Landaverde C, Medici V, Rossaro L. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Hernández-Guerra M, García-Pagán JC, Bosch J. Increased hepatic resistance: a new target in the pharmacologic therapy of portal hypertension. J Clin Gastroenterol. 2005;39:S131-S137. [PubMed] |
| 3. | Ferguson JW, Therapondos G, Newby DE, Hayes PC. Therapeutic role of vasopressin receptor antagonism in patients with liver cirrhosis. Clin Sci (Lond). 2003;105:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Sakaida I, Yamashita S, Kobayashi T, Komatsu M, Sakai T, Komorizono Y, Okada M, Okita K; ASCITES 14-Day Administration Study Group. Efficacy and safety of a 14-day administration of tolvaptan in the treatment of patients with ascites in hepatic oedema. J Int Med Res. 2013;41:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Ohki T, Sato K, Yamada T, Yamagami M, Ito D, Kawanishi K, Kojima K, Seki M, Toda N, Tagawa K. Efficacy of tolvaptan in patients with refractory ascites in a clinical setting. World J Hepatol. 2015;7:1685-1693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Kogiso T, Yamamoto K, Kobayashi M, Ikarashi Y, Kodama K, Taniai M, Torii N, Hashimoto E, Tokushige K. Response to tolvaptan and its effect on prognosis in cirrhotic patients with ascites. Hepatol Res. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Uojima H, Kinbara T, Hidaka H, Sung JH, Ichida M, Tokoro S, Masuda S, Takizawa S, Sasaki A, Koizumi K. Close correlation between urinary sodium excretion and response to tolvaptan in liver cirrhosis patients with ascites. Hepatol Res. 2017;47:E14-E21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Kawaratani H, Fukui H, Moriya K, Noguchi R, Namisaki T, Uejima M, Kitade M, Takeda K, Okura Y, Kaji K. Predictive parameter of tolvaptan effectiveness in cirrhotic ascites. Hepatol Res. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Chishina H, Hagiwara S, Nishida N, Ueshima K, Sakurai T, Ida H, Minami Y, Takita M, Kono M, Minami T. Clinical Factors Predicting the Effect of Tolvaptan for Refractory Ascites in Patients with Decompensated Liver Cirrhosis. Dig Dis. 2016;34:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Pérez-Ayuso RM, Arroyo V, Planas R, Gaya J, Bory F, Rimola A, Rivera F, Rodés J. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology. 1983;84:961-968. [PubMed] |
| 11. | Garcia N, Sanyal AJ. Ascites. Curr Treat Options Gastroenterol. 2001;4:527-537. [PubMed] |
| 12. | Singhal S, Baikati KK, Jabbour II, Anand S. Management of refractory ascites. Am J Ther. 2012;19:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Schouten J, Michielsen PP. Treatment of cirrhotic ascites. Acta Gastroenterol Belg. 2007;70:217-222. [PubMed] |
| 14. | Casado M, Bosch J, García-Pagán JC, Bru C, Bañares R, Bandi JC, Escorsell A, Rodríguez-Láiz JM, Gilabert R, Feu F. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296-1303. [PubMed] |
| 15. | Suzuki E, Chiba T, Ogasawara S, Saito T, Kanogawa N, Motoyama T, Ooka Y, Tawada A, Maruyama H, Ogawa M. Tolvaptan treatment for patients with decompensated cirrhosis and advanced hepatocellular carcinoma. Hepatol Res. 2015;45:E161-E162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Hirai K, Shimomura T, Moriwaki H, Ishii H, Shimoshikiryo T, Tsuji D, Inoue K, Kadoiri T, Itoh K. Risk factors for hypernatremia in patients with short- and long-term tolvaptan treatment. Eur J Clin Pharmacol. 2016;72:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Fukui H. Do vasopressin V2 receptor antagonists benefit cirrhotics with refractory ascites? World J Gastroenterol. 2015;21:11584-11596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Pedersen JS, Bendtsen F, Møller S. Management of cirrhotic ascites. Ther Adv Chronic Dis. 2015;6:124-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 20. | Chen C, Chen DP, Gu YY, Hu LH, Wang D, Lin JH, Li ZS, Xu J, Wang G. Vascular invasion in hepatitis B virus-related hepatocellular carcinoma with underlying cirrhosis: possible associations with ascites and hepatitis B viral factors? Tumour Biol. 2015;36:6255-6263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Pose E, Solà E, Piano S, Gola E, Graupera I, Guevara M, Cárdenas A, Angeli P, Ginès P. Limited Efficacy of Tolvaptan in Patients with Cirrhosis and Severe Hyponatremia: Real-Life Experience. Am J Med. 2017;130:372-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Nakagawa A, Atsukawa M, Tsubota A, Kondo C, Okubo T, Arai T, Itokawa N, Narahara Y, Iwakiri K. Usefulness of portal vein pressure for predicting the effects of tolvaptan in cirrhotic patients. World J Gastroenterol. 2016;22:5104-5113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem. 1995;270:21021-21027. [PubMed] |