Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5333
Peer-review started: January 19, 2017
First decision: February 23, 2017
Revised: March 27, 2017
Accepted: May 9, 2017
Article in press: May 9, 2017
Published online: August 7, 2017
Processing time: 201 Days and 11.9 Hours
To investigate the capability of salvianolic acid B (Sal B) to protect hepatocytes from hydrogen peroxide (H2O2)/carbon tetrachloride (CCl4)-induced lysosomal membrane permeabilization.
Cell Counting Kit-8 assay was used to measure cell viability. Apoptosis and death were assayed through flow cytometry. BrdU incorporation was used to detect cell proliferation. Serum alanine aminotransferase activity and liver malondialdehyde (MDA) content were measured. Liver histopathological changes were evaluated using hematoxylin-eosin staining. Lysosomal membrane permeability was detected with LysoTracker Green-labeled probes and acridine orange staining. The levels of protein carbonyl content (PCC), cathepsins (Cat)B/D, and lysosome-associated membrane protein 1 (LAMP1) were evaluated through western blotting. Cytosol CatB activity analysis was performed with chemiluminescence detection. The mRNA level of LAMP1 was evaluated through quantitative real-time polymerase chain reaction.
Results indicated that H2O2 induced cell injury/death. Sal B attenuated H2O2-induced cell apoptosis and death, restored the inhibition of proliferation, decreased the amount of PCC, and stabilized the lysosome membrane by increasing the LAMP1 protein level and antagonizing CatB/D leakage into the cytosol. CCl4 also triggered hepatocyte death. Furthermore, Sal B effectively rescued hepatocytes by increasing LAMP1 expression and by reducing lysosomal enzyme translocation to the cytosol.
Sal B protected mouse embryonic hepatocytes from H2O2/CCl4-induced injury/death by stabilizing the lysosomal membrane.
Core tip: Lysosomal membrane permeabilization leads to the release of luminal contents, such as proteases and protons, into the cytosol, resulting in cell death. Salvianolic acid B (Sal B), a water-soluble compound extracted from Salvia miltiorrhiza, exerts a cell protective effect by anti-oxidation. In the present study, we elucidate that Sal B protects hepatocytes from H2O2/CCl4 -induced injury/death by stabilizing the lysosomal membrane.
- Citation: Yan XF, Zhao P, Ma DY, Jiang YL, Luo JJ, Liu L, Wang XL. Salvianolic acid B protects hepatocytes from H2O2 injury by stabilizing the lysosomal membrane. World J Gastroenterol 2017; 23(29): 5333-5344
- URL: https://www.wjgnet.com/1007-9327/full/v23/i29/5333.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i29.5333
Lysosomes are cytoplasmic membrane-enclosed organelles that contain various hydrolytic enzymes; they complete the degradation process of cellular components and macromolecules[1]. The function of lysosomes is critically dependent on soluble lysosomal hydrolases, including cathepsin (Cat) B and CatD[2], as well as membrane integrity due to highly glycosylated lysosomal membrane proteins, including lysosome-associated membrane proteins (LAMPs)[3].
Lysosomes are potentially harmful to cells when damage occurs on the lysosomal membrane under pathological conditions. This occurrence is called lysosomal membrane permeabilization (LMP), which allows the release of luminal contents, such as proteases, into the cytosol and results in lysosomal alkalization and cell death. The oxidative stress caused by the imbalance between reactive oxygen species (ROS) production and clearance is associated with numerous pathological consequences, including necrosis, autophagy, and apoptosis[4]. Hydrogen peroxide (H2O2) is a key mediator underlying cellular oxidative stress and is involved in various pathological processes[5,6]. In carbon tetrachloride (CCl4)-induced liver injury, oxidative stress is one of the main mechanisms[7].
Hepatocytes make up a systemic hub, integrating the entire body’s metabolic demand and detoxification processes, all of which are redox-regulated[8]. An increase in oxidative stress sensitizes hepatocytes to subsequent necrosis and/or apoptosis and ultimately results in a massive loss of mature hepatocytes with subsequent inflammation, fibrosis, cirrhosis, and even hepatocellular carcinoma[9]. However, how hepatocyte death is caused by oxidative stress-induced LMP remains unclear.
Salvianolic acid B (Sal B), a water-soluble compound extracted from the herb of Salvia miltiorrhiza, has been widely used in many Asian countries for the treatment of cardiovascular disorders and cerebrovascular diseases for thousands of years[10]. Sal B exerts significant free radical scavenging effects[11]. Several recent reports have indicated that Sal B produces hepatoprotective effects in chronic alcoholic liver disease[12], non-alcoholic fatty liver disease[13], and non-alcoholic steatohepatitis[14]. Furthermore, Sal B can effectively inhibit hepatocyte apoptosis by regulating death receptor and mitochondrial pathways[15]. However, whether the anti-oxidation effects of Sal B are related to LMP in hepatocytes still requires elucidation.
In the present study, H2O2/CCl4-induced injury/death in the BNL CL.2 hepatocyte cell line was used to determine if LMP leads to the leakage of lysosomal contents into the cytosol and triggers cell death in hepatocytes and if Sal B protects cells from death.
The BNL CL.2 mouse embryonic hepatocyte cell line was purchased from the Cell Bank of Shanghai Institutes for Biological Sciences and routinely grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/mL of penicillin, and 100 μg/mL of streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C. The culture media were changed once every 2 d.
Cell viability was assessed with the Cell Counting Kit-8 (CCK8) assay (Dojindo, Kumamoto, Japan). Four thousand cells were seeded onto 96-well plates and incubated for 24 h at 37 °C in 5% CO2. After incubation with different concentrations of Sal B, 10 μL of the CCK8 reagent was added to each well and incubated for 2 h at 37 °C. The absorbance at 450 nm was measured with a microplate reader (Bio-Tek, Hercules, CA, United States). The mean absorbance values from four wells after each treatment were used to represent the cell viability index. The experiments were repeated at least thrice. Cell viability (%) was obtained as follows: (treated group absorbance - blank group absorbance)/(control group absorbance - blank group absorbance) × 100%.
After growing 4000 cells on a 96-well plate treated with 10 μmol/L of Sal B for 24 h and with 500 μmol/L of H2O2 for another 2 h, the medium was changed to a final concentration of 10 μmol/L of 5-bromo-2-deoxyuridine (BrdU; Sigma, St. Louis, MO, United States) for 24 h at 37 °C. After fixing with cold 4% paraformaldehyde for 15 min at room temperature and washing with phosphate-buffered saline (PBS), the cells were incubated with 0.2% TritonX-100 for 20 min to permeabilize the membranes and blocked with 10% horse serum in PBS. A primary antibody (CST, Boston, MA, United States) was added at 1:100 dilution to the blocking solution and incubated at 4 °C overnight. After incubating, a secondary antibody (AlexaTM-594 donkey anti-mouse; Jackson ImmunoResearch, West Grove, PA, United States) was added at 1:100 dilution to the blocking solution for 1 h at room temperature. The cells were labelled with 5 μg/mL of Hoechst 33342 (CST) for 1 h at 37 °C. BrdU-positive proliferating cells were expressed as a percentage of the control level.
Cells treated with 10 μmol/L of Sal B for 12 h or 24 h followed by 2 h with 500 μmol/L of H2O2 were harvested through trypsinization. After washing with cold PBS three times, the cells were resuspended in 100 μL of binding buffer at a density of 1.2 × 106 cells per mL. Then, 5 μL of Annexin V-FITC (BD Pharmingen, Santiago, CA, United States) and 5 μL of PI (BD Pharmingen) were added to each sample and incubated in the dark for 15 min. Fluorescence was detected immediately after staining by using a flow cytometer (Ex = 488 nm; Em = 530 nm). The results were presented as the percentage of cells that were viable (Ann-V-/PI-), early apoptotic (Ann-V+/PI-), late apoptotic (Ann-V+/PI+), or nonviable (Ann-V-/PI+).
At the end of the designated treatments, 20 μg of cellular protein was separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, United States) blocked with 5% non-fat milk for 2 h. The membranes were probed with the designated primary and secondary antibodies, developed using enhanced chemiluminescence (Thermo Scientific, Waltham, MA, United States), and visualized using an FCM gel imaging device (Protein Simple, San Francisco, CA, United States).
The protein carbonyl content (PCC) was assayed with an Oxyblot kit (Millipore). Briefly, 5 μL of cell lysates, including 20 μg of protein mixed with 5 μL of 12% SDS, were supplemented with 10 μL of 2,4-dinitrophenylhydrazine in 2 N HCl for 15 min at room temperature to derive carbonyl groups from the protein side chains. Then, the lysates were separated via 12% SDS-PAGE. Western blotting was performed using the 2,4-dinitrophenylhydrazine antibody (1:50). Given the presence of multiple bands, the average value of all the bands within each lane was used to provide an overall measure of PCC.
A total of 10000 cells were seeded on a 60 mm confocal plate. After the designated treatments, the cells were incubated with 75 nmol/L of LysoTracker Green (Invitrogen, Waltham, MA, United States) or 5 mg/mL of acridine orange (AO) (Sigma) for 30 min at 37 °C. Fluorescence was examined with a confocal microscope, with the excitation wavelength set at 488 nm. Two separate emission bands (505-570 nm and 615-754 nm) for AO staining and one for LysoTracker staining (504-511 nm) were obtained, used simultaneously, and photographed.
Cytosolic/particulate separation was based on the protocol described in the Cytosol/Particulate Rapid Separation Kit (BioVision, Milpitas, CA, United States). Briefly, cells were harvested in 15 mL tubes and centrifuged at 600 × g for 5 min. The pellets were resuspended in 40 μL of cell suspension buffer and mixed with 40 μL of cytosol releasing buffer, including 1 mmol/L of PMSF. The cell suspension was carefully layered on 500 μL of an oil layer with 40 μL of a particulate layer at the bottom on ice for 30 s. After centrifugation at 1400 × g for 1 min, the cytosol was separated from the membrane particles by the oil layer and transferred to new tubes. The protein concentration was used with a BCA total protein assay kit (Biomiga, San Diego, CA, United States).
Total RNA was isolated from cells (1 × 106) by using the TRIzol reagent (Invitrogen). Equal amounts of RNA were reverse transcribed into cDNA. The cDNA was amplified through RT-PCR, and the housekeeping gene 18S rRNA was used as an internal standard to quantify the levels of the target mRNA. The cycling conditions were as follows: hold: 95 °C for 10 s; cycling: 95 °C for 5 s, 60 °C for 30 s, 40 cycles; and melt for 65-95 °C. The nucleotide sequences for the primers used were as follows: 18S rRNA: Forward: 5’-GTAACCCGTTGAACCCCATT-3’ and Reverse: 5’-CCATCCAATCGGTAGTAGCG-3’; LAMP1: Forward: 5’-GATACAGTGGGGTTTGTGGG-3’ and Reverse: 5’-CTGTCGAGTGGCAACTTCAG-3’.
A total of 20 μL of cytoplasmic protein was added to 20 μL of CatB reaction buffer (100 mmol/L of sodium acetate, 200 mmol/L of NaCl, 4 mmol/L of EDTA, 10 mmol/L of DTT, and 10 mmol/L of Z-RR-AMC) at 37 °C for 30 min. The fluorescence released was measured at 37 °C for 10 min using a cytofluorimeter plate reader with excitation and emission wavelengths of 355 and 450 nm, respectively. The fluorescence increment value within 10 min was regarded as the relative value of enzyme activity in 20 μL of cytosol protein. Protein concentration was assayed by BCA, and CatB activity in each sample was corrected according to the protein concentration.
All procedures involving animals were reviewed and approved by the Local Ethics Committee for Animal Research Studies at Shanghai University of Traditional Chinese Medicine (IACUC protocol number: 20140911). The mice were maintained in a specific pathogen-free facility under controlled laboratory conditions (23 °C, 12 h light/12 h dark cycle, 50% humidity, and ad libitum access to food and water) for 2 wk prior to experimentation, and were treated humanely. Intragastric gavage was performed on conscious animals by using straight gavage needles that were appropriate for the animal size (20 g of body weight: 22 gauge, 1 inch length, 1.25 mm ball diameter). In total, 24 male BALB/c mice were initially and randomly divided into four groups: control group (n = 6), CCl4-treated group (n = 6), Sal B/CCl4-treated group (n = 6), and N-acetylcysteine (NAC)/CCl4-treated group (n = 6).
Before CCl4 injection, the mice in the control and CCl4 groups were injected intragastrically with 0.9% NaCl. The mice in the Sal B/ CCl4-treated and NAC/CCl4-treated groups were intragastrically administered with Sal B and NAC at a dosage of 10 mg/kg body weight and 100 mg/kg body weight, respectively, for 3 d. The mice in the CCl4-treated, Sal B/CCl4-treated, and NAC/CCl4-treated groups were given intraperitoneal injections of 5% CCl4 (10 μL/g weight), and the mice in the control group were intraperitoneally injected with olive oil (10 μL/weight). All mice were sacrificed on day 7 of the experiment. The livers were excised, and samples were obtained for the following investigation.
The fresh liver tissue samples were fixed in 10% formalin and embedded in paraffin. Then, the samples were cut into 4 μm thick sections and stained with hematoxylin and eosin (H and E) staining solution. The stained sections were subject to visualization of histopathological changes under a light microscope.
Serum alanine aminotransferase (ALT) activity was assayed according to the recommendations of the Institute of Biological Products Nanjing Jiancheng (Nanjing, China). The malondialdehyde (MDA) in the tissues was determined according to the manufacturer’s instructions (Cayman, Ann Arbor, MI, United States).
All data are presented as mean ± SD. Statistical analysis was performed using Student’s t-test and one-way analysis of variance. Statistical significance was set to P < 0.05. At least three independent experiments were performed for each condition.
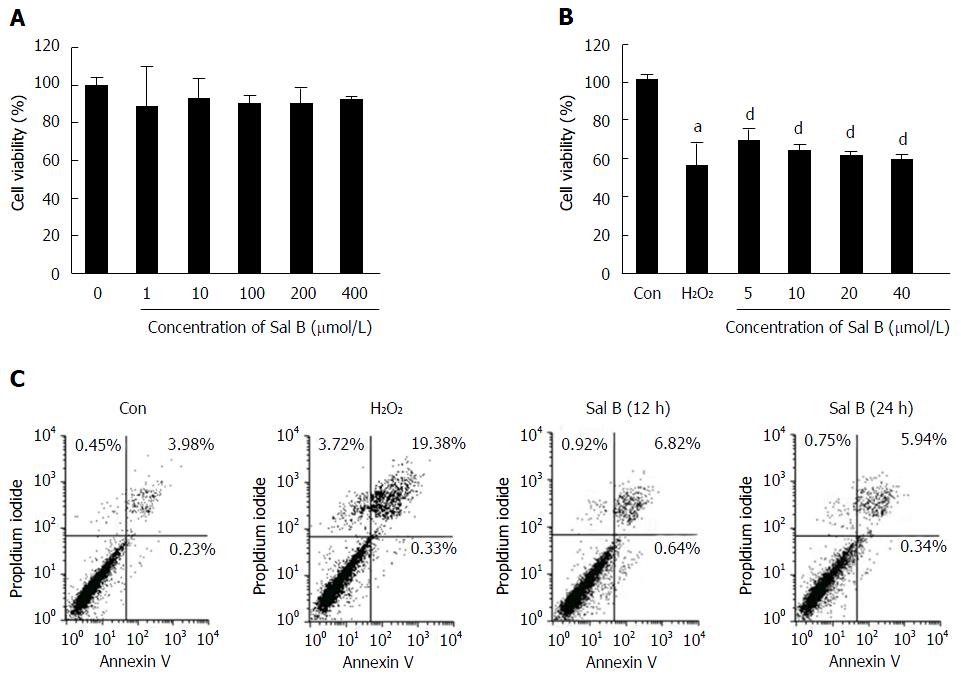
Cells were exposed to Sal B at concentrations of 0, 1, 10, 100, 200 and 400 μmol/L for 24 h to assess the safety of Sal B. The results revealed that Sal B concentrations from 1 μmol/L to 400 μmol/L were not cytotoxic to cells (Figure 1A). The inhibition rate of 500 μmol/L of H2O2 in cell viability was approximately 50%. However, Sal B effectively revised H2O2-inhibited cell viability at different concentrations, ranging from 5 μmol/L to 40 μmol/L (Figure 1B).
As shown in Figure 1C, in the untreated control, only 3.98% of the cells were late apoptotic cells and 0.45% were dead cells. By contrast, in the H2O2-treated group, 19.38% of the cells were late apoptotic cells and 3.72% were dead cells. This result indicates an approximately 5-fold increase in the number of late apoptotic or dead cells in the H2O2-treated group compared with the untreated control group. After 12 h of treatment with 10 μmol/L of Sal B and 500 μmol/L of H2O2, 6.82% of the cells were late apoptotic cells and 0.92% were dead cells. At the 24 h time point, 5.94% of the cells were late apoptotic cells and 0.75% were dead cells in the Sal B treatment group. These results demonstrate that Sal B protected hepatocytes from H2O2-induced apoptosis and death.
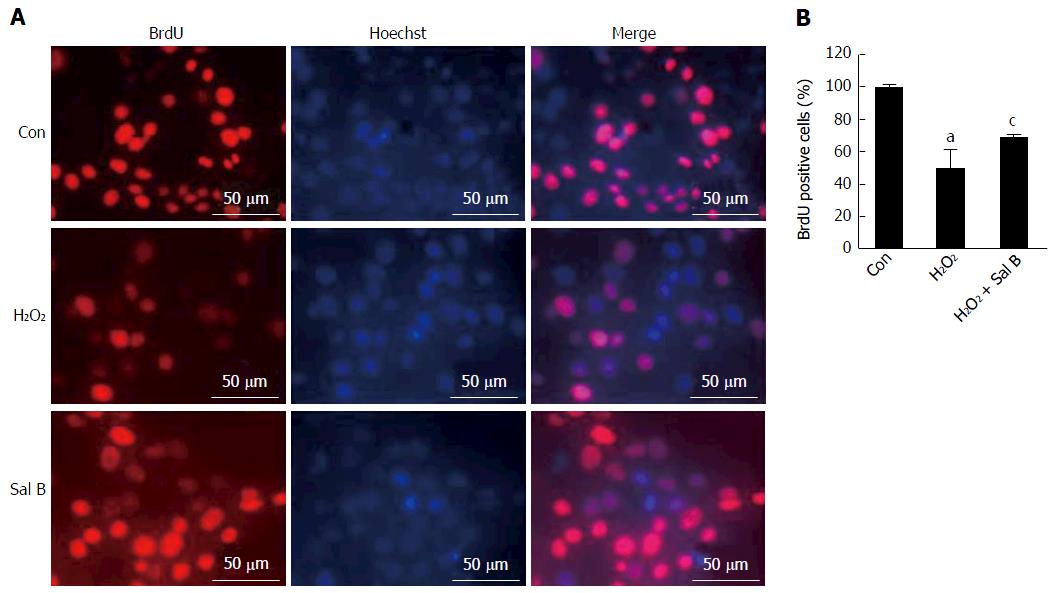
Instead of thymidine, BrdU was incorporated into the nuclear DNA during the S-phase of the cell cycle to evaluate cell proliferation[16]. As shown in Figure 2, after exposure to H2O2, the number of BrdU-positive cells significantly decreased to 50.1% of the control. However, pretreatment with 10 μmol/L of Sal B significantly increased the number of BrdU-positive cells to 68.6% of the control.
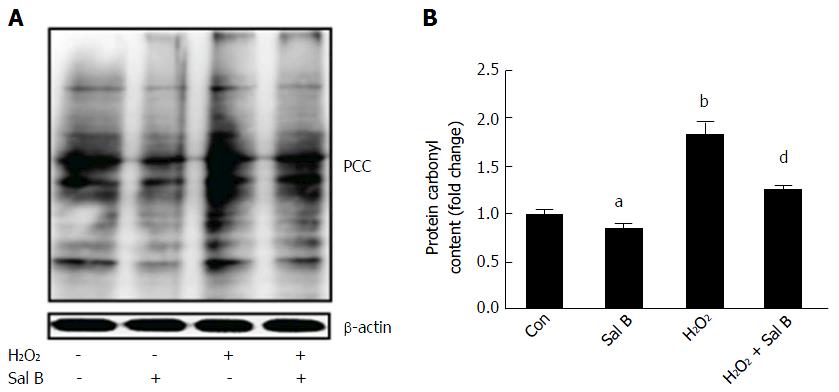
Proteins are the major target of ROS because amino acids are susceptible to oxidation. Carbonylation is the most common oxidative alteration of proteins. The results in Figure 3 demonstrate that the amount of PCC in cells increased by nearly 2-fold after H2O2 treatment. However, with or without H2O2, Sal B reduced the content of PCC in the cells. Evidently, Sal B diminished the oxidative stress of cells.
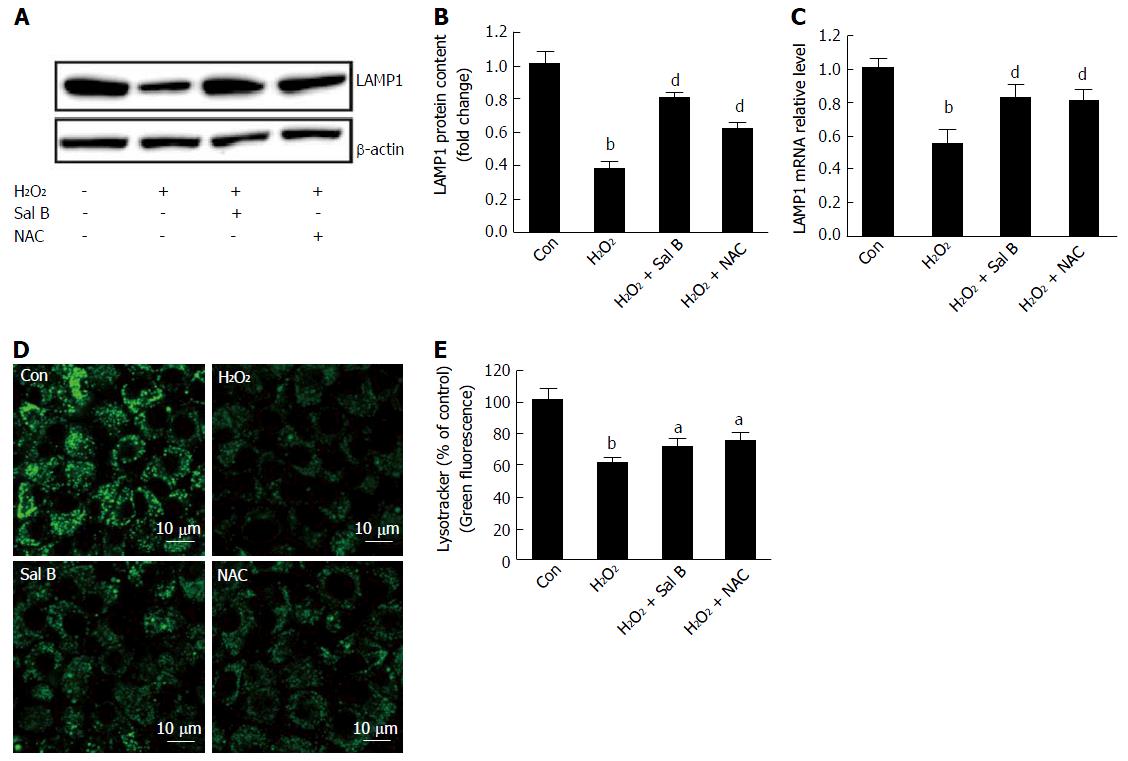
LAMP1 is highly expressed in lysosomes, and its primary localization is at the endosome-lysosomal membrane[17]. The results of this study revealed that LAMP1 mRNA and protein levels significantly decreased with H2O2 treatment. By contrast, for cells pretreated with Sal B or NAC, either the LAMP1 mRNA level or the protein level increased (Figure 4A-C). LysoTracker green, a lysosomal marker that is selectively retained in the partially acidic lysosome and reflects lysosomal membrane permeability[18], was used to evaluate the integrity of the lysosome membrane. The intensity of green fluorescence decreased with H2O2 treatment. However, the fluorescence intensity increased with Sal B pretreatment. NAC was used as a positive antioxidant and showed a similar result as Sal B (Figure 4D and E).
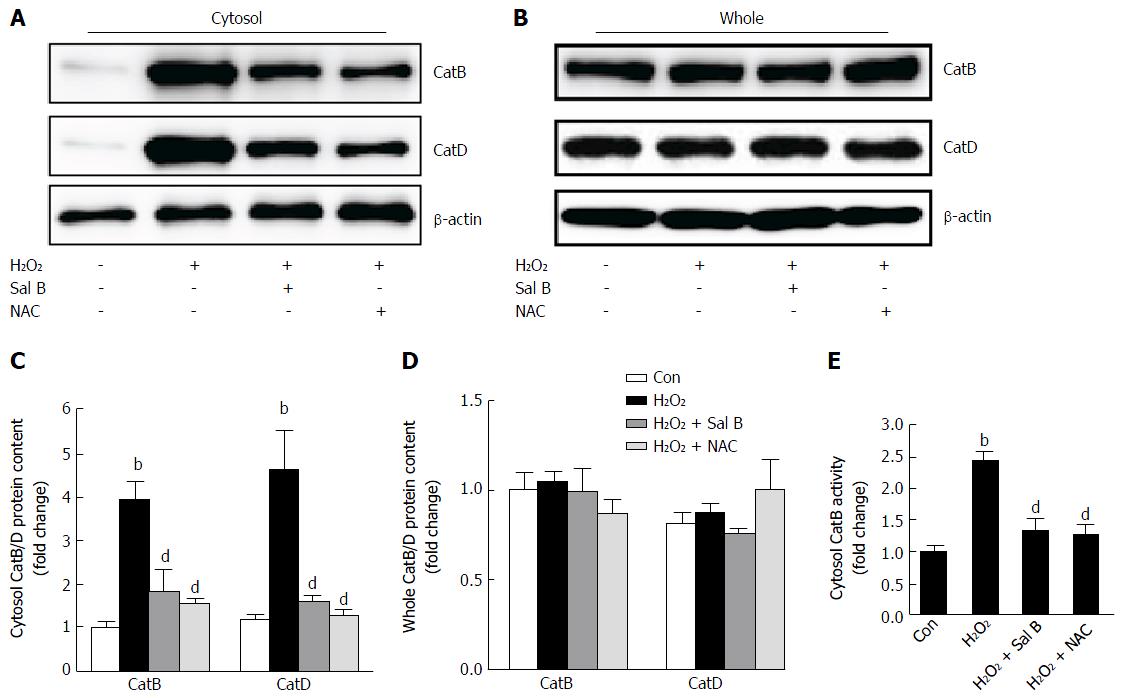
To further investigate whether catalytic enzymes in the lysosome leaked into the cytosol and caused the inhibition of cell viability, the content and activity of CatB and CatD in the cytosol and entire cells were assessed. The contents of CatB and CatD in the cytosol increased significantly after exposure to H2O2 compared with that in the control (Figure 5A and C). By contrast, the content detected in the entire cells remained unchanged (Figure 5B and D). Sal B and NAC suppressed cytoplasmic CatB and CatD contents (Figure 5A-D). The CatB activity in the cytosol obviously increased with H2O2 treatment. However, either Sal B or NAC suppressed cytoplasmic CatB activity (Figure 5E).
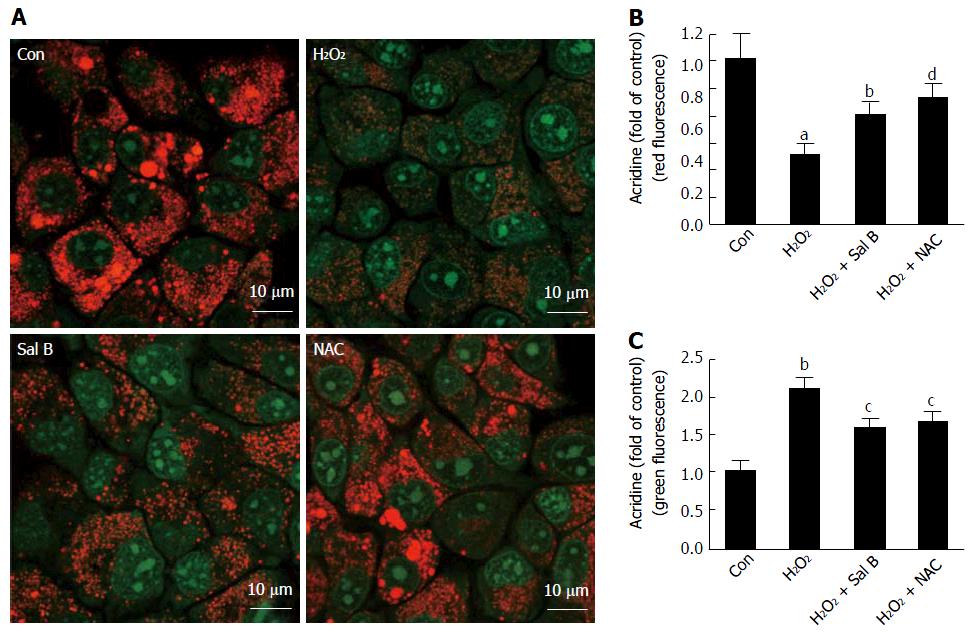
AO is a cell-permeable fluorescent dye with a concentration-dependent fluorescence emission that ranges from red (when the concentration is high in the lysozyme) to green (when the concentration is low in the cytosol). AO is positively charged at a low pH, which consequently hinders the ability of AO molecules to cross the vesicular membrane and escape into the surrounding cytoplasm. In the case of LMP, AO is released from the lysosomes into the cytosol, where it emits an enhanced green fluorescence that can be monitored by fluorescence microscopy[18]. After the delivery of H2O2 to the cells, a significant reduction in red fluorescence was observed, suggesting that H2O2 induced LMP at the cellular level (Figure 6A and C). By contrast, Sal B and NAC were associated with an increase in red fluorescence and concomitant suppression of green fluorescence compared with the situation in the H2O2-treated cells (Figure 6A-C).
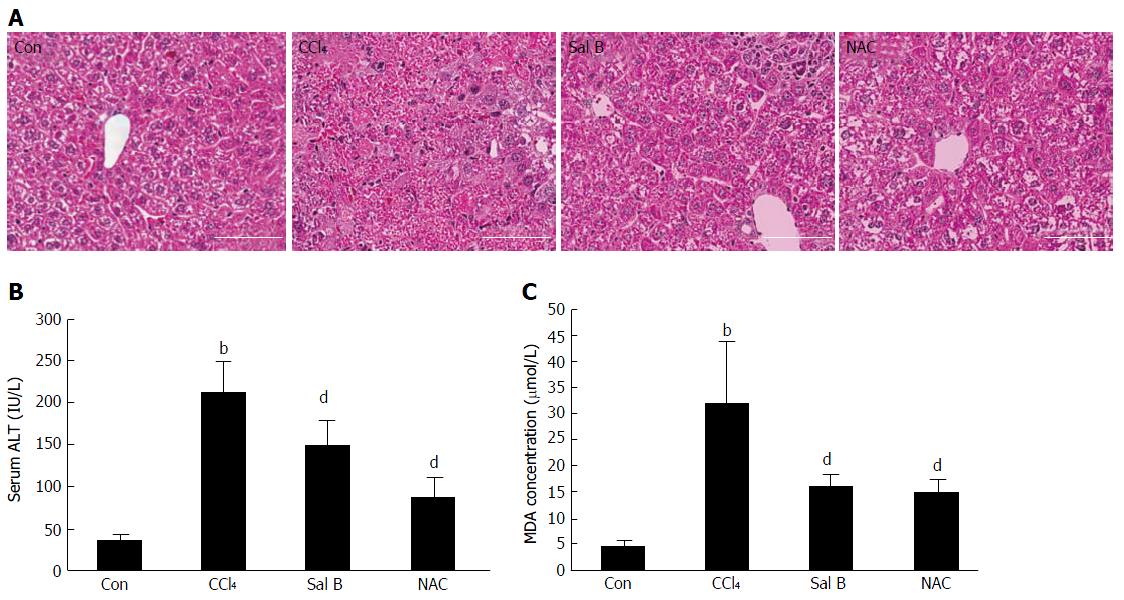
Hematoxylin-eosin staining revealed a normal hepatic architecture within the clear hepatic lobule and the absence of dead hepatocytes in the control group of mice. By contrast, a large area of dead hepatocytes was observed in the central vein area of the CCl4-treated mice. Compared with the CCl4-treated group, the CCl4 group treated with Sal B or NAC exhibited improvements in CCl4-associated pathological changes (Figure 7A). ALT has been used as an index of liver injury in clinics and in vivo studies[19]. The mice in the CCl4-treated group exhibited signs of liver injury. The ALT activity in these mice was significantly higher than that in the control group (P < 0.01), although Sal B or NAC interventions reduced the activity of ALT (P < 0.01) (Figure 7B).
MDA level has been widely used as a marker of lipid peroxidation in cells and body fluids in clinical and experimental studies[20]. The MDA concentration in the CCl4-treated group was significantly higher than that in the control group (P < 0.01). Pretreatment with Sal B or NAC reduced the MDA levels (P < 0.01) (Figure 7C).
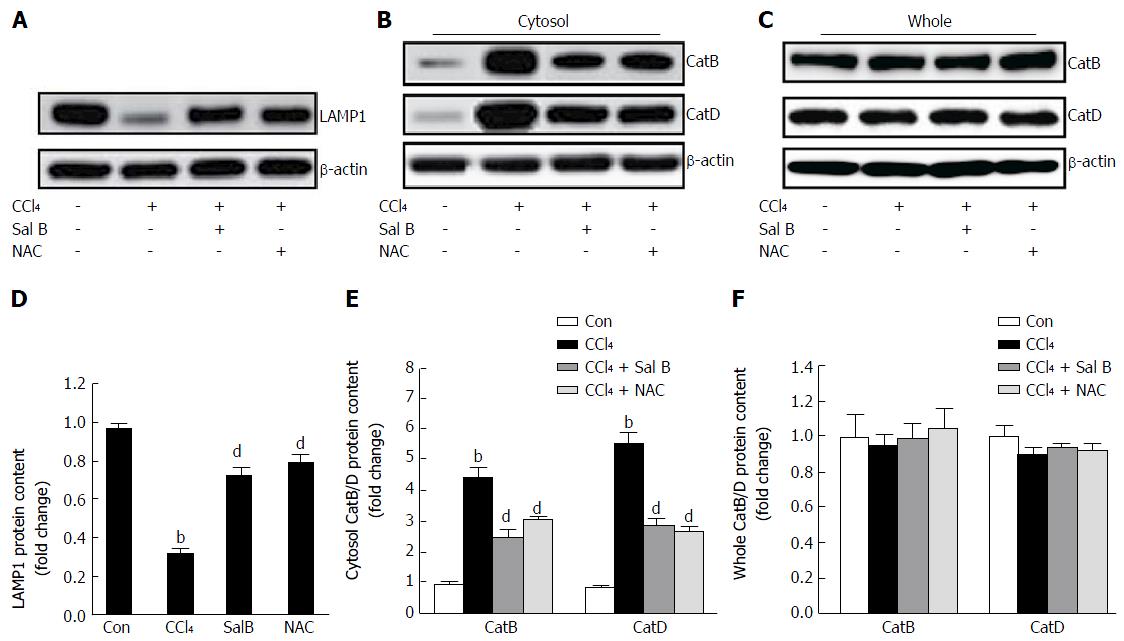
LAMP1 is used as a lysosomal marker and contributes to the protection of the lysosomal membrane and its proteins from hostile constituents, such as hydrogen ions and proteases[21]. LAMP1 protein levels decreased significantly after CCl4 treatment (P < 0.01), and Sal B or NAC pretreatment increased the protein levels (P < 0.01) (Figure 8A and D).
To further confirm if CCl4 induces liver injury triggered by lysosomal disintegration, the leakage of lysosomal enzymes into the cytosolic fractions was investigated. Surprisingly, the contents of CatB and CatD in the cytosol increased significantly after CCl4 treatment (P < 0.01), and Sal B or NAC pretreatment decreased the protein levels (P < 0.01) (Figure 8B and E). By contrast, the content detected in the entire cells remained unchanged (Figure 8C and F).
Hepatocytes, which are in charge of the detoxification of exogenous xenobiotics, drugs, viral infections and chronic alcoholism, have the largest amount of cells in the liver. Regardless of etiology (e.g., toxic chemicals, drugs and virus), hepatocyte injury/death is involved in all pathological stages of hepatitis, fibrosis, cirrhosis, and even liver cancer[22]. Therefore, hepatocytes act as a common therapeutic target in various liver diseases. Sal B is a major water-soluble polyphenolic antioxidant in Radix Salviae Miltiorrhizae and has been extensively used in clinics in China[23-25]. Sal B possesses efficient radical scavengers and antioxidants[14,26,27]. Previous reports have shown that Sal B exerts therapeutic effects in treating liver fibrosis[28,29]. In this study, the effect of Sal B on H2O2-induced hepatocytes was investigated in vitro.
CCl4 is a potent hepatotoxicant that is commonly used to generate liver injury in animal models[30]. In hepatocytes, CCl4 is metabolized by cytochrome P450 to produce highly reactive free radicals[31]. Therefore, oxidative stress is one of the main mechanisms of CCl4-induced liver injury[7]. The present results suggest that the levels of lipid peroxidation in livers and serum ALT activity in hepatocytes after cell injury/death increased after CCl4 treatment. The results of hepatic histological staining in CCl4-treated mice also showed large areas of dead hepatocytes.
LAMP1 is a highly glycosylated protein located in the lysosomal membrane; it controls the integrity of the lysosomal membrane[21]. In this study, LAMP1 detected in the livers was low, and CatB and CatD levels in the cytoplasm increased but were unchanged in whole tissues after CCl4 treatment. However, after Sal B treatment, the structure of the liver tissues returned to normal. Meanwhile, serum ALT decreased, the levels of lipid peroxidation diminished, and the levels of LAMP1 were enhanced in the livers. Thus, the hepatotoxicant effect of CCl4 is attributed to its induction of oxidative stress followed by lysosomal membrane permeabilization, which mediates hepatocyte injury/death. Sal B protected the livers by interfering with lysosome membrane permeabilization.
Consistent with the in vivo results, H2O2-induced cytotoxicity is a common method used for measuring potential antioxidants. In the in vitro study, treatments with 500 μmol/L of H2O2 for 2 h induced a decrease in cell viability, and the rate of inhibition was approximately 50%. This concentration was used for the subsequent experiments. After oxidative damage, cell viability was inhibited, and cell proliferation diminished. Sal B decreased the number of apoptotic and necrotic cells and restored cell proliferation.
In cultured cells, a large amount of H2O2 diffuses into the lysosome and results in LMP[32-34], which is attributed to the reduced H2O2-induced expression of LAMP1[35]. Upon LMP, hydrolytic enzymes[36,37] and protons[38,39] in the lysosomes are released into the cytoplasm, resulting in acidification and triggering lysosome-induced cell death[40] Hepatocyte death is accompanied by an increase in LMP in acute liver injury[41]. According to the cell type and dosages of the inducing factors, lysosomal apoptosis or necrosis occurs[42]. Cathepsins, including CatB and CatD, exhibit catalytic activity for a few hours even under near-neutral pH conditions[43]. CatB leakage from the lysosomes is also associated with apoptosis[44,45].
The current study found that the levels of CatB and CatD in the cytoplasm increased considerably after exposure to H2O2 for 2 h. The activity of CatB in cytoplasm increased and reached nearly 2.5-fold. Meanwhile, the results of LysoTracker and AO staining confirmed that LMP occurred during H2O2 treatment. After Sal B treatment, the levels of CatB/D and CatB enzyme activity in cytoplasm decreased, and the fluorescence intensity of LysoTracker and AO in the lysosome was enhanced. Thus, leakage of lysosome contents was improved, lysosome membrane integrity was protected, and cell injury/death was inhibited.
NAC is a sulfhydryl donor antioxidant that contributes to the regeneration of glutathione[46] and commonly used as a positive antioxidant control. (-)-epigallocatechin-3-gallate (EGCG) promotes the production of intracellular ROS upstream of LMP and cell death, and NAC protects against EGCG-mediated LMP by decreasing the level of ROS[47]. Bxpc3 cell death is also involved in LMP and oxidative stress, which is protected against by NAC[21]. In the present study, the content of CatB/D and CatB enzyme activity in the cytosol after NAC treatment showed a significant decrease compared with that in the H2O2 treatment group. The fluorescence intensity of LysoTracker Green and the LAMP1 level in the cells treated with NAC increased. These results show that NAC protected hepatocytes from H2O2-induced apoptosis and death by inhibiting LMP. The effects of NAC were similar to those of the antioxidant Sal B.
In sum, Sal B protected the integrity of the lysosomal membrane by up-regulating the expression of LAMP1, which reduced CatB/D leakage into the cytosol and protected hepatocytes from death.
Highly glycosylated lysosome-associated membrane proteins (LAMPs), especially LAMP1, on the inner surface of the lysosomal membrane serve as a barrier to soluble hydrolases and keep the integrity of membrane. The loss of membrane integrity leads to lysosomal membrane permeabilization (LMP), allowing release of the luminal contents into the cytosol, resulting in cellular injury/death. Oxidative stress causes LMP in hepatocytes, leading to cellular injury/death. Salvianolic acid B (Sal B) performs anti-inflammatory and antioxidant functions and elicits protective effects on hepatocytes.
Lysosomes filled with more than 50 soluble acid hydrolases are the cellular recycling center and play an important role in cells. Upon LMP, the leakage of enzymes from lysosomes into cytosol causes cellular injury/death. Hepatocytes are the most frequent cells in the liver and the target of oxidative stress, and the resulting injury/death is involved in all liver diseases. Herein, hepatocytes served as a target of therapy.
Upon H2O2 attacking, the expression of LAMP1 was significantly decreased and destroyed the integrity of the lysosome membrane in hepatocytes and contributed to cellular injury/death. Sal B elicits protective effects on hepatocytes by scavenging reactive oxygen species and enhancing LAMP1 expression.
Sal B can protect lysosome membrane integrity by diminishing oxidative stress and can elicit a protective effect on hepatocytes. This study provided a theoretical basis for the clinical application of Sal B to treat liver diseases.
LMP is the leakage of lysosome contents due to the destruction of lysosome membrane integrity and causes cell injury/death. Sal B is a major water-soluble polyphenolic antioxidant in Radix Salviae Miltiorrhizae and also elicits protective effects on hepatocytes.
The paper is very interesting. Moreover, whereas the authors target the effect of Sal B on the protection of the integrity of the lysosomal membrane by upregulating the expression of LAMP1, via reduction of cathepsin B/D leakage into the cytosol, and protects hepatocytes from apoptosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cubero FJ, Ling C, Yu TF S- Editor: Qi Y L- Editor: Filipodia E- Editor: Li D
| 1. | Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1249] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 2. | Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 784] [Cited by in RCA: 949] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 3. | Howe CL, Granger BL, Hull M, Green SA, Gabel CA, Helenius A, Mellman I. Derived protein sequence, oligosaccharides, and membrane insertion of the 120-kDa lysosomal membrane glycoprotein (lgp120): identification of a highly conserved family of lysosomal membrane glycoproteins. Proc Natl Acad Sci USA. 1988;85:7577-7581. [PubMed] |
| 4. | Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal. 2012;16:1323-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 411] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 5. | Celi P, Gabai G. Oxidant/Antioxidant Balance in Animal Nutrition and Health: The Role of Protein Oxidation. Front Vet Sci. 2015;2:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 727] [Cited by in RCA: 746] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 7. | Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Mello T, Zanieri F, Ceni E, Galli A. Oxidative Stress in the Healthy and Wounded Hepatocyte: A Cellular Organelles Perspective. Oxid Med Cell Longev. 2016;2016:8327410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Mishra A, Younossi ZM. Epidemiology and Natural History of Non-alcoholic Fatty Liver Disease. J Clin Exp Hepatol. 2012;2:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Wang J, Xiong X, Feng B. Cardiovascular effects of salvianolic Acid B. Evid Based Complement Alternat Med. 2013;2013:247948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Tsai MK, Lin YL, Huang YT. Effects of salvianolic acids on oxidative stress and hepatic fibrosis in rats. Toxicol Appl Pharmacol. 2010;242:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Zhang N, Hu Y, Ding C, Zeng W, Shan W, Fan H, Zhao Y, Shi X, Gao L, Xu T. Salvianolic acid B protects against chronic alcoholic liver injury via SIRT1-mediated inhibition of CRP and ChREBP in rats. Toxicol Lett. 2017;267:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Zeng W, Shan W, Gao L, Gao D, Hu Y, Wang G, Zhang N, Li Z, Tian X, Xu W. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci Rep. 2015;5:16013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Wang YC, Kong WZ, Jin QM, Chen J, Dong L. Effects of salvianolic acid B on liver mitochondria of rats with nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:10104-10112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Yan X, Zhou T, Tao Y, Wang Q, Liu P, Liu C. Salvianolic acid B attenuates hepatocyte apoptosis by regulating mediators in death receptor and mitochondrial pathways. Exp Biol Med (Maywood). 2010;235:623-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Duque A, Rakic P. Different effects of bromodeoxyuridine and [3H]thymidine incorporation into DNA on cell proliferation, position, and fate. J Neurosci. 2011;31:15205-15217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Madan R, Rastogi R, Parashuraman S, Mukhopadhyay A. Salmonella acquires lysosome-associated membrane protein 1 (LAMP1) on phagosomes from Golgi via SipC protein-mediated recruitment of host Syntaxin6. J Biol Chem. 2012;287:5574-5587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Pierzyńska-Mach A, Janowski PA, Dobrucki JW. Evaluation of acridine orange, LysoTracker Red, and quinacrine as fluorescent probes for long-term tracking of acidic vesicles. Cytometry A. 2014;85:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 19. | Li W, Qu XN, Han Y, Zheng SW, Wang J, Wang YP. Ameliorative effects of 5-hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on alcoholic liver oxidative injury in mice. Int J Mol Sci. 2015;16:2446-2457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Zeng T, Guo FF, Zhang CL, Zhao S, Dou DD, Gao XC, Xie KQ. The anti-fatty liver effects of garlic oil on acute ethanol-exposed mice. Chem Biol Interact. 2008;176:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Hornick JR, Vangveravong S, Spitzer D, Abate C, Berardi F, Goedegebuure P, Mach RH, Hawkins WG. Lysosomal membrane permeabilization is an early event in Sigma-2 receptor ligand mediated cell death in pancreatic cancer. J Exp Clin Cancer Res. 2012;31:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Cao L, Quan XB, Zeng WJ, Yang XO, Wang MJ. Mechanism of Hepatocyte Apoptosis. J Cell Death. 2016;9:19-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Lee HJ, Cho JY, Moon JH. Comparison of the inhibitory effect against copper ion-induced oxidation in rat plasma after oral administration of salvianolic acid B and its decocted solutions. J Med Food. 2013;16:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Zhao CQ, Zhou Y, Ping J, Xu LM. Traditional Chinese medicine for treatment of liver diseases: progress, challenges and opportunities. J Integr Med. 2014;12:401-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Wang X, Wang N, Cheung F, Lao L, Li C, Feng Y. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J Integr Med. 2015;13:142-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Shu T, Pang M, Rong L, Liu C, Wang J, Zhou W, Wang X, Liu B. Protective Effects and Mechanisms of Salvianolic Acid B Against H2O2-Induced Injury in Induced Pluripotent Stem Cell-Derived Neural Stem Cells. Neurochem Res. 2015;40:1133-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Hu Y, E Q, Zuo J, Yang L, Liu W. Salvianolic acid B inhibits mitochondrial dysfunction by up-regulating mortalin. Sci Rep. 2017;7:43097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Yu F, Lu Z, Chen B, Wu X, Dong P, Zheng J. Salvianolic acid B-induced microRNA-152 inhibits liver fibrosis by attenuating DNMT1-mediated Patched1 methylation. J Cellular Molecular Med. 2015;19:2617-2632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Yu F, Lu Z, Huang K, Wang X, Xu Z, Chen B, Dong P, Zheng J. MicroRNA-17-5p-activated Wnt/β-catenin pathway contributes to the progression of liver fibrosis. Oncotarget. 2016;7:81-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Teng H, Chen M, Zou A, Jiang H, Han J, Sun L, Feng C, Liu J. Hepatoprotective effects of licochalcone B on carbon tetrachloride-induced liver toxicity in mice. Iran J Basic Med Sci. 2016;19:910-915. [PubMed] |
| 31. | Dong D, Yin L, Qi Y, Xu L, Peng J. Protective Effect of the Total Saponins from Rosa laevigata Michx Fruit against Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Nutrients. 2015;7:4829-4850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Ullio C, Casas J, Brunk UT, Sala G, Fabriàs G, Ghidoni R, Bonelli G, Baccino FM, Autelli R. Sphingosine mediates TNFα-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. J Lipid Res. 2012;53:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Malekova L, Tomaskova J, Novakova M, Stefanik P, Kopacek J, Lakatos B, Pastorekova S, Krizanova O, Breier A, Ondrias K. Inhibitory effect of DIDS, NPPB, and phloretin on intracellular chloride channels. Pflugers Arch. 2007;455:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Lee SJ, Cho KS, Koh JY. Oxidative injury triggers autophagy in astrocytes: the role of endogenous zinc. Glia. 2009;57:1351-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Lee SJ, Park MH, Kim HJ, Koh JY. Metallothionein-3 regulates lysosomal function in cultured astrocytes under both normal and oxidative conditions. Glia. 2010;58:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Owa C, Messina ME, Halaby R. Triptolide induces lysosomal-mediated programmed cell death in MCF-7 breast cancer cells. Int J Womens Health. 2013;5:557-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Marques C, Oliveira CS, Alves S, Chaves SR, Coutinho OP, Côrte-Real M, Preto A. Acetate-induced apoptosis in colorectal carcinoma cells involves lysosomal membrane permeabilization and cathepsin D release. Cell Death Dis. 2013;4:e507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Deng D, Jiang N, Hao SJ, Sun H, Zhang GJ. Loss of membrane cholesterol influences lysosomal permeability to potassium ions and protons. Biochim Biophys Acta. 2009;1788:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Nilsson C, Roberg K, Grafström RC, Ollinger K. Intrinsic differences in cisplatin sensitivity of head and neck cancer cell lines: Correlation to lysosomal pH. Head Neck. 2010;32:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Brandstetter C, Mohr LK, Latz E, Holz FG, Krohne TU. Light induces NLRP3 inflammasome activation in retinal pigment epithelial cells via lipofuscin-mediated photooxidative damage. J Mol Med (Berl). 2015;93:905-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 41. | Chang CP, Yang MC, Lei HY. Concanavalin A/IFN-gamma triggers autophagy-related necrotic hepatocyte death through IRGM1-mediated lysosomal membrane disruption. PLoS One. 2011;6:e28323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Repnik U, Hafner Česen M, Turk B. Lysosomal membrane permeabilization in cell death: concepts and challenges. Mitochondrion. 2014;19 Pt A:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 43. | Appelqvist H, Wäster P, Kågedal K, Öllinger K. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5:214-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 559] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 44. | Oberle C, Huai J, Reinheckel T, Tacke M, Rassner M, Ekert PG, Buellesbach J, Borner C. Lysosomal membrane permeabilization and cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes. Cell Death Differ. 2010;17:1167-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Colletti GA, Miedel MT, Quinn J, Andharia N, Weisz OA, Kiselyov K. Loss of lysosomal ion channel transient receptor potential channel mucolipin-1 (TRPML1) leads to cathepsin B-dependent apoptosis. J Biol Chem. 2012;287:8082-8091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta. 2013;1830:4117-4129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 592] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 47. | Zhang Y, Yang ND, Zhou F, Shen T, Duan T, Zhou J, Shi Y, Zhu XQ, Shen HM. (-)-Epigallocatechin-3-gallate induces non-apoptotic cell death in human cancer cells via ROS-mediated lysosomal membrane permeabilization. PLoS One. 2012;7:e46749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |