INTRODUCTION
Herbal medicines are becoming increasingly popular in modern societies and substantial research has been conducted to establish their pharmacological properties according to scientific principles[1]. Since ancient times, people have used herbal medicines to treat and prevent disease. Due to their natural origins, herbal medicines have mild activities and few side effects; for this reason, many people are becoming increasingly interested in traditional herbal medicines[2-5].
Hwangryunhaedok-tang (HHT; Huang-Lian-Jie-Tang in Chinese or Oren-gedoku-to in Japanese) is a preparation that consists of Rhizoma Coptidis, Radix Scutellariae, Cortex Phellodendri and Fructus Gardenia[6]. HHT has been used to treat various symptoms, including inflammatory diseases[7,8], cardiovascular diseases[9], diabetes mellitus[10], liver diseases[11,12], brain injury[13,14] and obesity[15]. HHT is also used to prevent or ameliorate gastrointestinal (GI) diseases[16-19]. HHT has been shown to have a preventive effect on the development of water immersion restraint (WIR) stress-induced acute gastric lesions in rats[16] and it suppresses the symptoms of inflammatory bowel disease by reducing levels of interleukin-8 (IL-8), leukotriene B4 (LTB4) and prostaglandin E2 (PGE2)[17]. In addition, HHT has been shown to prevent lethal indomethacin (IND)-induced enteropathy by decreasing adenosine deaminase (ADA) and, thus, increasing the adenosine levels in female BALB/c mice[18].
Interstitial cells of Cajal (ICCs) contribute to normal GI function, such as by generating electrical slow waves and mediating neuromuscular signaling[19-21]. Damage to ICCs or reductions in ICC numbers have been described in many GI motility disorders[22-24]; therefore, it is very important to understand the mechanisms of ICC pacemaker activity. However, no scientific evidence of a relationship between HHT and ICCs has been presented until now. In the present study, we investigated the effects of water extracts of HHT and its components on the pacemaker potentials of cultured ICCs from murine small intestine.
MATERIALS AND METHODS
Preparation of HHT extract
HHT was purchased from I-WORLD Pharmaceuticals (Incheon, South Korea). HHT (3.67 g) consists of Coptidis Rhizoma (0.67 g), Scutellariae Radix (1.00 g), Phellodendri Cortex (1.00 g) and Gardeniae Fructus (1.00 g). The materials were authenticated by Prof. Hyungwoo Kim (Division of Pharmacology, Pusan National University, School of Korean Medicine, Yangsan, Korea). HHT extract (HHTE) was prepared by boiling HHT in water for three hours. The standard adult dose of HHTE is 10-15 g (crude material) per day. More information about HHTE can be found at the I-WORLD Pharmaceuticals Homepage (http://i-pharm.koreasme.com). HHTE was dissolved in distilled water at a concentration of 0.5 g (crude drug)/mL and stored in a refrigerator until required.
Reagents
Geniposide, berberine chloride, baicalin and wogonin were purchased from Wako (Japan). High-performance liquid chromatography (HPLC)-grade water and methanol were obtained from JT Baker Inc. (Phillipsburg, NJ, United States). Trifluoroacetic acid was purchased from Sigma-Aldrich (St. Louis, MO, United States).
Preparation of standard solutions
Each standard compound (geniposide, berberine chloride, baicaline and wogonin) was accurately weighed and dissolved in methanol at a concentration of 1000 g/mL and the solution was 10-fold diluted before injection.
Chromatographic conditions
An Agilent 1200 (Agilent Technologies, Santa Clara, CA, United States) equipped with a quaternary pump, autosampler, column oven and diode-array detector was used. Acquired data were processed using Chemstation software (Ver. B. 03. 02). Chromatographic separation was performed on an XDB C18 column (4.6 mm × 150 mm, 5 μm; Agilent, United States) at 35 °C. The mobile phase consisted of water with 0.1% trifluoroacetic acid (A) and methanol (B). The following program was used: 30% (B) for 1 min, 30%-80% (B) over 1 min-13 min and 80% (B) for 1 min, which was followed by a re-equilibration to 30% (B). The flow rate was set at 0.8 mL/min and the injection volume was 10 μL. The detection wavelengths were 240 nm for geniposide, 260 nm for berberine chloride and 275 nm for baicalin and wogonin.
Identification of standard compounds in HHTE
Standard compounds were detected in the chromatogram of HHTE at the following retention times: 5.2 min for geniposide, 9.1 min for berberine chloride, 10.1 min for baicalin and 14.7 min for wogonin (Figure 1).
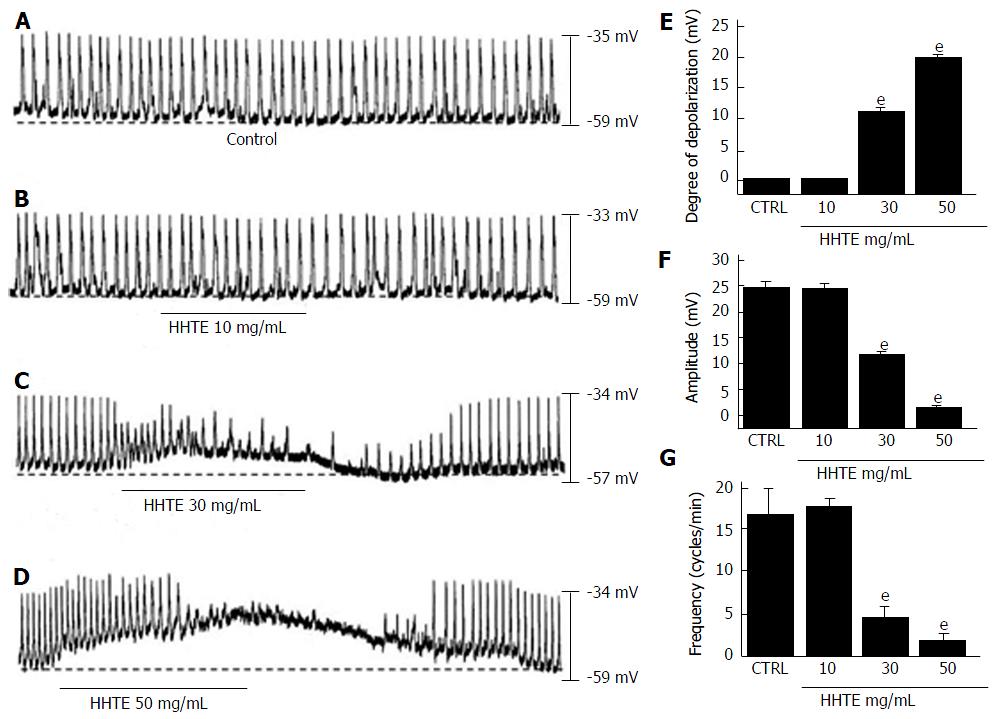
Figure 1 Effects of water extract of Hwangryunhaedok-tang on the pacemaker potentials of cultured interstitial cells of Cajal from murine small intestine.
A-D: HHTE (0-50 mg/mL) induced pacemaker potential depolarizations and decreased amplitudes in a concentration-dependent manner. Responses to HHTE are summarized (E-G). Bars indicate the means ± SEMs. eP < 0.001: significantly different from the control. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang.
Ethics
We conducted the animal care and experiments in accordance with the guidelines issued by the Institutional Animal Care and Use Committee (IACUC) at Pusan National University (Busan, Republic of Korea; Approval no. PNU-2015-1036) and those issued by the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Preparation of ICC and ICC clusters
ICR mice (Samtako Bio Korea Co., Ltd., Osan, South Korea; 3-7 day-old; weighing 1.9 g-2.2 g) of either sex were used and kept under controlled conditions (20 °C ± 3 °C, RH 49% ± 6% and light on 7 a.m.-7 p.m.). The small intestines were removed and opened; then, the luminal contents were cleaned using Krebs-Ringer bicarbonate solution and the obtained tissues were pinned to the bases of Sylgard dishes. Mucosae were removed and small tissue strips of intestine muscle were equilibrated for 25 min in Ca2+-free Hank’s solution with the following solutions (in mmol/L): KCl 5.36, NaCl 125, NaOH 0.34, Na2HCO3 0.44, glucose 10, sucrose 2.9 and HEPES 11 (pH 7.4). Cells were then dispersed in an enzyme solution [1.3 mg/mL collagenase (Worthington Biochemical, Lakewood, NJ, United States), 2.1 mg/mL bovine serum albumin (Sigma-Aldrich, St. Louis, MO, United States), 2.6 mg/mL trypsin inhibitor (Sigma-Aldrich, St. Louis, MO, United States) and 0.50 mg/mL ATP (Sigma-Aldrich, St. Louis, MO, United States)]. Cells were then cultured at 37 °C in a 95% O2-5% CO2 incubator in smooth muscle growth medium (SMGM) (Clonetics, San Diego, CA, United States) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, United States) and 5 ng/mL of murine stem cell factor (SCF) (Sigma-Aldrich, St. Louis, MO, United States). All experiments were performed on ICC cells that were cultured for < 12 h. ICCs were identified immunologically using an anti-c-kit antibody (phycoerythrin (PE)-conjugated rat anti-mouse c-kit monoclonal antibody) (eBioscience, San Diego, CA, United States) at a dilution of 1:50 for 20 min[25]. Because ICCs differed morphologically from other cell types in culture, they were identified by phase contrast microscopy after incubation with anti c-kit antibody.
Patch-clamp experiments
The cells were transferred onto a solution chamber on the stage of an inverted microscope (IX70, Olympus, Japan). The whole-cell patch-clamp technique was used to record the membrane potentials (current clamp) of cultured ICCs as described previously[21,22] and an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, United States) was used to amplify membrane potentials. The bath solution [Normal Tyrode (NT)] contained 135 mmol/L NaCl, 5 mmol/L KCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L glucose and 10 mmol/L HEPES, with a pH adjusted to 7.4 using NaOH. The internal solution contained 145 mmol/L Cs-glutamate, 8 mmol/L NaCl, 10 mmol/L BAPTA and 10 mmol/L HEPES-CsOH, with a pH adjusted to 7.2 with CsOH. All experiments were performed at 30-32 °C.
Drugs
Y25130, RS39604 and SB269970 were purchased from Tocris Bioscience (Bristol, United Kingdom) and all other drugs were obtained from Sigma-Aldrich (St. Louis, MO, United States). For stock solutions, all drugs were dissolved in distilled water or dimethylsulfoxide (DMSO) and stored at -20 °C. The final concentration of DMSO in the bath solution was always < 0.1%. Furthermore, the addition of these chemicals did not alter the bath solution pH.
Statistical analysis
The results are expressed as the means ± SEMs. N values refer to the numbers of cells used in the experiments. For multiple comparison analysis, we used one-way ANOVA with Bonferroni’s post hoc comparison. For statistical analyses, we used Prism 6.0 (GraphPad Software Inc., La Jolla, CA, United States) and Origin version 8.0 (OriginLab Corporation, Northampton, MA, United States). P values < 0.05 were considered statistically significant.
RESULTS
Functional components of HHTE
The presence of geniposide, berberine chloride, baicalin and wogonin in HHTE was established by HPLC and their levels were quantified using calibration curves obtained with purchased standards[26]. Validation of the method confirmed its reliability and stability. The contents of the four marker components of HHTE by HPLC were 2.684% ± 0.014% in geniposide, 0.993% ± 0.006% in berberine chloride, 5.081% ± 0.027% in baicalin and 0.040% ± 0.003% in wogonin.
Effects of HHTE on the pacemaker potentials of ICCs
Using the whole-cell patch clamp technique, we investigated the characteristics of cell clusters. Cell clusters formed network-like structures after culture for < 12 h and were viable as demonstrated by the generation of spontaneous rhythmic contractions. ICCs were immunologically identified using an anti-c-kit antibody[25]. All experiments were performed at 30 °C-32 °C. Under the current clamp mode (I = 0), ICCs generated pacemaker potentials (Figure 1A) with a mean resting membrane potential of -58.3 ± 1.5 mV and a mean amplitude of 24.9 ± 1.1 mV. Initially, we examined the effects of HHTE on the pacemaker potentials of ICCs. HHTE (10-50 mg/mL) was found to depolarize pacemaker potentials and decrease their amplitudes in a concentration-dependent manner (Figure 1B-D). In the presence of HHTE, the mean degrees of depolarization were 0.1 ± 0.1 mV at 10 mg/mL, 11.1 ± 0.9 mV at 20 mg/mL and 19.6 ± 1.1 mV at 30 mg/mL (Figure 1E, n = 17), the mean amplitudes were 24.8 ± 1.0 mV at 10 mg/mL, 12.1 ± 0.3 mV at 20 mg/mL and 1.7 ± 0.6 mV at 30 mg/mL (Figure 1F, n = 17) and the mean frequencies were 17.5 ± 1.0 cycles/min at 10 mg/mL, 4.5 ± 1.3 cycles/min at 20 mg/mL and 1.8 ± 0.9 cycles/min at 30 mg/mL (Figure 1G, n = 17). The summarized values showing the effects of HHTE on pacemaker potentials are provided in Figure 1E, 1F and 1G. These results show HHTE dose-dependently depolarizes the ICC pacemaker potentials.
HHTE mechanisms of action on ICCs
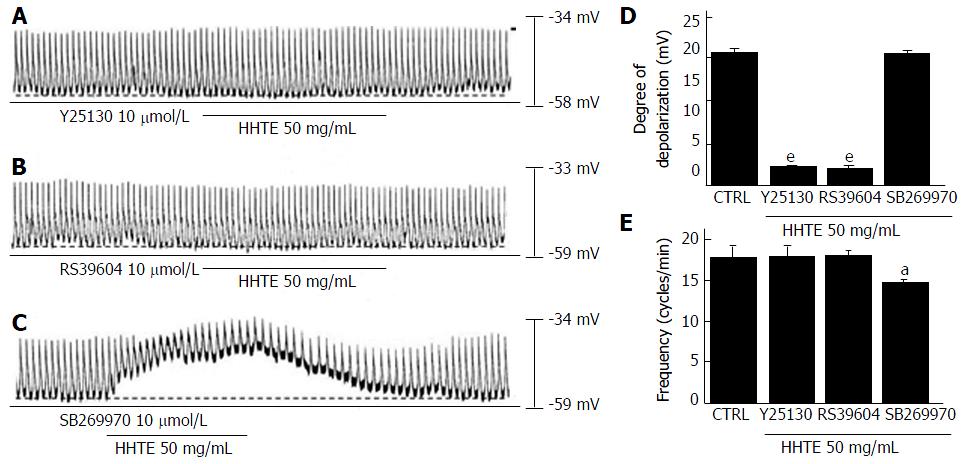
To identify the connection between HHTE and its receptors in cultured ICCs, we studied the correlation of 5-HT receptors because these receptors are involved in GI tract motility, which is strongly associated with prokinetic activity[27]. In previous studies, 5-HT has been shown to interact with seven 5-HT receptor subtypes in the GI tract and only murine small intestine ICCs express three of these receptors (5-HT3, 4 and 7)[28,29]. To confirm the 5-HT receptor subtypes involved in the effects of HHTE, we used the various 5-HT receptor antagonists and then treated samples with HHTE. A 5-HT3 receptor antagonist (Y25130), a 5-HT4 receptor antagonist (RS39604), or a 5-HT7 receptor antagonist (SB269970) was used to pretreat the samples at 10 μmol/L for 5 min; then, HHTE was added. In the case of pretreatment with Y25130, HHTE-induced pacemaker potential depolarization was blocked (Figure 2A) and HHTE-induced pacemaker potential depolarization in the presence of Y25130 was 0.8 ± 0.5 mV (n = 5; Figure 2D and E). RS39604 also blocked HHTE-induced pacemaker potential depolarization. HHTE-induced pacemaker potential depolarization in the presence of RS39604 was 0.5 ± 0.6 mV (n = 5; Figure 2B, D and E). However, pretreatment with SB269970 did not block the HHTE-induced pacemaker potential depolarization. HHTE-induced pacemaker potential depolarization in the presence of SB269970 was 19.4 ± 1.8 mV (n = 5; Figure 2C-E). These results indicate that HHTE affects ICCs in the murine small intestine through the 5-HT3 and 5-HT4 receptors.
Figure 2 Effects of 5-HT receptor antagonists on water extract of Hwangryunhaedok-tang-induced pacemaker potential depolarizations in the cultured interstitial cells of Cajal of murine small intestine.
A: Y25130 (a 5-HT3 receptor antagonist) inhibited HHTE-induced responses. B: A 5-HT4 receptor antagonist, RS39604, blocked HHTE-induced responses. C: A 5-HT7 receptor antagonist, SB269970, did not block HHTE-induced responses. D and E responses to 5-HT receptor antagonists are summarized. Bars indicate the means ± SEMs. aP < 0.05 and eP < 0.001: Significantly different from nontreated controls. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang.
Connection of G protein in HHTE-induced pacemaker potential depolarization in ICCs
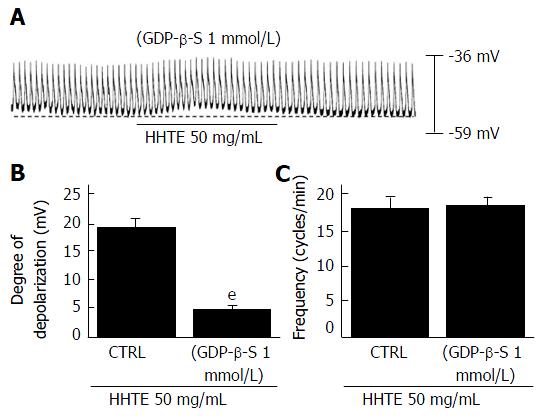
To determine whether G proteins are involved in HHTE-induced pacemaker potential depolarization, we used GDP-β-S (a non-hydrolysable guanosine 5’ diphosphate analogue), which inhibits G-protein activation[30,31]. HHTE (50 mg/mL) induced pacemaker potential depolarization in ICCs (Figure 1D); however, when GDP-β-S (1 mmol/L) was applied in the pipette solution, HHTE (50 mg/mL) only caused slight pacemaker potential depolarization (5.2 ± 0.3 mV, n = 5; Figure 3A and B). These results indicate that G protein is involved in HHTE-induced ICC pacemaker potential depolarization.
Figure 3 Effects of GDP-β-S on water extract of Hwangryunhaedok-tang-induced pacemaker potential depolarizations in cultured interstitial cells of Cajal.
A: When GDP-β-S (1 mmol/L) was applied in the pipette, HHTE did not depolarize the pacemaker potential. B and C: HHTE-induced responses are summarized. Bars indicate the means ± SEMs. eP < 0.001: Significantly different from nontreated controls. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang.
Effects of external and internal Ca2+ on HHTE-induced pacemaker potential depolarization in ICCs
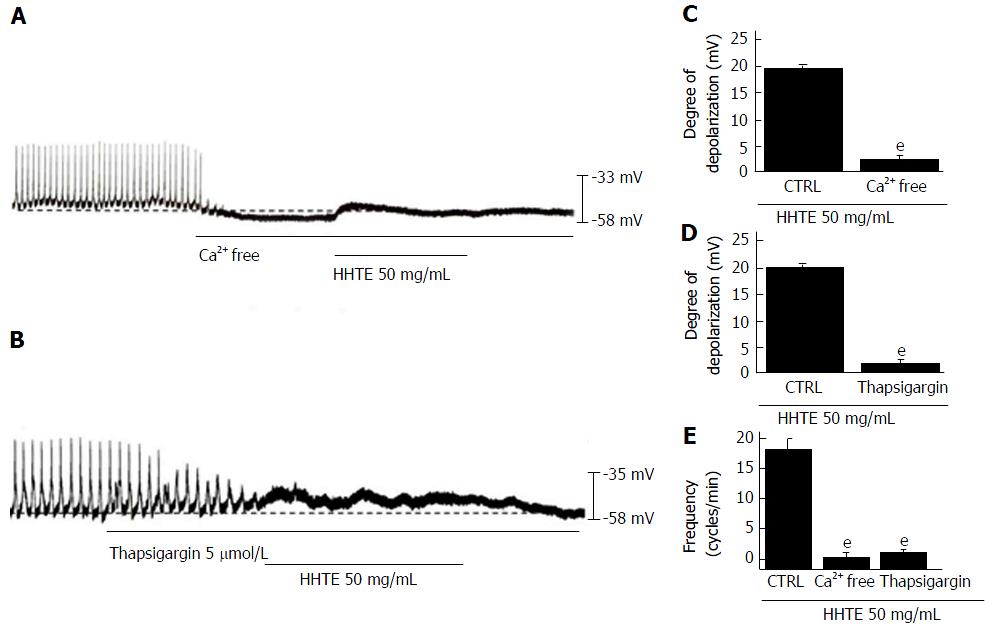
Regulation of external and internal Ca2+ has an important role in mediating GI smooth muscle contraction and the generation of pacemaker potentials in ICCs[32]. To identify the roles of Ca2+, HHTE was applied in external Ca2+-free conditions or in the presence of an inhibitor of Ca2+-ATPase in the endoplasmic reticulum (thapsigargin). In the external Ca2+-free conditions, pacemaker potentials were abolished; under this condition, HHTE did not induce pacemaker potential depolarization (n = 6; Figure 4A and C). In addition, pretreatment with thapsigargin abolished pacemaker potentials and under this condition, HHTE also did not induce pacemaker potential depolarization (n = 5; Figure 4B and D). These results show that HHTE-induced pacemaker potential depolarization depends on internal and external Ca2+.
Figure 4 Effects of external and internal Ca2+ on water extract of Hwangryunhaedok-tang-induced pacemaker potential depolarization in cultured interstitial cells of Cajal from murine small intestine.
A: In external Ca2+-free conditions, the pacemaker potentials were abolished and HHTE had no effect. B: Thapsigargin abolished the pacemaker potentials and HHTE had no effects; C-E: HHTE-induced pacemaker potential depolarizations are summarized. Bars represent the means ± SEMs. eP < 0.001: Significantly different from untreated controls. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang.
Pathways mediating HHTE-induced pacemaker potential depolarization in ICCs
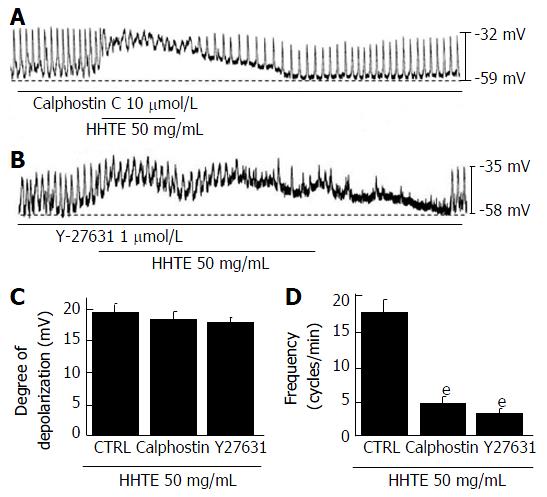
To determine whether HHTE-induced pacemaker potential depolarization is associated with protein kinase C (PKC) or Rho kinase, we pretreated ICCs with a PKC inhibitor (calphostin C, 10 μmol/L, Figure 5A) or a Rho kinase inhibitor (Y27631, 1 μmol/L, Figure 5B) for 5 min. HHTE-induced pacemaker potential depolarizations were not inhibited by calphostin C or Y27632 (Figure 5C). We confirmed that treatment with these inhibitors alone did not affect pacemaker potential depolarization. These results suggest that HHTE-induced pacemaker potential depolarization is not mediated by PKC or Rho kinase in the cultured ICCs of murine small intestine.
Figure 5 Effects of protein kinase C inhibitor and Rho kinase inhibitor on water extract of Hwangryunhaedok-tang-induced pacemaker potential depolarizations in cultured interstitial cells of Cajal from murine small intestine.
Both (A) calphostin C (a PKC inhibitor) and (B) Y27631 (a Rho kinase inhibitor) had no effects on the pacemaker potentials; under these conditions, HHTE induced pacemaker potential depolarizations. C and D responses to HHTE in the presence of calphostin C or Y27631 are summarized. Bars represent the means ± SEMs. eP < 0.001: significantly different from the nontreated control. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang; PKC: Protein kinase C.
Effects of Coptidis Rhizoma, Scutellariae Radix, Phellodendri Cortex and Gardeniae Fructus extracts in ICCs
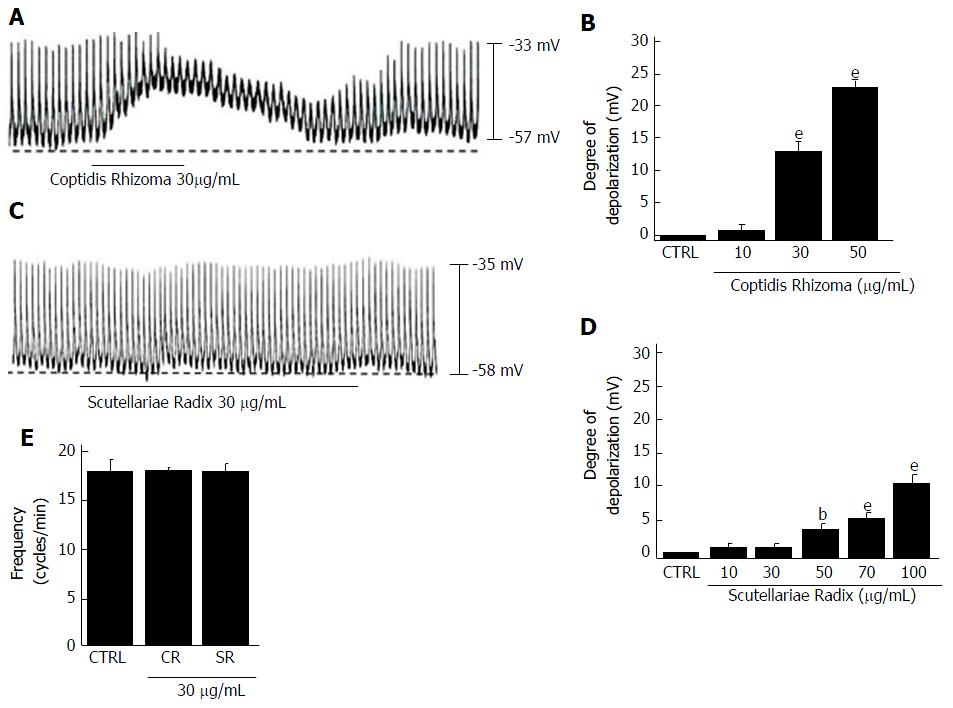
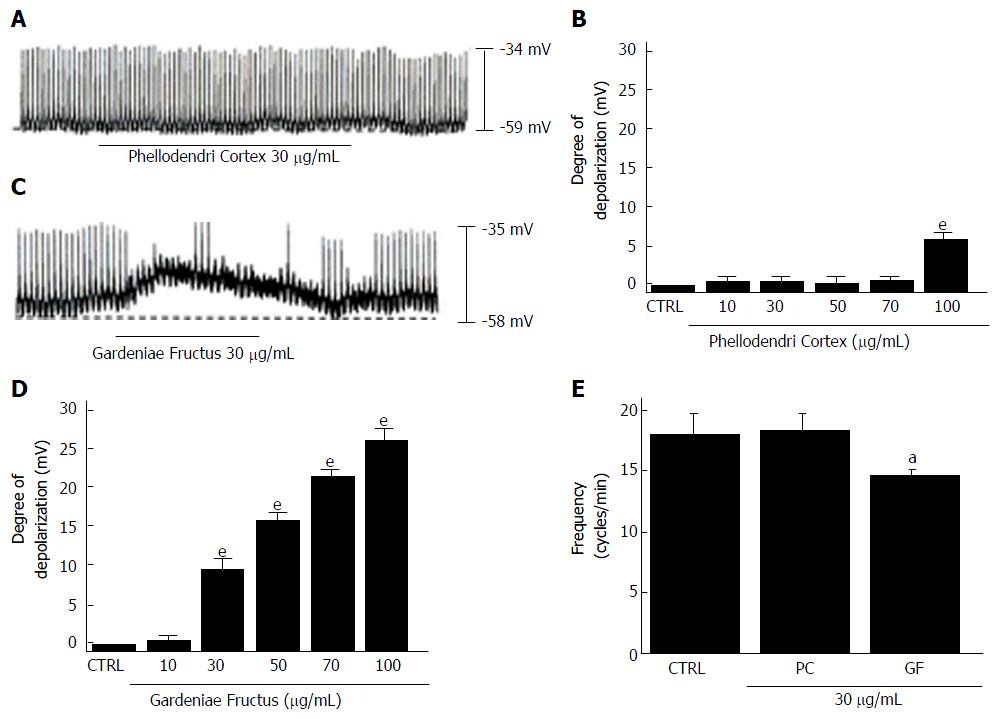
We investigated the effects of the four components of HHT, Coptidis Rhizoma, Scutellariae Radix, Phellodendri Cortex and Gardeniae Fructus[6], in cultured ICCs from murine small intestine. All four components depolarized the pacemaker potentials and decreased the amplitudes of the pacemaker potentials (Figures 6 and 7) in a concentration-dependent manner. At a concentration of 30 μg/mL, Coptidis Rhizoma had a mean degree of depolarization of 13.1 ± 2.2 mV (Figure 6A, n = 7), Scutellariae Radix had a mean degree of depolarization of 0.6 ± 0.4 mV (Figure 6C, n = 7), Phellodendri Cortex had a mean degree of depolarization of 0.7 ± 0.6 mV (Figure 7A, n = 6) and Gardeniae Fructus had a mean degree of depolarization of 9.7 ± 1.5 mV (Figure 7C, n = 7). The summarized values and bar graph showing the effects of these components on pacemaker potentials are provided in Figures 6B, 6D, 6E, 7B, 7D and 7E. These results suggest that Coptidis Rhizoma and Gardeniae Fructus are the main components responsible for HHTE-induced pacemaker potential depolarizations in the cultured ICCs of murine small intestine.
Figure 6 Effects of Coptidis Rhizoma and Scutellariae Radix on pacemaker potentials in cultured interstitial cells of Cajal from murine small intestine.
A: Pacemaking activities of ICCs exposed to Coptidis Rhizoma (30 μg/mL) in current-clamp mode (I = 0); B: Coptidis Rhizoma (10-50 μg/mL) concentration-dependently induced pacemaker potential depolarization. Responses to Coptidis Rhizoma are summarized; C: Pacemaking activities of ICCs exposed to Scutellariae Radix (30 μg/mL) in the current-clamp mode (I = 0); D: Scutellariae Radix (10-100 μg/mL) concentration-dependently induced pacemaker potential depolarization. Responses to Scutellariae Radix are summarized; E: Frequency changes to Coptidis Rhizoma and Scutellariae Radix are summarized. Bars represent the means ± SEMs. bP < 0.01 and eP < 0.001: Significantly different from the control. CTRL: Control; CR: Coptidis Rhizoma; SR: Scutellariae Radix; ICCs: Interstitial cells of Cajal.
Figure 7 Effects of Phellodendri Cortex and Gardeniae Fructus on pacemaker potentials in cultured interstitial cells of Cajal from murine small intestine.
A: Pacemaking activities of ICCs exposed to Phellodendri Cortex (30 μg/mL) in the current-clamp mode (I = 0); B: Phellodendri Cortex (10-100 μg/mL) concentration-dependently induced pacemaker potential depolarization. Responses to Phellodendri Cortex are summarized; C: Pacemaking activities of ICCs exposed to Gardeniae Fructus (30 μg/mL) in the current-clamp mode (I = 0); D: Gardeniae Fructus (10-100 μg/mL) concentration-dependently induced pacemaker potential depolarizations. Responses to Gardeniae Fructus are summarized; E: Frequency changes to Phellodendri Cortex and Gardeniae Fructus are summarized. Bars represent the means ± SEMs. aP < 0.05 and eP < 0.001: Significantly different from the control. CTRL: Control; PC: Phellodendri Cortex. GF: Gardeniae Fructus.
DISCUSSION
ICCs are located within and around smooth muscle layers of the GI tract and they control GI motility by acting as pacemaker cells[20-22,33,34]. The pacemaker functions of these cells result from the electrical slow waves generated and propagated in smooth muscles[20,35]. Additionally, ICCs are connected to both nerve and smooth muscle cells and they are involved in mechanosensation and neurotransmission[32,34,36]. Many GI motility disorders, such as achalasia and inflammatory bowel disease, have been associated with ICC dysfunctions[37]. Therefore, understanding the mechanisms of ICC and pacemaker activity is very important. In the present study, we show that HHTE depolarized ICC pacemaker potentials through 5-HT3 and 5-HT4 receptors via external and internal Ca2+ regulation and via G protein-, PKC- and Rho kinase- independent pathways. Additionally, we tested the effects of HHTE on GI motility in mice in vivo and found that HHTE increased the GI motility in mice[26].
HHT has long been used as an herbal medicine to treat GI disorders in Asia[16-18]. Ohta et al[16] suggested that oral HHT prevents stress-induced acute gastric lesions in rats. Additionally, Zhou and Mineshita[17] reported that HHT showed anti-inflammatory and anti-ulcerative effects on trinitrobenzene-sulfonic acid (TNB)-induced colitis in rats. In that study, HHT was found to inhibit myeloperoxidase activity and to significantly decrease the IL-8, PGE2 and LTB4 levels. Therefore, the authors suggested that HHT may be useful for treating inflammatory bowel disease. In addition, Watanabe-Fukuda et al[18] observed that HHT prevented lethal indomethacin (IND)-induced enteropathy by downregulating adenosine deaminase (ADA) and thus upregulating adenosine (an anti-inflammatory nucleoside) levels. Furthermore, these authors reported that berberine, which is a major ingredient of HHT, suppressed ADA expression and reduced the incidence of lethality.
In the present study, we found that 5-HT3 or 5-HT4 receptors, via external and internal Ca2+ regulation and via G protein, were involved in HHTE-induced responses in ICCs. Of the seven 5-HT receptor subtypes, the 5-HT3 and 5-HT4 receptors are the most highly expressed and best understood 5-HT receptor subtypes in the GI tract[38,39]. 5-HT3 receptors are ligand-gated cation channels that can initiate peristalsis and secretion in the GI tract[38]. Additionally, 5-HT4 receptors are G-protein-coupled metabotropic receptors and their activation regulates GI motility[39]. Furthermore, these receptors are coupled to G proteins, which have Ca2+/calmodulin-dependent activation[40,41]. Additionally, in the present study, HHT-induced pacemaker potential depolarizations via 5-HT3 and 5-HT4 receptors were not inhibited by the Rho kinase inhibitor, Y27632, or the PKC inhibitor, calphostin (Figure 5). These results suggest that HHT-induced pacemaker potential depolarizations are independent of PKC and Rho kinase activation.
5-HT has played a major role in gut function regulation[42]. Among 5-HT receptors, 5-HT3 and 5-HT4 receptors have been the most widely investigated for gut motility[27]. 5-HT3 receptors are mainly targeted to treat diarrhea and abdominal pain[27,43,44] and 5-HT4 receptors are targeted to treat constipation[44,45]. Although each agonist and antagonist has been used effectively, pharmacological agents of 5-HT receptors have problems in their clinical use because of deleterious side effects. In the present study, HHTE affects ICC pacemaker potentials through 5-HT3 and 5-HT4 receptors; therefore, HHTE may be used to treat diarrhea, abdominal pain and constipation, among other symptoms. Of note, future studies will have to be designed to determine the side effects of HHT.
The 5-HT3 receptor channels are the only ionotropic receptors within the 5-HT receptor family[46-48]. 5-HT3 receptors are involved in many pathophysiological processes, such as GI motility disorders and the development of nausea and vomiting[48-50]. Therefore, 5-HT3 receptors play an important role in GI motility functions. In this paper, we found that HHTE may activate 5-HT3 and 5-HT4 receptors; among them, 5-HT4 receptors may have the main role in regulating the ICC, whereas 5-HT3 receptors may have a supportive role. Additionally, when GDP-β-S (1 mmol/L) was applied in the pipette solution, we found that HHTE caused slight pacemaker potential depolarization (Figure 3), which might be due to the 5-HT3 receptor signaling pathway.
GI disorders are observed in Western and Eastern countries[51]. Because GI disorders are not lethal, the medications used to treat these disorders have a higher safety threshold than those for medications used to treat non-GI diseases. However, recent studies have highlighted the substantial health impacts of GI disorders[52]. Furthermore, several GI treatments have significant adverse effects, which has led to the withdrawal of cisapride and tegaserod from the market[53,54]. Of note, herbal products are often used to treat GI disorders like irritable bowel syndrome[55,56] and herbal medications have a desirable role in the treatment of GI disorders.
HHT consists of Coptidis Rhizoma, Scutellariae Radix, Phellodendri Cortex and Gardeniae Fructus[6]. We investigated the effects of these four constituents on the pacemaker potentials of ICCs to identify the components that are primarily responsible for the effect of HHT on ICC pacemaker potentials. All four components depolarized pacemaker potentials and decreased the amplitudes of pacemaker potentials in a concentration-dependent manner (Figures 6 and 7). However, Coptidis Rhizoma and Gardeniae Fructus were observed to have greater depolarizing effects. We will perform future experiments to determine the detailed effects of the HHT active elements (Coptidis Rhizoma and Gardeniae Fructus) and identified compounds (geniposide, berberine chloride, baicalin and wogonin) on ICC pacemaking potential.
ICCs are pacemaker cells that appear to play important roles in the determination and regulation of GI motility[19,20]. HHTE depolarized the pacemaker potentials of ICCs in a G protein- and Ca2+-dependent manner. Based on the findings described in this study, we propose the following model of the effects of HHTE in ICCs. HHTE binds 5-HT3 and 5-HT4 receptors and HHTE-induced pacemaker potential depolarizations are G protein- and Ca2+-dependent in ICCs. An increase in intracellular Ca2+ induces membrane depolarizations on ICCs[57,58]. In addition, these pacemaker potential depolarizations might be regulated by transient receptor potential melastatin 7 (TRPM7) and Ca2+-activated Cl- (ANO1) channels[20,59].
In the present study, HHTE was found to regulate ICC pacemaker potential depolarization in a G protein- and Ca2+-dependent manner. Accordingly, HHT may play an excitatory role in GI motility regulation. Additionally, because GI motility regulations are highly integrated behaviors between smooth muscle cells, ICCs and enteric nervous systems, further studies are needed to investigate the effects of HHT on other cells in vitro and in vivo.
In summary, our findings suggest that HHT can depolarize the pacemaker potentials of cultured ICCs from mouse small intestine. Future research should attempt to identify the components responsible for the effects of HHT and to determine their mechanisms of action in vitro and in vivo.