Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5246
Peer-review started: December 17, 2016
First decision: January 10, 2017
Revised: February 21, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: July 28, 2017
Processing time: 224 Days and 14.4 Hours
Hepatic encephalopathy (HE) remains a diagnosis of exclusion due to the lack of specific signs and symptoms. Refractory HE is an uncommon but serious condition that requires the search of hidden precipitating events (i.e., portosystemic shunt) and alternative diagnosis. Hypothyroidism shares clinical manifestations with HE and is usually considered within the differential diagnosis of HE. Here, we describe a patient with refractory HE who presented a large portosystemic shunt and post-ablative hypothyroidism. Her cognitive impairment, hyperammonaemia, electroencephalograph alterations, impaired neuropsychological performance, and magnetic resonance imaging and spectroscopy disturbances were highly suggestive of HE, paralleled the course of hypothyroidism and normalized after thyroid hormone replacement. There was no need for intervention over the portosystemic shunt. The case findings support that hypothyroidism may precipitate HE in cirrhotic patients by inducing hyperammonaemia and/or enhancing ammonia brain toxicity. This case led us to consider hypothyroidism not only in the differential diagnosis but also as a precipitating factor of HE.
Core tip: Hepatic encephalopathy (HE) remains a diagnosis of exclusion due to the lack of specific signs and symptoms. Refractory HE requires the search of hidden precipitating events and alternative diagnosis. We describe a patient with refractory HE who presented with large portosystemic shunt and post-ablative hypothyroidism. Her cognitive impairment, hyperammonaemia, electroencephalograph alterations, impaired neuropsychological performance and magnetic resonance imaging and spectroscopy disturbances suggestive of HE paralleled the course of hypothyroidism and improved after thyroid hormone replacement. The case findings support that hypothyroidism may precipitate HE in cirrhotic patients by inducing hyperammonaemia and/or enhancing ammonia brain toxicity.
- Citation: Díaz-Fontenla F, Castillo-Pradillo M, Díaz-Gómez A, Ibañez-Samaniego L, Gancedo P, Guzmán-de-Villoria JA, Fernández-García P, Bañares-Cañizares R, García-Martínez R. Refractory hepatic encephalopathy in a patient with hypothyroidism: Another element in ammonia metabolism. World J Gastroenterol 2017; 23(28): 5246-5252
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5246.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5246
Hepatic encephalopathy (HE) is a brain dysfunction that frequently affects patients with chronic liver failure. Its symptoms and signs are not specific and may also be present in other causes of brain dysfunction[1]. In cirrhotic patients, an episode of HE is usually triggered by a clinical event that exacerbates the toxic effects of ammonia and other substances on the brain. The search and adequate treatment of such precipitating factors are fundamental to revert the HE of these patients. Refractory HE is an uncommon but serious condition that requires the search of alternative diagnosis and non-identified precipitating factors.
Hypothyroidism shares clinical manifestations with HE, such as disturbances in memory and attention, and is considered in the differential diagnosis of HE. However, little is known on whether hypothyroidism coexists with or precipitates HE. Herein, we describe a patient with a large portosystemic shunt who developed refractory HE during post-ablative hypothyroidism. The case’s clinical manifestations, electroencephalographic pattern and magnetic resonance imaging (MRI) and spectroscopy (MRS) findings were consistent with HE. Treatment with thyroid hormone normalized ammonia levels and improved clinical alterations and electroencephalograph (EEG). MRI and MRS showed an improvement in markers of ammonia toxicity, suggesting that hypothyroidism might increase brain exposition to ammonia and precipitate HE. This case led us to consider hypothyroidism not only in the differential diagnosis, but also as a precipitating factor of HE.
A 58-year-old woman with the diagnosis of chronic and refractory HE in the setting of liver cirrhosis and large portosystemic shunt was referred to our Liver Unit from a secondary hospital. Her liver disease was diagnosed in 2011 when she presented her first episode of overt HE (type C, grade II). Due to a history of alcohol abuse for years and other causes of liver disease being ruled out, a diagnosis of alcoholic cirrhosis Child-Pugh B7 was established. The patient had no ascites nor hepatocellular carcinoma, and primary prophylaxis with propranolol because of oesophageal varices grade II-III/IV was started. At that time, the patient abandoned alcohol consumption, and remained asymptomatic and compensated from the liver point of view.
As comorbidities, the patient had diabetes mellitus, arterial hypertension and multinodular goitre diagnosed in 2011 in a pre-toxic state. In January 2012, she started carbimazole and received radioiodine therapy in February 2012 because of hyperthyroidism. Carbimazole was stopped after 4 mo, and treatment with levothyroxine (October 2012-April 2013) was subsequently started because of post-ablative hypothyroidism. In August 2013, carbimazole was restarted due to recurrent toxic multinodular goitre, and a second session of radioiodine was administered in November 2014. Carbimazole was stopped at the end of November 2014 and levothyroxine was again prescribed in middle of January 2015 because of subclinical hypothyroidism (thyroid-stimulating hormone (TSH) 8 mUI/L, normal range: 0.5-4.5 mUI/L).
In February 2015, the patient was admitted to another hospital because of a subacute neurological syndrome consistent with daily hypersomnia, dysarthria, mental slowness, bizarre behaviour (“she wanted to turn on the TV with her keys”) and diminished voluntary movements. At the physical exam, she was conscious and oriented, although she presented mental slowness and slow speech rate, shortened attention span and irritability. The patient had asterixis, with no other evident motor or neurological alterations. Laboratory test showed mild macrocytosis, with no other abnormalities and the brain computed tomography (CT) scan was also normal. Precipitating factors were not found (no infection, gastrointestinal bleeding or recent alcohol abuse was documented) and oral and rectal lactulose was started, producing adequate laxative response. However, rifaximin was added because of lack of clinical efficacy.
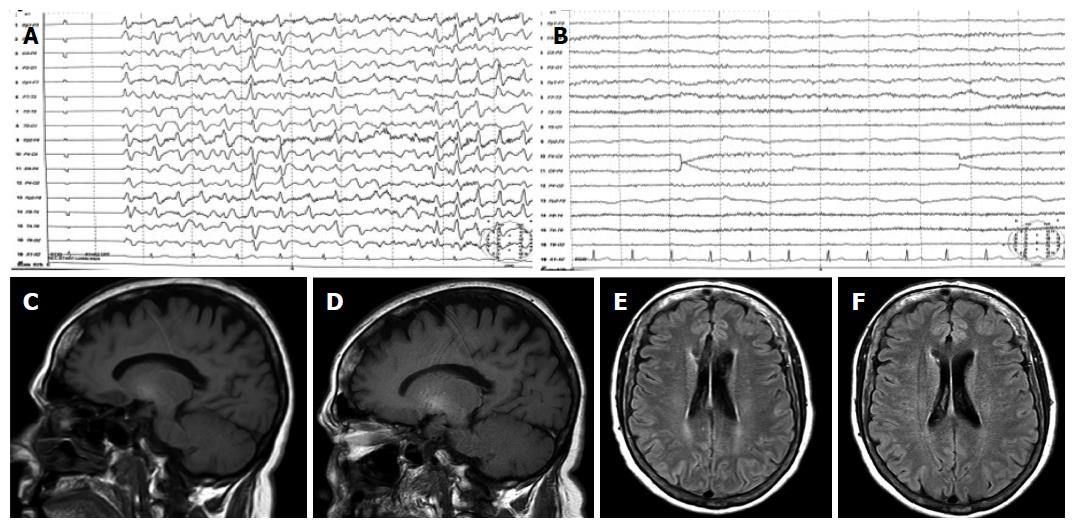
An EEG was performed and showed slow activity with the presence of generalized triphasic waves (Figure 1A). Because of persistent neurological impairment consistent with HE, an abdominal CT scan searching for portosystemic shunts was performed and revealed a large shunt communicating portal and left renal veins. The patient was then referred to our tertiary hospital for shunt embolization.
At admission, the patient showed similar neurological status with hypersomnia, mental slowness, shortened attention span, and memory difficulties, but was oriented in time and space. Asterixis and temporal disorientation was occasionally present but other neurological abnormalities were not observed. She reported pronounced constipation and gained weight of 3 kg within the last weeks. During her stay in the hospital, she remained neurologically stable despite high doses of oral lactulose (with adequate daily bowel movements) and rifaximin, developing occasionally confusion, disorientation and asterixis without clear precipitating factor and with partial response to lactulose intensification.
Laboratory testing (Table 1) showed macrocytosis without anaemia and hyperammonaemia and thyroid test demonstrated hypothyroidism (TSH 70 mUI/L and T4 0.6 ng/dL; normal range: 0.6-1.4 ng/dL). A Psychometric Hepatic Encephalopathy Score (PHES) was performed and showed a pathological performance with a score of 9 SD below the reference population (Table 1). MRI and MRS were performed using 1.5T equipment (Figure 1C-F). T1-weighted hyperintensity in basal ganglia (Figure 1C) and periventricular white matter high signals on FLAIR sequence were shown (Figure 1E).
| March 2015 | May 2016 | |
| Anthropometric | ||
| Weight in kg | 75 | 72 |
| BMI | 28.2 | 27.7 |
| Laboratory | ||
| Hb in g/dL | 12.7 | 15.3 |
| MCV | 103.8 | 98.8 |
| Leucocytes as 103/μL | 3.9 | 5.7 |
| Platelets as 103/μL | 120 | 165 |
| INR | 1.18 | 1.14 |
| AST in U/L | 33 | 31 |
| ALT in U/L | 41 | 34 |
| GGT in U/L | 145 | 165 |
| AP in U/L | 90 | 116 |
| Albumin in g/dL | 3.3 | 4.3 |
| Bilirubin in mg/dL | 1 | 1.3 |
| Creatinine in mg/dL | 0.59 | 0.5 |
| Na in mmol/L | 139 | 144 |
| Child-Pugh | B (7) | A (6) |
| MELD | 8 | 9 |
| Ammonia in μmol/L | 62 | 23 |
| Thyroid function | ||
| TSH in mUI/L | 69.86 | 2.66 |
| T4free in ng/dL | 0.6 | |
| PHES | ||
| Symbol digit test as points | 8 | 34 |
| Number connection test A in s | 78 | 25 |
| Number connection test B in s | 514 | 73 |
| Serial dotting test in s | 79 | 37 |
| Line tracing test as seconds + errors | 200 | 100 |
| Final score | -9 | 1 |
| Magnetic resonance spectroscopy | ||
| Glx/Cr | 1.884 | 0.439 |
| Cho/Cr | 0.934 | 1.204 |
| mI/Cr | 0.064 | 0.529 |
A therapeutic decision of treating and resolving first hypothyroidism and re-evaluating the patient afterwards was based on three aspects: (1) time course (neurological impairment is coincident with the progression of thyroid disturbance); (2) lack of previous neurological impairment attributable to porto-systemic shunt (completely asymptomatic over the past 4 year); and (3) although many symptoms of the patient could be attributable to either hypothyroidism or HE, some others (constipation and weight gain) were highly indicative of thyroid dysfunction that could potentially contribute to the clinical syndrome.
Increasing levothyroxine dose resulted in steady clinical and laboratory improvements. At 1 wk later, the patient was discharged under levothyroxine 75 μgr/d, rifaximin 600 mg/12 h, lactulose and carvedilol 6.25 mg/12 h leading to clinical improvement and restoration of thyroid function in the following 4 wk. She had no admission or decompensations during the next months, rifaximin was removed and lactulose was used occasionally.
The patient was re-evaluated 1 year later. She was completely asymptomatic, fully active, back to work without mental complaints, as reported by her and her relatives. She had stable liver disease (Table 1), normal thyroid function and venous ammonia levels. Her performance at PHES improved dramatically in all the tests, with a final score within the reference population. Similarly, an improvement in her EEG was observed (Figure 1B). MRI showed no changes in the hyperintensity in basal ganglia on T1-weighted images (Figure 1D) but it showed an improvement in periventricular white matter hyperintensities on FLAIR images (Figure 1F). Single voxel proton MRS located in the right centrum semiovale (Table 1) showed a decrease in the glutamate-glutamine/creatine peak (Glx/Cr), together with an increase in the myoinositol/creatine (mI/Cr) and choline/creatine (Cho/Cr) peaks at short echo-time (23 ms).
We considered that currently there was no need for an intervention over the porto-systemic shunt. Her routine follow-up showed that she is clinically stable and asymptomatic under medical treatment (carvedilol 6.25 mg/12 h, levothyroxine 112 μg/d, sitagliptin 50 mg/d and lactulose occasionally).
Since hypothyroidism shares symptoms with HE, it is considered within the differential diagnosis of HE. However, there is scarce information on whether hypothyroidism may coexist and even precipitate HE and on how to attribute brain dysfunction to one or the other entity. The present report supports the role of hypothyroidism as a precipitating factor of HE by inducing hyperammonaemia and illustrates the difficulties in diagnosing HE when it coexists with other causes of brain dysfunction.
The lack of specific symptoms or laboratory tests that ensure the diagnosis of HE make therapeutic decisions sometimes difficult. A practical approach would be treating all the potential aetiologies of brain dysfunction, but this may not be the optimal strategy if it involves aggressive treatments with high risk of serious complications. Shunt embolization may be a good option in patients with HE secondary to portosystemic shunts, but such patients should be carefully selected since a rise in portal pressure with risk of variceal bleeding and ascites may occur.
The question, whether the neurological impairment was caused by the hypothyroidism or was attributable to the large portosystemic shunt, was very important to make further therapeutic decisions. Although both hypothyroidism and HE may present with mental slowness and memory impairment[1], asterixis and disorientation are more commonly observed in HE. Our patient’s EEG pattern (slow activity and generalized triphasic waves) is characteristic of, although not specific for, HE[2] and has seldom been reported in hypothyroidism[3]. Furthermore, MRI depicted on T1-weighted images high signal in basal ganglia, which is frequently seen in patients with cirrhosis or portal-systemic shunts. Besides, FLAIR sequences showed high signal intensity in hemispheric white matter. This finding has been shown to improve after resolution of episodic HE or liver transplantation (LT) and has been attributed to mild brain oedema[4]. All these findings support the diagnosis of HE. However, the parallelism between the time course of the cognitive impairment and the hypothyroidism, the lack of response to hypoammonaemic measures, and the neurological improvement following normalization of thyroid function implies a relevant role for hypothyroidism.
Ammonia is thought to have an important role in HE. In normal conditions, ammonia is mainly generated in the gut, then is extracted in the liver and eliminated as urea by the kidney. In liver failure and/or in patients with portal-systemic shunts, ammonia reaches the systemic circulation and the central nervous system[1]. Ammonia is metabolized to glutamine in astrocytes through the glutamine synthetase enzyme and both ammonia and glutamine exert their toxic effects on the brain. Muscle and kidney glutamine synthetase, together with kidney glutaminase, also regulate plasma ammonia levels and they are especially relevant in the context of liver failure. Patients with cirrhosis characteristically show an increase in Glx/Cr peak and a decrease of Cho/Cr and mI/Cr in MRS of the brain. Abnormalities in Glx/Cr have been interpreted as an increase in brain glutamine secondary to the ammonia detoxification in astrocytes, whereas disturbances in Cho/Cr and mI/Cr have been interpreted as a compensatory response to the increased intracellular osmolality caused by glutamine accumulation. The severity of these disturbances has been associated with HE, and they are reversible following successful LT[5].
A potential interaction between thyroid status and ammonia metabolism arose in our patient. Hyperammonaemia in patients with hypothyroidism has been seldom described over the last two decades, and only in sporadic cases reports[6-12] (Table 2). Six out of seven of these patients (86%) are patients with liver cirrhosis. All those cirrhotic patients exhibited clinical manifestations suggestive of HE (grade I to IV), whereas the subject without liver disease had not. A common finding in all these cirrhotic patients with hypothyroidism was that neurological disturbances were refractory to hypoammonaemic therapy and improved with thyroid hormone replacement.
| Year | Ref. | Clinical presentation | Thyroid disease | Liver disease | Evolution |
| 1992 | Hitoshi et al[6] | Mild dementia, slow speech, hyperreflexia, dysmetria, asterixis with hyperammonaemia. | Hypothalamic hypothyroidism | Cirrhosis and portal hypertension | Thyroid hormone replacement improved: |
| Progression to coma despite lactulose treatment. | Hypothyroidism | ||||
| Hyperammonaemia | |||||
| NRL disturbances | |||||
| 1999 | De Nardo et al[7] | Hoarseness, fatigue, tongue swelling, myopathy. Hyperammonaemia. | Primary hypothyroidism | none | Thyroid hormone replacement improved: |
| Hypothyroidism | |||||
| Hyperammonaemia | |||||
| Systemic symptoms and myopathy | |||||
| 2000 | Thobe et al[8] | Coma, hyperammonaemia. | Primary hypothyroidism | Compensated cirrhosis | Thyroid hormone replacement improved: |
| Unresponsive to lactulose. | Hypothyroidism | ||||
| Hyperammonaemia | |||||
| NRL disturbances | |||||
| 2001 | Yamamoto et al[9] | Dysarthria, disorientation. | Primary hypothyroidism | Decompensated cirrhosis | Thyroid hormone replacement improved: |
| Hypothyroidism | |||||
| Hyperammonaemia | |||||
| NRL disturbances | |||||
| 2007 | Rimar et al[10] | Coma, hyperammonaemia. Unresponsive to lactulose. | Primary hypothyroidism | Compensated cirrhosis | Thyroid hormone replacement improved: |
| Hypothyroidism | |||||
| Hyperammonaemia | |||||
| NRL disturbances | |||||
| 2007 | Khairy et al[11] | Grade III hepatic encephalopathy. | Primary hypothyroidism | Decompensated cirrhosis | Thyroid hormone replacement improved: |
| Hypothyroidism | |||||
| NRL disturbances | |||||
| 2012 | Redkar et al[12] | Coma, hyperammonaemia. Unresponsive to lactulose. | Primary hypothyroidism | Decompensated cirrhosis | Thyroid hormone replacement improved: |
| Hypothyroidism | |||||
| Hyperammonaemia | |||||
| NRL disturbances |
The mechanisms by which hypothyroidism may induce hyperammonaemia are not identified. In a rodent model, hypothyroidism was associated with increased urea cycle enzyme activity and decreased glutamine synthetase in the liver[13]. However, a decreased urea synthesis rate was found in women with hypothyroid[14], suggesting a decrease in ammonia clearance via urea synthesis during hypothyroidism. We are not aware of data regarding a potential effect of thyroid hormone on muscle glutamine synthetase or gut glutaminase, which could also influence ammonia metabolism. A potential mechanism could involve thyroid hormone impact on glutamine synthetase activity not only in liver but also in muscle and kidney. A decrease in glutamine synthetase activity during hypothyroidism could contribute to hyperammonaemia that might revert with thyroid function restitution.
Hashimoto’s encephalopathy, an autoimmune disease of the thyroid gland, may manifest as a wide range of neurological symptoms. It could lead to hypothyroidism and should be considered when evaluating patients with neurological disturbances refractory to standard therapy.
As in previous reports, our patient exhibited clinical manifestations suggestive of HE as well as hyperammonaemia refractory to hypoammonaemic measures, both of which reversed with thyroid hormone. Additionally, thyroid hormone replacement was also linked to an improvement in the periventricular white matter hyperintensities on FLAIR images and in MRS results, with a decreased in the Glx/Cr peak and an increase in the mI/Cr and Cho/Cr peaks. This novel observation supports that neurological disturbances were concomitant with hyperammonaemia and higher brain exposition to ammonia consistent with HE.
In conclusion, this clinical case shows that hypothyroidism is associated with hyperammonaemia and enhanced ammonia brain exposition. In agreement with the few cases previously reported, these data support that hypothyroidism may precipitate HE in cirrhotic patients by inducing hyperammonaemia and/or enhancing ammonia brain toxicity. Therefore, patients with cirrhosis and refractory HE should be evaluated for hypothyroidism, particularly if aggressive treatments are being considered.
We acknowledge our gratitude to the patient who gave informed consent for this case report’s publication. The authors are grateful to Dr. Javier Vaquero for his critical review of the manuscript and helpful advice.
A 58-year-old woman with cognitive impairment consistent with persistent hepatic encephalopathy (HE) and portosystemic shunt refractory to standard treatment was referred to our hospital.
Refractory cognitive impairment consistent with HE that paralleled the course of post-ablative hypothyroidism.
The main differential diagnosis was neurological disturbances secondary to hypothyroidism vs refractory HE secondary to portosystemic shunt. The clinical, laboratory, electroencephalograph (EEG) and magnetic resonance imaging findings, together with the clinical resolution following thyroid replacement, support the role of hypothyroidism as a precipitating event of HE.
Laboratory tests demonstrated hypothyroidism, hyperammonaemia and alterations consistent with cirrhosis.
T1-weighted hyperintensities in basal ganglia and periventricular white matter high signals on FLAIR sequence, together with markers of brain ammonia exposition in magnetic resonance, supported the diagnosis of HE.
The patient was refractory to standard HE treatment. Thyroid hormone replacement was associated with clinical resolution of the syndrome, together with normalization of plasma ammonia levels, neuropsychological performance, EEG and magnetic resonance markers of brain ammonia exposition.
Very few case reports linked refractory neurological impairment in cirrhotic patients with hypothyroidism. This case shows an improvement in hyperammonaemia and brain ammonia exposition following thyroid hormone replacement, which was associated with clinical resolution of neurological impairment.
HE is a brain dysfunction that frequently affects patients with chronic liver failure. Its symptoms and signs are not specific and may be present in other causes of brain dysfunction.
In cirrhotic patients, hypothyroidism may precipitate HE by enhancing ammonia brain toxicity.
This is an excellent case report describing an incidence whereby hypothyroidism was a precipitating factor that contributed to a recurrence of HE in a patient with liver cirrhosis. Overall, the report was very well written, with a clear description of the time line of events followed by a thorough discussion of the literature and known interactions between hypothyroidism and HE.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: DeMorrow S, Moini M, Olesen SS, Schwabl P S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1408] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 2. | Guerit JM, Amantini A, Fischer C, Kaplan PW, Mecarelli O, Schnitzler A, Ubiali E, Amodio P; members of the ISHEN commission on Neurophysiological Investigations. Neurophysiological investigations of hepatic encephalopathy: ISHEN practice guidelines. Liver Int. 2009;29:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | River Y, Zelig O. Triphasic waves in myxedema coma. Clin Electroencephalogr. 1993;24:146-150. [PubMed] |
| 4. | Rovira A, Alonso J, Córdoba J. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol. 2008;29:1612-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Garcia-Martinez R, Cordoba J. Brain Imaging in Hepatic Encephalopathy. In: Mullen KD and Prakash RK, eds. Hepatic Encephalopathy. New York: Springer, 2012: 123-137. . [DOI] [Full Text] |
| 6. | Hitoshi S, Terao Y, Sakuta M. Portal-systemic encephalopathy and hypothalamic hypothyroidism: effect of thyroid hormone on ammonia metabolism. Intern Med. 1993;32:655-658. [PubMed] |
| 7. | De Nardo D, Franconi G, Sabino D. [Hyperammonemia during hypothyroidism: an unusual biohumoral finding normalized by hormonal replacement treatment]. Ann Ital Med Int. 1999;14:196-201. [PubMed] |
| 8. | Thobe N, Pilger P, Jones MP. Primary hypothyroidism masquerading as hepatic encephalopathy: case report and review of the literature. Postgrad Med J. 2000;76:424-426. [PubMed] |
| 9. | Yamamoto T, Takeuchi K, Okuda C, Nagashima N, Honjo H, Sakurai N, Yoshizaki H, Kuyama Y. Symptomatic hypothyroidism in decompensated liver cirrhosis. J Clin Gastroenterol. 2001;33:172-173. [PubMed] |
| 10. | Rimar D, Kruzel-Davila E, Dori G, Baron E, Bitterman H. Hyperammonemic coma-barking up the wrong tree. J Gen Intern Med. 2007;22:549-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Khairy RN, Mullen KD. Hypothyroidism as a mimic of liver failure in a patient with cirrhosis. Ann Intern Med. 2007;146:315-316. [PubMed] |
| 12. | Redkar N, Bendle M, Maidapwad V, Chavan R, Shriwastav R. Hepatic encephalopathy masking myxedema coma. J Assoc Physicians India. 2012;60:70-71. [PubMed] |
| 13. | Marti J, Portoles M, Jimenez-Nacher I, Cabo J, Jorda A. Effect of thyroid hormones on urea biosynthesis and related processes in rat liver. Endocrinology. 1988;123:2167-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Marchesini G, Fabbri A, Bianchi GP, Motta E, Bugianesi E, Urbini D, Pascoli A, Lodi A. Hepatic conversion of amino nitrogen to urea nitrogen in hypothyroid patients and upon L-thyroxine therapy. Metabolism. 1993;42:1263-1269. [PubMed] |