Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5115
Peer-review started: February 8, 2017
First decision: March 16, 2017
Revised: March 31, 2017
Accepted: May 9, 2017
Article in press: May 9, 2017
Published online: July 28, 2017
Processing time: 172 Days and 2.6 Hours
To assess dietary myo-inositol in reducing stem cell activation in colitis, and validate pβ-cateninS552 as a biomarker of recurrent dysplasia.
We examined the effects of dietary myo-inositol treatment on inflammation, pβ-cateninS552 and pAkt levels by histology and western blot in IL-10-/- and dextran sodium sulfate-treated colitic mice. Additionally, we assessed nuclear pβ-cateninS552 in patients treated with myo-inositol in a clinical trial, and in patients with and without a history of colitis-induced dysplasia.
In mice, pβ-cateninS552 staining faithfully reported the effects of myo-inositol in reducing inflammation and intestinal stem cell activation. In a pilot clinical trial of myo-inositol administration in patients with a history of low grade dysplasia (LGD), two patients had reduced numbers of intestinal stem cell activation compared to the placebo control patient. In humans, pβ-cateninS552 staining discriminated ulcerative colitis patients with a history of LGD from those with benign disease.
Enumerating crypts with increased numbers of pβ-cateninS552 - positive cells can be utilized as a biomarker in colitis-associated cancer chemoprevention trials.
Core tip: We report that dietary myo-inositol reduced inflammation and intestinal stem cell activation in both genetic and chemically-induced mouse models of colitis. In a limited clinical trial of colitis patients with a history of recurrent low grade dysplasia (LGD), myo-inositol reduced the number of intestinal crypts with activated stem cells. This study demonstrated for the first time that nuclear pβ-cateninS552 staining discriminates between patients with inflammation and those with a history of LGD, suggesting that nuclear pβ-cateninS552 staining may reflect local expansions of activated stem cells with neoplastic potential. Based on these data, we propose a more extensive clinical trial.
- Citation: Bradford EM, Thompson CA, Goretsky T, Yang GY, Rodriguez LM, Li L, Barrett TA. Myo-inositol reduces β-catenin activation in colitis. World J Gastroenterol 2017; 23(28): 5115-5126
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5115.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5115
Chronic inflammation in ulcerative colitis (UC) and Crohn’s disease (CD) contributes to an elevated risk of colitis-associated cancer (CAC)[1,2]. Patients with UC have an overall 11-fold relative risk of cancer and a 38-fold increased risk if they are diagnosed before age 30[1]. Similarly, patients with CD have an 18-fold increased risk of developing colorectal cancer[2]. In these diseases, chronic inflammation increases the risk of dysplasia through a variety of mechanisms. Oxidative stress, eicosanoid production and signaling through the cyclooxygenase/lipoxygenase pathways[3,4], apoptosis, altered metabolism, and signaling through phosphoinositide 3-kinase (PI3K) and Akt[5-7] all contribute to the transition from inflammation to dysplasia.
β-catenin translocation into the nucleus is a hallmark of colorectal cancer. Mutations in the genes encoding proteins in the phosphatase and tensin homolog (PTEN)/PI3K/Akt and Wnt/β-catenin pathways lead to colorectal cancer[8-11]. Mutations in adenomatous polyposis coli promote dysplasia and cancer by reducing β-catenin degradation and enhancing nuclear accumulation, detected by immunohistochemical staining. Additionally, Akt phosphorylation of β-catenin at serine 552 (pβ-cat) increases nuclear translocation of pβ-cat, upregulates TCF/LEF transcription, and increases in vitro tumor invasion[8]. Data from our group showed that mutations in PTEN enhance PI3K/Akt signaling and pβ-cat levels, leading to increased polyp formation[10]. These data suggest that alterations in PI3K and β-catenin signaling directly affect stem cell activation in dysplasia.
Under conditions of chronic inflammation, cells undergo neoplastic transformation after acquiring founder mutations. Volgelstein and others coined the term “field effect” to reflect the clonal expansion of mutated cells that cause areas of dysplasia to appear in the vicinity of sentinel areas of sporadic cancer or polyps[9,12]. In many cancers, including CAC, clonal fields of cells harboring DNA mutations surround visible dysplasia or carcinoma. Based on analysis of colitis-induced dysplasia, Brentnall and colleagues proposed that populations of intestinal epithelial cells[13] carrying p53 mutations spread to adjacent crypts by migrating over colonic surfaces[14]. Clevers and others suggest that the field effect originates in mutated intestinal stem cells[15,16]. We hypothesize that staining for nuclear pβ-cat provides a useful biomarker in identifying such a field effect.
Myo-inositol, a naturally abundant 6-carbon polyalcohol isolated from cereals (e.g., rough coat of rice), and its derivatives regulate a variety of intracellular pathways. Inositol polyphosphates are important as second messengers in signal transduction, act as anti-oxidants, and mediate calcium regulation in membrane signaling. Nuclear inositol signaling may also play a role in DNA repair and chromatin remodeling[11,17-21]. Due to these effects, inositol has been tested in a wide range of human clinical trials, including cancer prevention, autism, and psychiatric disorders.
Lam et al[22] conducted a dose escalation trial of myo-inositol in smokers with bronchodysplasia. They found that myo-inositol (9 g twice a day) reduced dysplasia, as well as Akt and phospho-ERK staining in dysplastic tissues[23]. Given our previous data linking PI3K signaling to β-catenin activation we considered the possibility that myo-inositol could reduce dysplasia by reducing ISC activation in colitis. However, before launching a large scale clinical trial, we wished to develop the means for detecting elevated numbers of activated ISC in colitis patients using pβ-cat staining. Additionally, we wished to determine whether this marker would serve as a reliable read-out of ISC chemoprevention.
C57BL/6 (WT) and C57BL/6 IL-10-/- (IL-10-/-) mice were purchased from Jackson Laboratories (Bar Harbor, ME, United States). Mice were maintained under specific pathogen free conditions at the University of Kentucky. All experiments were approved by the University of Kentucky IACUC.
Serum myo-inositol was measured using an enzymatic spectrophotometric assay as previously described[24]. Briefly, blood was collected by cardiac perfusion into untreated 1.5 mL tubes, incubated at room temperature for 1 h, centrifuged, and serum was collected and stored at -80 °C. Serum samples were treated with glucose oxidase and catalase (Calbiochem, San Diego, CA, United States), deproteinized with perchloric acid, and subsequently neutralized. Enzymatic determination of myo-inositol was done by reading the absorbance change at 546 nm from the reduction of Fe3+ - to Fe2+ -bathophenanthroline disulphonic acid (VWR, Radnor, PA, United States) from the NAD+-dependent oxidation of myo-inositol by myo-inositol dehydrogenase (Sigma, St.Louis, MO, United States). Myo-inositol concentration was determined from a calibration curve prepared with aqueous standards.
Thirteen IL-10-/- mice were divided into three groups: control (IL-10-/-), piroxicam-treated (IL-10-/- Px), and piroxicam-treated plus myo-inositol (IL-10-/- Px/myo-inositol). Piroxicam-containing chow was made by Harlan-Teklad (Madison, WI, United States). As previously described, 8-10 wk old IL-10-/- mice were fed chow containing 65 mg/250 g piroxicam for one week to synchronize colitis, followed by 85 mg/250 g piroxicam to induce colitis[25]. Mice were sacrificed 14 or 42 d later. For the group treated with myo-inositol, mice were treated with 1% (w/v) myo-inositol (Sigma) in the drinking water for one week prior to piroxicam treatment and for the duration of the experiment. For experiments of dextran sodium sulfate (DSS)-induced colitis, WT mice were treated with 2% (w/v) DSS (MP Biomedical, Santa Ana, CA, United States) for eight cycles. A single cycle of DSS consisted of 7 d of DSS in the drinking water followed by a recovery period of 14 d of regular water. Mice were treated with 1% myo-inositol during the recovery periods.
IL-10-/- mice undergoing treatment were scored daily or every other day using a standard disease activity index (DAI) based on diarrhea, fecal blood (Beckman Coulter SENSA Hemoccult Test), and percent weight loss as previously described[26]. Each criterion was assigned a score from 0 (no diarrhea, fecal blood or weight loss) to 4 (severe diarrhea, visible fecal blood, and up to 20% weight loss). The daily combined DAI score is the sum of each individual criterion.
Tissues were fixed in 4% neutral buffered formalin overnight, processed through paraffin, sectioned at 5 μm, and stained for hematoxylin and eosin (H&E). For murine tissues, colitis scores were calculated based on a graded scale of inflammation (0-3), extent (0-3), regeneration (0-4), crypt damage (0-4) and percent involvement (1-4) as previously described[27]. The combined colitis score is the sum of the scores for inflammation, extent, and crypt damage/regeneration.
For human sections, standard of care biopsies were processed, stained and analyzed by the pathology departments of the University of Chicago or Northwestern University. Slides were stained for H&E, β-catenin, p53 or Ki67 and staining patterns were interpreted by staff pathologists. For laboratory staining of anti-pβ-cateninS522 (pβ-cat) and Ki67, paraffin sections were rehydrated through graded alcohols and antigen retrieval was performed using Target Retrieval Solution (Dako, Carpinteria, CA, United States), pH 6.0, in a decloaking chamber. Sections were incubated with anti-pβ-cat (provided by Linheng Li, Stowers) or anti-Ki67 (TEC-3, Dako) followed by anti-rabbit or anti-mouse peroxidase-labeled polymer (Dako). Sections were developed using 3,3’-diaminobenzidine (DAB) tetrahydrochloride chromagen (Dako) and counterstained with hematoxylin.
IEC were isolated with EDTA and depleted of CD45 cells with sheep anti-rat IgG magnetic Dynabeads (Life Technologies, Grand Island, NY, United States) preloaded with rat anti-mouse CD45 antibody. For subcellular fractionation, all buffers contained ProteaseArrest™ protease inhibitor cocktail (G-Biosciences), and phosphatase inhibitor cocktails I and II (Sigma) in 1:100 dilution. IEC were homogenized in Buffer I (50 mmol/L Tris-HCl pH 7.4, 100 mmol/L NaCl, 0.01% digitonin), lysates were passed through 26G needle, then centrifuged at 4 °C for 10 min. Pellets were resuspended in Buffer II (50 mmol/L Tris-HCl pH 7.4, 2% Triton X100, 100 mmol/L NaCl) and incubated on ice for 30 min, then centrifuged as above. Pellets were dissolved in Buffer III (50 mmol/L Tris-HCl pH 7.4, 0.25% n-Dodecyl-D-maltoside, 100 mmol/L NaCl, 2 mmol/L MgCl2) and incubated with 2U of Benzonase (Sigma) per 100 μL of lysate for 30 min at room temperature. After centrifugation the resulting supernatant was used as the nuclear fraction.
Proteins were transferred to Immobilon FL (Millipore) membrane by semi-dry transfer (Bio-Rad, Hercules, CA) and membranes were blocked in Pierce Protein-free T20 blocking buffer (Thermo, Rockford, IL, United States) for 1 h. Primary antibodies for anti-pβ-cateninS522 (pβ-cat, provided by Linheng Li, Stowers), pAktS473 (Cell Signaling, Davers, MA, United States), and β-actin (Sigma) were diluted 1:1000 and incubated overnight at 4 °C. HRP-conjugated secondary antibodies (KPL, Gaithersburg, MD) were used at 0.02 μg/mL and blots were developed using West Pico ECL reagent (Thermo, Rockford, IL, United States).
NCM460 cells (normal derived colon mucosa) were received by a cell licensing agreement with INCELL Corporation (San Antonio, TX, United States), and were propagated in M3:10 medium with addition of the conditioned medium (30%) from previously cultured NCM460 cells. Cells were transfected with a reporter construct containing TCF/luc to evaluate β-catenin transcription[28]. Transfected cells were pre-treated for 3 h with 5 mmol/L myo-inositol and overnight with 5 mmol/L myo-inositol or 10 ng/mL of TNFα[29,30]. Luciferase was detected with Luciferase Reagent (Promega, Madison, WI, United States).
Colon biopsies were obtained from adult patients undergoing diagnostic or surveillance colonoscopy or surgical resection at Northwestern University or the University of Chicago. For the NCI myo-inositol clinical trial, all patients were diagnosed as having colitis-induced low grade dysplasia (LGD). Informed consent was obtained from every patient and samples were coded. Collection of all material was approved by Northwestern University IRB and sample analysis was approved by the University of Kentucky IRB. Some biopsies were collected under the NCI Myo-Inositol Chemoprevention in Colitis-Associated Dysplasia trial, #NWU09-13-02.
Three patients with LGD completed the 90-d clinical trial; 1 was given placebo and 2 were given myo-inositol. Colon biopsies were collected at the start of the study and again 90 d later. Participants were provided a 90 d supply of either maltodextrin (placebo) or myo-inositol in 9 g packets. Myo-inositol was manufactured by Tsuno Rice Fine Chemicals Co., Ltd (Wakayama, Japan) and filled into pouches by PharmOps, Inc (Phillipsburg, NJ, United States). Myo-inositol and the matching placebo were provided by NCI, DCP. For the first 14 d, participants dissolved one 9 g single-dose packet of study agent in 8 ounces of juice once daily. Following this initial period, participants escalated to 18 g/d taken in divided dose - one 9 g packet in the morning and one 9 g packet in the evening for the remaining 76 d. Patients were instructed to dose at approximately the same time each day, 2 h prior to eating. Pre- and post-study biopsies were assessed for general histological findings, IHC of p53 and Ki67, and patterns of nuclear pβ-cat.
Slides were scanned using an Aperio ScanScope XT™ slide scanner and visualized using ImageScope v11. A modified nuclear quantitation algorithm was used to identify DAB-positive and DAB-negative epithelial nuclei. Slides were assessed for pβ-cat staining using the following three criteria; (1) the number of crypts containing 2, 3, 4 or 5 pβ-cat-positive cells; (2) the number of high powered fields of view containing 3 or more crypts, each containing 2 or more pβ-cat-positive cells (clustering or “high frequency” fields of view); and (3) the number of pβ-cat-positive cells per 100 IEC. For analysis of proliferation, numbers of Ki67-positive cells per 100 IEC were counted in high frequency fields of view as well as adjacent tissue.
All pairwise comparisons were made using a Student’s t-test; P < 0.05 was considered statistically significant. Comparisons among multiple groups were made using a one-way ANOVA with post-hoc Tukey’s test.
Results from prior work indicate that myo-inositol inhibits PI3K and ERK signaling as evidenced by reduced p-Akt and p-ERK staining in dysplastic tissues[23]. Given our previous data linking PI3K signaling to β-catenin activation we considered the possibility that myo-inositol limits ISC activation in colitis[31]. The effects of myo-inositol on β-catenin activation were initially examined in the IL10-/- and DSS colitis models, which are known to progress from inflammation to CAC.
Initially, to establish the relationship between dietary intake and serum levels, myo-inositol levels was measured in untreated IL-10-/- mice given 1% w/v myo-inositol in their drinking water. Baseline myo-inositol concentrations in IL-10-/- mice were 2.0 ± 0.2 μmol/L, while provision of 1% myo-inositol in the drinking water raised serum myo-inositol concentrations 2.4-fold, to 4.9 ± 0.5 μmol/L, P = 0.02. Although our experiments were conducted with 1% myo-inositol, we tested the effect of 2% myo-inositol as well, and serum levels increased to 14.6 ± 1.1 μmol/L (P = 0.01 relative to 1% myo-inositol). These data document the correlation between dietary and serum levels of myo-inositol as used in these studies.
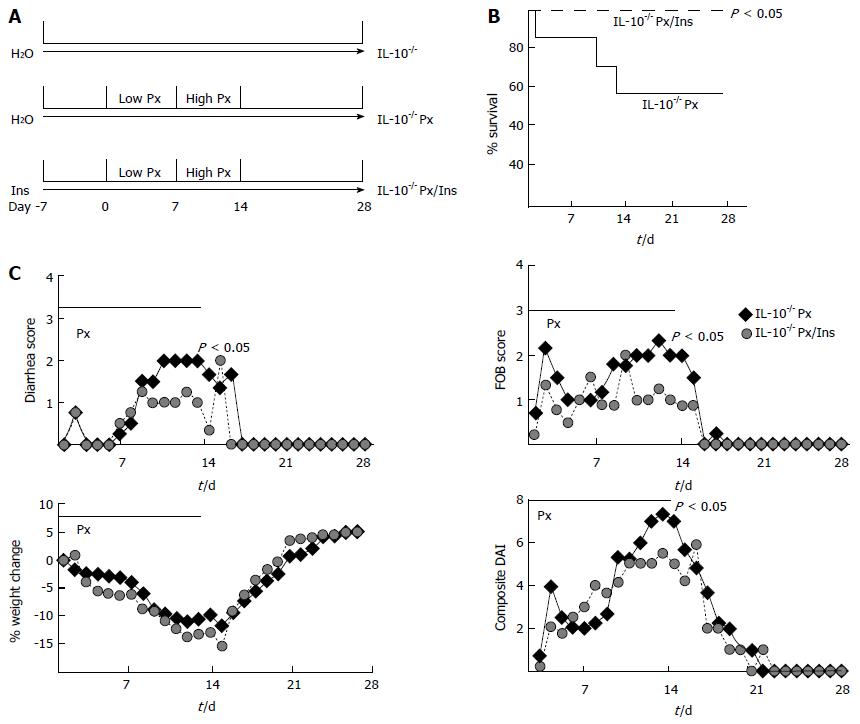
Piroxicam (Px) was used to induce colitis in IL-10-/- mice, and a cohort of these mice was treated with 1% myo-inositol in the drinking water (Figure 1A). While no IL-10-/- or IL-10-/- Px/myo-inositol mice died during the study, 42% of the IL-10-/- Px mice died during the second week of Px treatment (Figure 1B). Myo-inositol significantly reduced mean diarrhea scores in IL-10-/- Px mice from 2.3 ± 0.6 to 0.8 ± 0.3 (P = 0.02), and occult fecal blood scores from 2.0 ± 0.4 to 0.9 ± 0.3 (P = 0.02) (Figure 1C). There was no significant change in body weight between myo-inositol-treated and untreated mice, though myo-inositol may have contributed to increased caloric intake. Composite DAI scores were reduced by myo-inositol treatment (Figure 1C). Given that myo-inositol has anti-inflammatory effects[32], we next examined tissue histology in IL-10-/- Px and IL-10-/- Px/myo-inositol mice.
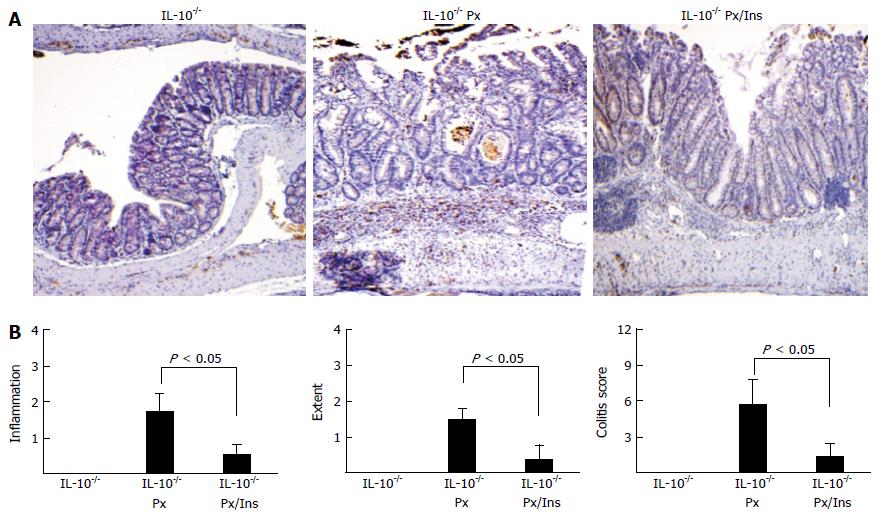
Tissue analysis revealed that myo-inositol significantly reduced inflammation in colonic tissue from IL-10-/- Px mice (Figure 2A and B). Images show that myo-inositol normalizes crypt distortion, transmural inflammation, and ulceration induced by Px in IL-10-/- mice. These changes correlate with increased myeloperoxidase (MPO) staining in IL-10-/- Px mice and greatly reduced MPO staining in IL-10-/- Px/myo-inositol mice (Figure 2A). This reduction was particularly evident near the junction of the proximal and distal colon, where IL-10-/- Px mice develop large lymphoid aggregates, ulcerated thickened mucosa, and crypt distortion. Histological grading of colitis (Methods) revealed that myo-inositol reduces inflammation scores, the extent of colitis, and composite colitis scores (Figure 2B). Inflammation scores were reduced in myo-inositol-treated IL-10-/- Px mice from 3.3 ± 1.9 to 1.0 ± 0.7 (P < 0.05) and myo-inositol completely abrogated crypt damage. Treatment with myo-inositol reduced the extent of colitis in IL-10-/- Px mice from 2.5 ± 1.2 to 0.5 ± 0.5 (P < 0.05).
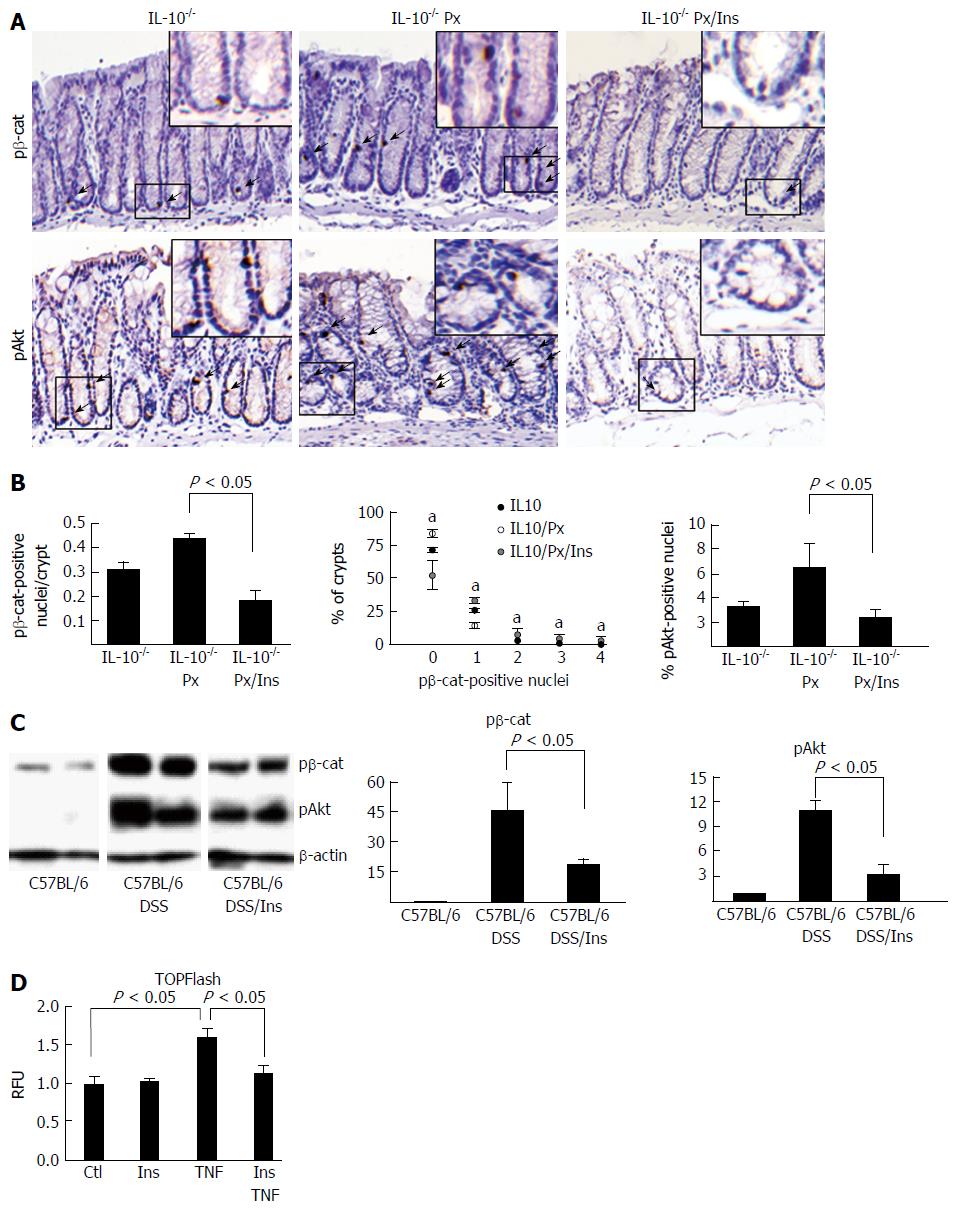
pβ-cat staining can be used as a marker of activated stem and progenitor cells[25]. Thus, we examined the effect of myo-inositol on pβ-cat and pAkt levels in the IL-10-/-mice (Figure 3A and B). Results show that myo-inositol abrogates colitis-induced pβ-cat induction. Cell counting revealed that the number of pβ-cat-positive nuclei dropped by 63% in myo-inositol-treated IL-10-/- Px mice from 0.3 ± 0.02 to 1.9 ± 0.02 (P = 0.004). Analysis of the crypt distribution of pβ-cat demonstrated that in IL-10-/- Px mice there were significantly more crypts with > 2 pβ-cat positive nuclei in mice with active colitis. By comparison, myo-inositol treatment normalized pβ-cat levels in IL-10-/-/Px mice (Figure 3B). Similar effects of myo-inositol were observed with pAkt staining, in which myo-inositol reduced pAkt nuclear staining from 6.4 ± 2.0 to 2.3 ± 0.7 (P < 0.05) percent. Together these data suggest that myo-inositol impairs PI3K and β-catenin activation in colitis.
Previously we (GY) found that myo-inositol feeding reduced the incidence of cancer in a model of chronic DSS colitis[32]. To determine whether myo-inositol reduced levels of pβ-cat in these mice, we assessed protein levels of pβ-cat in isolated IEC from controls and mice treated with eight cycles of DSS with or without myo-inositol (Figure 3C). Levels of pAkt were assessed to determine if myo-inositol reduces this potential activator of b-catenin signaling. WB data show that myo-inositol reduced pβ-cat levels by 59%, from 45 ± 14-fold to 18 ± 4-fold, relative to DSS-treated controls (P = 0.04). Nuclear pAkt was reduced by 73%, from 11 ± 1-fold to 3 ± 1-fold (P = 0.006). Together, data from two colitis models indicate that oral myo-inositol reduces both pβ-cat and PI3K signaling in colitis.
To determine if myo-inositol can reduce inflammation-induced β-catenin activation, we used the in vitro TOPFlash assay to assess β-catenin activity in response to treatment with TNF and myo-inositol. Myo-inositol treatment alone had no discernible effect on baseline β-catenin activation. NCM cells treated with 10 ng/mL TNF showed a 60% increase in β-catenin activation (1.00 ± 0.08 vs 1.6 ± 0.12, P < 0.05), which was abrogated by co-treatment with 5 mmol/L myo-inositol (1.6 ± 0.12 vs 1.13 ± 0.09, P < 0.05; Figure 3D). These findings provide evidence that myo-inositol directly impacts Wnt/β-cat signaling.
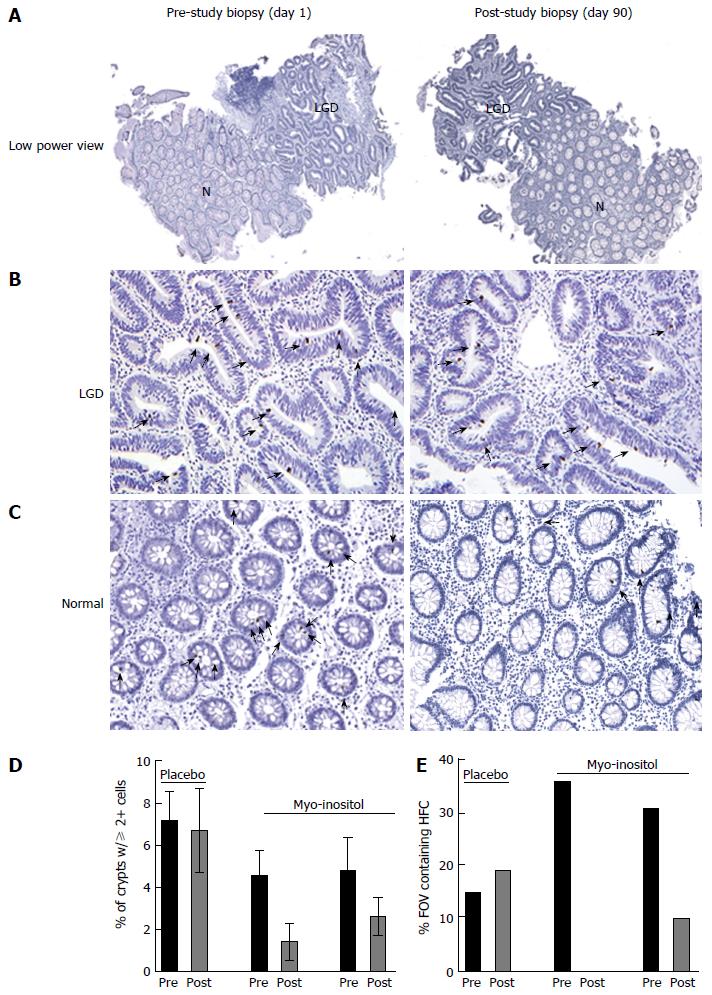
Several studies, in both human and mice, have shown the ability of myo-inositol to reduce dysplasia[23,32]. Given the chemopreventive effects of myo-inositol on CAC in mice[32] and the inhibition of β-catenin and PI3K signaling reported here, we postulated that myo-inositol may also reduce ISC activation and CAC in patients with longstanding colitis and a history of recurrent dysplasia. To examine whether myo-inositol can be chemopreventive in CAC, we proposed a 3 mo trial in which myo-inositol was given to UC patients with LGD. We proposed that the frequency of pβ-cat-positive IEC would decrease in response to dietary myo-inositol treatment. Due to the challenges of finding colitis patients with current LGD at the time of consent, we were able to enter only five patients into the trial. One patient dropped out due to adverse events (diarrhea, abdominal pain, flatulence, hemorrhoids and increased serum bilirubin), though it is not clear that these were study-related. One patient had the area of LGD removed with the initial biopsy, precluding further analysis at the completion of the study. Three patients completed the study; 1 treated with placebo and 2 treated with myo-inositol. Statistical analysis of this small study size is not possible. However, the results of the staining were used to identify a trend of myo-inositol-induced reductions in the number of crypts with high numbers of pβ-cat-positive intestinal epithelial cells.
Data from the myo-inositol-treated patients who completed the study did not show reductions in established dysplasia (as in Figure 4A and B). However, in myo-inositol-treated patients there was a global reduction in the percentage of crypts containing two or more pβ-cat-positive cells in areas adjacent to LGD, which was not observed in the placebo-treated patient (Figure 4C). While analyzing the data from the 3 patients in the study, we observed “clusters” of crypts containing 2 or more pβ-cat-positive cells in non-dysplastic areas where pβ-cat staining was otherwise very sparse (Figure 4C). This aggregation of crypts with higher numbers of pβ-cat-positive cells is suggestive of a field effect, in which foci of activated ISC are produced by expansion of mutated cells[14]. In order to quantify this effect, we counted the percentage of high power fields of view (20X) in which there were at least 3 crypts containing 2 or more pβ-cat-positive cells, henceforth defined as high frequency crypts (HFC). In the placebo-treated patient, the percent of HFC at day 1 and day 90 was 15% and 19%, respectively. However, in the patients treated with myo-inositol, the percentages of HFC at day 1 were 36% and 31%, which were reduced to 0% and 10%, respectively, by day 90 (Figure 4D). Although these findings are far from conclusive, they support the notion that myo-inositol reduces the frequency of activated stem cells in patients at high risk for CAC.
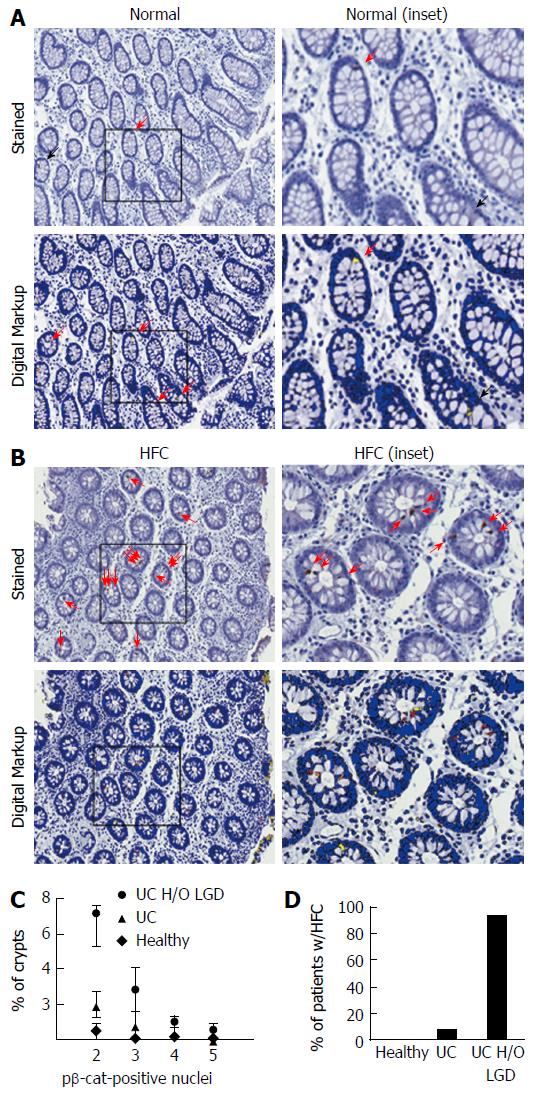
We previously observed that numbers of pβ-cat-positive IEC are increased in patients within areas of colitis-induced LGD and cancer, and inflammation[25]. However, the observation of pβ-cat-positive cell clustering within non-dysplastic tissue adjacent to areas of colitis-induced dysplasia led us to speculate that patients with a history of recurrent LGD could be stratified based on the presence of HFC. To test this hypothesis, a digital algorithm was developed to objectively distinguish pβ-cat-positive and negative nuclei in human biopsies. This allowed us to more accurately compare staining of tissues that were processed and stained by a variety of institutions and laboratory personnel.
Digital analysis of sections improved the quality of data generated by discriminating true positive and true negative cells (Figure 5A and B). In some cases, digital imaging identified positive cells that were not detected by trained observers (Figure 5A, black arrow). In other sections, the computer algorithm correctly discriminated false positives. The ability to accurately identify positive and negative nuclei significantly increased the sensitivity of pβ-cat staining.
To test the hypothesis that HFC could be used to stratify patients with a history of LGD, biopsies from healthy control patients (n = 3), patients with a diagnosis of active UC (n = 12), and UC patients with a history of LGD (n = 16) were stained for pβ-cat. In patients with a history of LGD, biopsies were taken from histologically normal areas of prior LGD, usually near mucosal tattoos marking resected LGD. In these tissues, we assessed the number of pβ-cat-positive cells within individual crypts and portrayed the relative distribution of crypts with distinct numbers of pβ-cat-positive cells/crypt (e.g., percentage of total crypts with 2,3,4 or 5 pβ-cat-positive cells/crypt) (Figure 5C). In order to stratify patients, histologically normal tissues from areas of previous dysplasia were evaluated for HFC (Figure 5D). When we calculated the percentage of patients that had at least one HFC, 8.3% (1 out of 12) patients with benign, active UC had HFC whereas 94% of patients with a history of LGD had at least one HFC (15 out of 16). These findings suggest that focal increases in pβ-cat staining identify patients at risk for dysplasia. If further validated in more extensive clinical trials, this biomarker may provide a useful means for testing chemopreventive agents such as myo-inositol.
The data presented in this study suggest that myo-inositol is a valuable chemopreventive agent that abrogates aberrant activation of ISC during inflammation and reduces the transition to dysplasia. Additionally, these data demonstrate that pβ-cat staining can be used to identify HFC within “pre-dysplastic” regions in patients with a previous history of colitis-induced dysplasia. A central hypothesis addressed in these studies was that myo-inositol reduces intestinal stem cell activation, detected by reduced nuclear pβ-cat and pAkt staining. Previous studies indicate that inositol compounds have both anti-inflammatory and chemopreventive properties[22,23,32-35]. Data here show that myo-inositol treatment reduces Akt activation in colitis. Additionally, we show that myo-inositol reduces β-catenin signaling, a key step in ISC activation. Prior data from our group demonstrated that Akt cooperates with Wnt to activate β-catenin[25]. In the models tested here, it is possible that PI3K inhibition by myo-inositol impaired Wnt signaling via reduced β-cat activation.
Because we were interested in early effects of myo-inositol during inflammation-induced neoplastic transformation, we analyzed data from IL-10-/- mice 14 d after the induction of colitis, 4 wk before the onset of dysplasia. The data presented here demonstrate that myo-inositol reduced inflammation, Akt activation, and pβ-cat nuclear localization. Additionally, in experiments with WT mice on 8 cycles of DSS, analysis of proteins from isolated IEC revealed that myo-inositol reduced nuclear pβ-cat and pAkt. In the same model, we reported that myo-inositol reduced tumor incidence, tumor multiplicity, and tumor volume[32]. Based on these results we propose that myo-inositol may reduce CAC by decreasing the numbers of activated ISC that may harbor pro-oncogenic genetic alterations.
5-ASA, a common therapeutic used in the treatment of UC, is a potent oxygen radical scavenger and has anti-proliferative and pro-apoptotic effects. 5-ASA reduces both pAkt and pβ-cat nuclear localization in human biopsies and in the IL-10-/- Px model of CAC[36,37]. However, in areas of established dysplasia, 5-ASA did not reduce proliferation or staining for pβ-cat and pAkt. We propose that like 5-ASA, myo-inositol reduces Akt/β-catenin signaling in the early stages of dysplastic transformation but not once it is established. We posit that 5-ASA and myo-inositol reduce CAC both by reducing inflammation-induced Wnt signaling in activated ISC at risk for acquiring founder mutations.
To address the question of whether a reduction in pβ-cat staining could be a biomarker of “fields” of pre-dysplastic cells in patients, we analyzed colonic biopsies from UC patients with benign disease or a history of LGD. We detected more regions with a high frequency of pβ-cat -positive nuclei (HFC) in tissue from patients with a history of LGD compared to those with benign disease. Using the criterion that tissue sections must have high powered fields of view with three or more crypts containing two or more pβ-cat-positive IEC, we were able to distinguish sections from UC patients from those who had a history of LGD and those who did not. We hypothesize that these data reflect local expansions of activated ISC harboring mutations that affect Wnt/β-catenin signaling. Inflammation-induced carcinogenic genetic and epigenetic changes may transmit a survival advantage to select ISC populations. Thus, our observations suggest that higher numbers of activated ISC may be a biomarker for areas of tissue at higher risk for neoplastic transformation. What remains unclear is whether myo-inositol primarily affects ISC by reducing inflammation, pro-carcinogenic inflammatory insults and/or by altering critical ISC signaling pathways (e.g., PI3K, Wnt/β-catenin) relevant to carcinogenesis. In unpublished studies, we found that enhanced nuclear staining of pβ-cat correlates highly with increased TCF4/LEF immunoprecipitiation and increased expression of downstream TCF4 target genes. We propose that reductions of β-catenin activity in stem cells by myo-inositol are an essential mechanism for its chemopreventive properties. Because dysplasia typically arises from expansion of clonal stem cells that harbor mutations in key oncogenic processes[9,38,39], reducing the numbers of activated stem cells in colitis would presumably lower the risk for generating neoplastic crypts. Additionally, myo-inositol is a potent anti-oxidant, and as such it may also reduce DNA damage caused by reactive oxygen species. We hypothesize that myo-inositol may exert chemopreventive properties through both of these mechanisms.
In order to test the hypothesis that myo-inositol can be used as a chemopreventive in CAC, we propose a revised clinical trial. In this trial, UC patients would be recruited who have history of recurrent LGD and evidence of elevated pβ-cat staining in areas adjacent to prior dysplasia. Myo-inositol would be given long-term (1 year or more) and the primary endpoints would include (1) a reduction in the recurrence of LGD; and (2) normalized or reduced pβ-cat staining in areas proximal to LGD. The primary goal would not be the reversal of LGD, but rather the prevention of stem cell activation in “high risk” areas of pre-dysplastic fields resulting in reduced emergence of new LGD. The goal would be to abort the progression to recurrent dysplasia in patients who have a history of recurrent disease. The clear benefit would be the provision of a safe, low cost agent for patients who are found to have recurrent LGD during surveillance colonoscopy for colitis.
Patients with inflammatory bowel disease (IBD) have an increased risk of dysplasia and colon cancer due to chronic inflammation. Developing a biomarker and identifying areas of the colon that harbor the potential for neoplastic transformation prior to the onset of histologically-identifiable dysplasia is critical for early treatment and cancer prevention. Additionally, there is a need for inexpensive, accessible pharmaceuticals to prevent recurrent dysplasia and the progression to cancer in IBD patients.
Colitis-associated cancer (CAC) is a significant concern for patients with chronic inflammatory diseases, such as IBD. Although largely preventable by stringent surveillance colonoscopy and endoscopic or surgical removal of dysplastic tissue, many patients with limited access to health care resources remain at high risk for developing cancer. Myo-inositol is a safe, affordable, accessible sugar alcohol with limited side-effects and an established history in many clinical trials. We posit that myo-inositol is an attractive treatment option for patients at high risk for recurrent colonic dysplasia.
This study demonstrated that nuclear staining patterns of Akt-phosphorylated β-catenin (pβ-cateninS552) positively correlate with levels of inflammation and proliferation in both mouse and human models of intestinal inflammation and IBD. Additionally, nuclear staining patterns of pβ-cateninS552 can be used to stratify patients with benign colitis and those with a history of dysplasia. In mouse models of colitis, oral myo-inositol significantly reduced inflammation and numbers of activated stem cells. In a limited clinical trial, myo-inositol treatment did not reduce dysplasia, but successfully altered the pattern of pβ-cateninS552 staining in areas adjacent to dysplasia, suggesting a role for oral myo-inositol in limiting stem cell activation during colitis.
In order to further test the hypothesis that myo-inositol can be used as a chemopreventive in CAC, the authors propose a revised clinical trial. In this trial, ulcerative colitis patients would be recruited who have history of recurrent low grade dysplasia (LGD) and evidence of elevated pβ-cat staining in areas adjacent to prior dysplasia. Myo-inositol would be given long-term and primary endpoints would include a reduction in the recurrence of LGD and normalization of pβ-cat staining in areas proximal to LGD. The primary goal would be the prevention of stem cell activation in “high risk” areas of pre-dysplastic fields resulting in reduced emergence of new LGD. The clear benefit would be the provision of a safe, low cost agent for patients who are found to have recurrent LGD during surveillance colonoscopy for colitis.
LGD: Mild architectural abnormalities characterized by basally-oriented nuclei, mild nuclear enlargement, and nuclear crowding.
This paper describe some important things about: pβ-cateninS552 staining faithfully reported the effects of myo-inositol in reducing inflammation and intestinal stem cell activation in mice.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Luo HS S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1198] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 2. | Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590-1592. [PubMed] |
| 3. | Sheehan KM, O’Connell F, O’Grady A, Conroy RM, Leader MB, Byrne MF, Murray FE, Kay EW. The relationship between cyclooxygenase-2 expression and characteristics of malignant transformation in human colorectal adenomas. Eur J Gastroenterol Hepatol. 2004;16:619-625. [PubMed] |
| 4. | Catalano A, Procopio A. New aspects on the role of lipoxygenases in cancer progression. Histol Histopathol. 2005;20:969-975. [PubMed] |
| 5. | Khaleghpour K, Li Y, Banville D, Yu Z, Shen SH. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3’-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426-7429. [PubMed] |
| 7. | Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497-5510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1330] [Cited by in RCA: 1448] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 8. | Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221-11229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 727] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 9. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] |
| 10. | He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 11. | York JD, Hunter T. Signal transduction. Unexpected mediators of protein phosphorylation. Science. 2004;306:2053-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Leedham SJ, Graham TA, Oukrif D, McDonald SA, Rodriguez-Justo M, Harrison RF, Shepherd NA, Novelli MR, Jankowski JA, Wright NA. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;136:542-550.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 13. | Kriebitz NN, Kiecker C, McCormick L, Lumsden A, Graham A, Bell E. PRDC regulates placode neurogenesis in chick by modulating BMP signalling. Dev Biol. 2009;336:280-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC, Burmer GC. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369-378. [PubMed] |
| 15. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2885] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 16. | Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep. 2014;15:62-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Steger DJ, Haswell ES, Miller AL, Wente SR, O‘Shea EK. Regulation of chromatin remodeling by inositol polyphosphates. Science. 2003;299:114-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Chi TH, Crabtree GR. Perspectives: signal transduction. Inositol phosphates in the nucleus. Science. 2000;287:1937-1939. [PubMed] |
| 19. | Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. Phosphorylation of proteins by inositol pyrophosphates. Science. 2004;306:2101-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Bennett M, Onnebo SM, Azevedo C, Saiardi A. Inositol pyrophosphates: metabolism and signaling. Cell Mol Life Sci. 2006;63:552-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Martelli AM, Follo MY, Evangelisti C, Falà F, Fiume R, Billi AM, Cocco L. Nuclear inositol lipid metabolism: more than just second messenger generation? J Cell Biochem. 2005;96:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Lam S, McWilliams A, LeRiche J, MacAulay C, Wattenberg L, Szabo E. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Han W, Gills JJ, Memmott RM, Lam S, Dennis PA. The chemopreventive agent myoinositol inhibits Akt and extracellular signal-regulated kinase in bronchial lesions from heavy smokers. Cancer Prev Res (Phila). 2009;2:370-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Dolhofer R, Wieland OH. Enzymatic assay of myo-inositol in serum. J Clin Chem Clin Biochem. 1987;25:733-736. [PubMed] |
| 25. | Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, May R, Yang GY, Ragheb JW, Evers BM. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869-881, 881.e1-881.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 27. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] |
| 28. | Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391-399. [PubMed] |
| 29. | Kim HJ, Jang YM, Kim H, Kwon YH. Apoptotic effect of IP(6) was not enhanced by co-treatment with myo-inositol in prostate carcinoma PC3 cells. Nutr Res Pract. 2007;1:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Treede I, Braun A, Jeliaskova P, Giese T, Füllekrug J, Griffiths G, Stremmel W, Ehehalt R. TNF-alpha-induced up-regulation of pro-inflammatory cytokines is reduced by phosphatidylcholine in intestinal epithelial cells. BMC Gastroenterol. 2009;9:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Brown JB, Cheresh P, Goretsky T, Managlia E, Grimm GR, Ryu H, Zadeh M, Dirisina R, Barrett TA. Epithelial phosphatidylinositol-3-kinase signaling is required for β-catenin activation and host defense against Citrobacter rodentium infection. Infect Immun. 2011;79:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Liao J, Seril DN, Yang AL, Lu GG, Yang GY. Inhibition of chronic ulcerative colitis associated adenocarcinoma development in mice by inositol compounds. Carcinogenesis. 2007;28:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Vucenik I, Shamsuddin AM. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J Nutr. 2003;133:3778S-3784S. [PubMed] |
| 34. | Vucenik I, Shamsuddin AM. Protection against cancer by dietary IP6 and inositol. Nutr Cancer. 2006;55:109-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Gustafson AM, Soldi R, Anderlind C, Scholand MB, Qian J, Zhang X, Cooper K, Walker D, McWilliams A, Liu G. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med. 2010;2:26ra25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 36. | Managlia E, Katzman RB, Brown JB, Barrett TA. Antioxidant properties of mesalamine in colitis inhibit phosphoinositide 3-kinase signaling in progenitor cells. Inflamm Bowel Dis. 2013;19:2051-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Brown JB, Lee G, Managlia E, Grimm GR, Dirisina R, Goretsky T, Cheresh P, Blatner NR, Khazaie K, Yang GY. Mesalamine inhibits epithelial beta-catenin activation in chronic ulcerative colitis. Gastroenterology. 2010;138:595-605, 605.e1-605.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Novelli MR, Williamson JA, Tomlinson IP, Elia G, Hodgson SV, Talbot IC, Bodmer WF, Wright NA. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science. 1996;272:1187-1190. [PubMed] |
| 39. | Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 861] [Article Influence: 66.2] [Reference Citation Analysis (0)] |