Published online Jul 21, 2017. doi: 10.3748/wjg.v23.i27.4942
Peer-review started: March 29, 2017
First decision: April 25, 2017
Revised: May 11, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: July 21, 2017
Processing time: 120 Days and 0.7 Hours
To evaluate maternal hepatitis B virus (HBV) DNA as risk for perinatal HBV infection among infants of HBV-infected women in California.
Retrospective analysis among infants born to hepatitis B surface antigen (HBsAg)-positive mothers who received post vaccination serologic testing (PVST) between 2005 and 2011 in California. Demographic information was collected from the California Department of Public Health Perinatal Hepatitis B Program databaseand matched to birth certificate records. HBV DNA level and hepatitis B e antigen (HBeAg) status were obtained from three large commercial laboratories in California and provider records if available and matched to mother infant pairs. Univariate analysis compared infected and uninfected infants. Multivariate analysis was restricted to infected infants and controls with complete maternal HBV DNA results using a predefined high HBV DNA level of > 2 × 107 IU/mL, a 5:1 ratio of cases to controls and a two-sided confidence level of 95%.
A total of 17687 infants were born to HBsAg positive mothers in California between Jan 1 2005 and Dec 31, 2011. Among 11473 infants with PVST, only 125 (1.1%) were found to be HBV infected. Among these infected infants, lapses in Advisory Committee on Immunization Practices recommended post exposure prophylaxis (PEP) occurred in only 9 infants. However, PEP errors were not significantly different between infected and uninfected infants. Among the 347 uninfected and infected infants who had maternal HBeAg and HBV DNA level, case-control analysis found HBeAg positivity (70.4% vs 28.9%, OR = 46.76, 95%CI: 6.05-361.32, P < 0.001) and a maternal HBV DNA level ≥ 2 × 107 IU/mL (92.6% vs 18.5%, OR = 54.5, 95%CI: 12.22-247.55, P < 0.001) were associated with perinatal HBV infection. In multivariate logistic regression, maternal HBV DNA level ≥ 2 × 107 IU/mL was the only significant independent predictor of perinatal HBV infection.
In California, transmission is low and most infected infants receive appropriate PEP and vaccination. Maternal HBV DNA ≥ 2 × 107 IU/mL is associated with high risk of perinatal infection.
Core tip: Most infants born to hepatitis B surface antigen positive woman in California received appropriate post exposure prophylaxis and vaccination but a low postvaccination serologic testing rate represents a missed opportunity to identify chronically infected infants needing lifelong medical care. Overall the perinatal transmission rate in California is low at 1.1% and only high maternal hepatitis B virus (HBV) DNA level predicts risk for perinatal transmission. Maternal HBV DNA is a vital prenatal screen if targeted antiviral therapy for high-risk mothers becomes a strategy to reduce transmission.
- Citation: Burgis JC, Kong D, Salibay C, Zipprich J, Harriman K, So S. Perinatal transmission in infants of mothers with chronic hepatitis B in California. World J Gastroenterol 2017; 23(27): 4942-4949
- URL: https://www.wjgnet.com/1007-9327/full/v23/i27/4942.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i27.4942
Perinatal hepatitis B virus (HBV) infection is associated with a 90% risk for chronic infection, which carries a 25% risk of death from liver failure and hepatocellular carcinoma[1]. The United States Advisory Committee on Immunization Practices (ACIP) recommends and California regulations require testing of all pregnant women for hepatitis B surface antigen (HBsAg)[2]. ACIP also recommends that infants born to HBsAg-positive mothers receive post-exposure prophylaxis (PEP) including hepatitis B immunoglobulin (HBIG) and the first dose of HBV vaccine within 12 h of birth; complete the HBV vaccine series; and receive postvaccination serologic testing (PVST), including testing for HBsAg (infection) and antibody to HBsAg (immunity) between 9-18 mo of age[3].
International studies have shown that maternal hepatitis B e antigen (HBeAg) positivity and high maternal HBV DNA levels (≥ 2 × 107 IU/mL or 108 copies/mL) are the most significant risk factors for perinatal transmission[4-7]. Perinatal HBV infection has been shown to correlate linearly with increasing HBV DNA levels[6,7]. Studies have not shown a consistent relationship between perinatal transmission and other risk factors such as delivery mode, invasive procedures during pregnancy, or breastfeeding[8-10]. Antiviral therapy for pregnant women with high HBV DNA levels has been proposed to reduce perinatal transmission, but its use is not yet standard of care[11-14]. The exact viral load at which to initiate antivirals to prevent transmission has yet to be established.
To explore risk factors for perinatal transmission in California, we conducted a retrospective analysis of infants born between 2005 and 2011 to HBsAg-positive mothers. Our objective was to evaluate, the effect of maternal HBeAg status and HBV DNA levels on perinatal HBV infection.
Each year, approximately 2500 pregnant women with chronic HBV infection are reported to the CDPH Perinatal Hepatitis B Prevention Program (PHPP). California local health jurisdictions monitor mother-infant pairs to ensure compliance with ACIP recommendations for the prevention of perinatal HBV infection and submit demographic data, laboratory results, times of HBIG and HBV vaccine administration at birth and completion of the HBV vaccine series, and PVST results to the CDPH PHPP. Data are entered and maintained at CDPH; internal logic checks and record reviews are used to verify data quality.
A retrospective study to determine risk factors for perinatal HBV infection was conducted. Eligible infants were those born to HBV-infected women between January 1, 2005 and December 31, 2011 whose HBV infection status was known via PVST. A case control analysis was conducted for mothers who had HBV DNA testing within one year of the infant’s birth. Case infants were those with positive HBsAg results indicating perinatal infection; control infants were those with negative HBsAg results. All control mothers also had HBeAg testing within one year of the infant’s birth. Infants born during the study period who were younger siblings of another case or control infant were excluded.
Birth certificate data on maternal race and ethnicity, birthplace, education level and insurance status during pregnancy and details about the delivery were obtained for all infants. Information on infant sex, gestational age at birth, birth weight, the administration of HBIG and HBV vaccine at birth, and completion of a three or four dose HBV vaccine series for all infants was obtained from the CDPH PHPP, local health jurisdictions, clinic and birth hospital records, and birth certificates. Based on ACIP recommendations, infants without documentation of administration of HBIG and HBV vaccine within 12 h birth were considered to have a PEP error. Infants without documentation of at least three doses of HBV vaccine by 8 mo of age (245 d) or 4 doses of vaccine by 12 mo of age (366 d) were considered to have a vaccine series error. Infants weighing less than 2000 g at birth without documentation of three HBV vaccine doses after the birth dose were also considered to have a vaccine series error[3].
HBeAg and HBV DNA test results for women of childbearing age 14 to 45 years between January 1, 2005 and December 31, 2011 were requested from three large reference laboratories that serve most patients in California: Quest Diagnostics (Madison, NJ, United States), LabCorp (Burlington, NC, United States), and ARUP Laboratories (Salt Lake City, UT, United States). These results were matched to PHPP records by mother’s name, date of birth, and proximity of mother’s residence zip code to the ordering provider’s zip code. Laboratory tests performed more than one year from the infant’s date of birth were excluded. When multiple laboratory results matched to a mother, the result closest to the infant’s date of birth was included. For all cases, maternal prenatal care providers were contacted to obtain any additional HBV DNA and HBeAg results that were available.
Given a limited case sample size of 27 infants, of whom 92% met the predefined exposure of HBV DNA level > 2 × 107 IU/mL, a 5:1 ratio of cases to controls, and a two-sided confidence level of 95%, our case control analysis had more than 95% power to detect an odds ratio of greater than 50. Group differences for continuous variables were examined using the Mann-Whitney test or Student’s t-test (α = 0.05, two-tail). OR and 95%CI were calculated for categorical variables using logistic regression. OR and CI for race were calculated using the Mantel Haenszel test. Variables with a P value < 0.10 in univariate analysis were included in a multivariate logistic regression analysis using the backward stepwise method with a removal standard of 0.05. Multivariate analysis was restricted to cases and controls with complete maternal HBV DNA results. All analyses were performed using SAS for Windows (version 9.3, SAS Institute, Cary, NC, United States). The statistical methods of this study were reviewed by Jennifer Zipprich from the Immunization Branch of the California Department of Public Health. Technical appendix, statistical code, and dataset are available upon request from Jennifer Zipprich (jennifer.zipprich@cdph.ca.gov). This study was reviewed by the California Health and Human Services Agency Committee for the Protection of Human Subjects and determined to be “not research” and was approved by the Stanford University Human Subjects Research and Institutional Review Board. A waiver of consent was granted for this study; the presented data are anonymized and the risk of identification is low. There are no conflicts of interest to disclose. No animal studies were conducted.
Of 17687 infants born and enrolled in PHPP during January 1, 2005 - December 31, 2011, 11473 (64.9%) had complete PVST results, and 347 (3%) of their mothers had HBV DNA testing within one year of the infant’s birth. Of the infants with PVST results, 125 (1.1%) were HBsAg-positive.
Of the 125 infected infants, 115 (92%) infants received appropriate PEP at birth and the PEP status of one adopted infant was unknown. Of the 9 infected infants who did not receive appropriate PEP at birth, 3 did not receive HBIG and 6 received PEP greater than 12 h after birth. Additionally, three infected infants did not complete the vaccine series in the appropriate time period.
Of the 125 infected infants, 27 (21.6%) had maternal HBV DNA results and were eligible for case selection. Of the 11348 uninfected infants, 320 (2.8%) had maternal HBV DNA and HBeAg results and were eligible for control selection. Of eligible controls, 135 were randomly selected for a ratio of 5 controls for every case with maternal HBV DNA results for the case control analysis.
The case (infected) and control (uninfected) infants were similar with respect to sex, birthweight and gestational age at birth (Table 1). There was no significant difference in PEP errors between case and control infants. The most common error noted was late or incomplete HBV vaccine series. There was also no significant difference in infant age at administration of HBIG and HBV vaccine at birth or vaccine series completion between cases and controls (data not shown, P = 0.49, P = 0.79, P = 0.43, respectively).
| Infant characteristics | Cases (n = 27) | Controls (n = 135) | OR | P value |
| Sex | ||||
| Male | 17 (63.0) | 73 (54.1) | Reference | |
| Female | 10 (37.0) | 62 (45.9) | 0.693 (0.296-1.623) | 0.398 |
| Birthweight1 | ||||
| > 2500 g | 25 (92.6) | 121 (89.6) | Reference | |
| < 2500 g | 2 (7.4) | 14 (10.4) | 0.692 (0.148-3.235) | 0.640 |
| Gestational age2 | ||||
| Full term | 25 (92.6) | 116 (85.9) | Reference | |
| Preterm | 2 (7.4) | 17 (12.6) | 0.546 (0.118-2.515) | 0.437 |
| Unknown | 0 (0) | 2 (1.5) | - | - |
| Errors with HBIG or Birth Dose | ||||
| None | 26 (96.3) | 132 (97.8) | Reference | |
| Any | 1 (3.7) | 3 (2.2) | 1.692 (0.169-16.912) | 0.654 |
| Late or incomplete series | ||||
| No | 24 (88.9) | 117 (86.7) | Reference | |
| Yes | 3 (11.1) | 18 (13.3) | 0.813 (0.222-2.978) | 0.754 |
Case and control mothers were similar with respect to age, foreign birth, education and insurance status during pregnancy (Table 2). There was no significant difference in maternal race with 96% of case mothers vs 81% of control mothers identified as API (OR = 4.790, 95%CI: 0.62-37.234, P = 0.129). Case mothers were more likely to be of Vietnamese or Hmong descent (OR = 19.6, 95%CI: 3.80-Undef, P < 0.001; OR = 10.75, 95%CI: 1.69-Undef, P = 0.031, respectively). There were no significant differences in gravidity, parity, or delivery mode. In addition, no bleeding risks (including placenta previa, placental abruption or need for maternal blood transfusion) were identified in either case or control mothers, but one case mother had missing information. There were also no differences between case and control mothers with respect to premature rupture of membranes (> 12 h) and prolonged labor (> 20 h) (data not shown).
| Maternal characteristics | Cases (n = 27) | Controls (n = 135) | OR | P value |
| Age (yr) | ||||
| < 25 | 5 (18.5) | 17 (12.6) | Reference | |
| 25-34 | 14 (51.9) | 75 (55.6) | 0.635 (0.201-2.002) | 0.438 |
| > 35 | 8 (29.6) | 43 (31.9) | 0.633 (0.181-2.209) | 0.473 |
| Race | ||||
| Not Asian/Pacific Islander | 1 (3.7) | 21 (15.6) | Reference | |
| Asian/Pacific Islander | 26 (96.3) | 114 (81.4) | 4.790 (0.616-37.234) | 0.129 |
| Foreign born | ||||
| No | 2 (7.4) | 17 (12.6) | Reference | |
| Yes | 25 (92.6) | 118 (87.4) | 1.801 (0.391-8.295) | 0.450 |
| Education | ||||
| Greater than High school | 14 (51.9) | 74 (54.8) | Reference | |
| High school or less | 13 (48.2) | 61 (45.2) | 1.126 (0.492-2.577) | 0.778 |
| Insurance Prenatal Care | ||||
| Non-government | 16 (59.3) | 63 (46.7) | Reference | |
| Government | 10 (37.0) | 72 (53.3) | 0.547 (0.232-1.292) | 0.169 |
| Unknown | 1 (3.7) | 0 (0) | - | - |
| Primigravid | ||||
| No | 12 (44.4) | 76 (56.3) | Reference | |
| Yes | 15 (55.6) | 59 (43.7) | 1.610 (0.701-3.699) | 0.262 |
| Nulliparous | ||||
| No | 11 (40.7) | 65 (48.2) | Reference | |
| Yes | 16 (59.3) | 70 (51.9) | 1.351 (0.584-3.124) | 0.482 |
| Delivery type | ||||
| Non cesarean | 21 (77.8) | 100 (74.1) | Reference | |
| Cesarean | 5 (18.6) | 35 (25.9) | 0.680 (0.239-1.942) | 0.472 |
| Unknown | 1 (3.7) | 0 (0) | - | - |
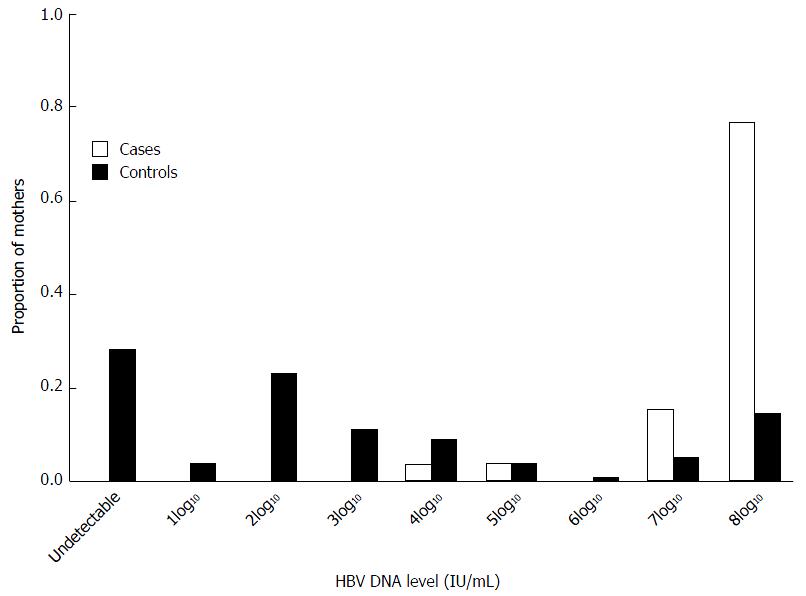
From provider reports to PHPP and electronically submitted data from three large references laboratories, HBV DNA results were only available for 27 (21.6%) mothers of the 125 infected infants, while 36 (28.8%) these mothers had HBeAg results. Among case mothers with HBV DNA results, all had detectable maternal HBV DNA levels compared to only 72.6% of control mothers (P < 0.001) (Figure 1). Case mothers were significantly more likely to have a HBV DNA level of ≥ 2 × 107 IU/mL than control mothers (92.6% vs 18.5%, OR = 54.50, 95%CI: 12.219-247.550, P < 0.001) (Table 3). Significantly more control mothers than case mothers had HBV DNA results reported prior to delivery (57.8% vs 33.3%, P = 0.02). Among all mothers with known HBeAg results, significantly more case mothers than control mothers were HBeAg-positive (70.4% vs 29.9%, OR = 46.76, 95%CI: 6.051-361.617, P < 0.001).
| Maternal laboratory results | Cases (n = 27) | Controls (n = 135) | OR | P value |
| HBV DNA Levels | ||||
| < 2 × 107 IU/mL | 2 (7.4) | 110 (81.5) | Reference | |
| > 2 × 107 IU/mL | 25 (92.6) | 25 (18.5) | 54.499 (12.219-247.550) | < 0.001 |
| HBeAg Results | ||||
| Negative | 1 (3.7) | 96 (71.1) | Reference | |
| Positive | 19 (70.4) | 39 (28.9) | 46.757 (6.051-361.317) | < 0.001 |
| Unknown | 7 (25.9) | 0 (0.0) | - |
Among both case and control mothers with detectable HBV DNA levels, mothers who were HBeAg-positive had higher median HBV DNA levels than those who were HBeAg-negative (1.1 × 108 IU/mL vs 178 IU/mL, P < 0.001). API mothers had significantly higher median HBV DNA levels (5745.50 IU/mL vs 162 IU/mL, P = 0.032) but there was no significant difference in the proportion of API mothers with HBV DNA levels above 2 × 107 IU/mL compared to other races (32.9% vs 18.2%, P = 0.217). API mothers were more likely to be HBeAg-positive than mothers of other races (38.6% vs 18.2%, P = 0.489) but this difference was not significant.
Variables with a P-value < 0.10 in the univariate analysis were included in the multivariate logistic regression. In the multivariate logistic regression analysis, only maternal HBV DNA level ≥ 2 × 107 IU/mL was a significant independent predictor of perinatal HBV infection.
Perinatal infection occurred in one female and one male infant with maternal HBV DNA levels < 2 × 107 IU/mL who received appropriate PEP at birth and completed the HBV vaccine series in the appropriate time period. Both infants were born at term via normal spontaneous vaginal deliveries. The mother of the female infant was HBeAg-negative and had an HBV DNA level of 1 × 104 IU/mL, although both laboratory results were only available 280 d after delivery. The mother of the male infant was HBeAg-positive, had an HBV DNA level of 1 × 105 IU/mL and laboratory results were available 24 d prior to delivery.
Our retrospective analysis and case-control study was designed to evaluate the role of maternal HBV DNA level in perinatal HBV infection. From 2005-2011, 17687 at-risk infants were born to HBsAg-positive mothers in California which created an ideal sampling frame given the largest API population of any state and more than a quarter of the foreign-born population in the United States, both with high prevalence of chronic HBV as well as the extensive PHPP records at CDPH.
The overall HBV infection rate of 1.1% among at-risk infants with PVST is low and demonstrates that current immunoprophylaxis strategies continue to protect most at-risk infants from chronic infection. However, despite appropriate PEP and completion of the vaccine series some infants still become infected. Our study showed that approximately one-third of at-risk infants did not have PVST, a striking deficiency even in a state with the majority of counties with resources for local health departments to provide surveillance for perinatal hepatitis B cases. Similar limitations have been seen in the United States despite comprehensive surveillance programs[15,16]. Infected infants could have been missed. This would have effectively limited our sample size but should not change the nature of the observed association.
New guidelines are proposed to provide anti-viral therapy in pregnancy to prevent perinatal transmission[17]. This requires both additional prenatal laboratory testing and a clear viral load threshold to offer therapy. Although retrospectively solicited, only 22% of case mothers of infected infants had HBV DNA results. During our study period, recommendations for routine prenatal screening did not specifically include HBV DNA PCR, but appropriate health care maintenance of chronic hepatitis B would have included this assessment. We relied upon matching laboratory results from three large reference laboratories that perform most of the hepatitis testing in California. Our matching algorithm has not been validated, but matches were individually verified by maternal name and date of birth. To optimize our yield, we included HBV DNA levels within one year of delivery. There is conflicting evidence regarding the variability of HBV DNA levels during pregnancy, as laboratory results obtained more distant from birth may not reflect the infant’s true risk at the time of delivery[18,19]. Our study demonstrated that mothers of infected infants with HBV DNA results were more likely to be API, HBeAg-positive, and have higher HBV DNA levels than mothers of uninfected infants. Increased risk was clearly evident with maternal HBV DNA level of ≥ 2 × 107 IU/mL.
We identified two cases of perinatal HBV infection in children born to women with lower HBV DNA levels including one with HBeAg-negative status. This differs from other studies that have reported no transmission among infants born to HBeAg-negative mothers or mothers with low HBV DNA levels[8,20-23]. Due to the fluctuations inherent in HBV infection, HBV DNA levels and HBeAg results obtained distant from delivery may not reflect risk at the time of delivery. Infected infants whose mothers were HBeAg-negative have been described; infection in these infants is possibly due to pre-core mutations[24,25].
Our study had several limitations. Since all eligible mothers were selected based on documented laboratory results, selection bias was possible in our sampling. However, univariate analyses restricted to cases with HBV DNA results showed no significant differences when all infected infants were included. Finally, the sample size of our case control evaluation was small and designed to evaluate maternal HBeAg status and HBV DNA levels as risk factors, so the ability to assess other risk factors was limited. Our unique study evaluates perinatal HBV infection in California, with the largest single state sample size in the United States. The results are consistent with findings from prior smaller North American and international studies identifying a similar HBV DNA threshold for transmission risk, although some studies in Asia find higher perinatal transmission rates [26-29].
In California there is an overall low transmission rate of HBV with current PEP strategies, but PVST is not completed for all at-risk infants creating missed opportunity to screen for chronic hepatitis B infection. Infants of mothers with high viral load are at increased risk of chronic infection, clearly evident with a maternal HBV DNA level of ≥ 2 × 107 IU/mL[17].
Appropriate PEP, completion of the HBV vaccine series, and completion of timely PVST still remain critical and first line strategy to prevent perinatal transmission. The use of antiviral therapy in the third trimester for highly viremic mothers has been shown to decrease the incidence of chronic infection in at-risk infants who received appropriate PEP and the HBV vaccine series[14]. Safety profiles of these medications, risk of viral resistance and maternal disease flare should be considered and may delay efforts to make this prevention strategy standard of care[30-32]. Our study further corroborates existing evidence that a high maternal HBV DNA level is a significant risk factor for perinatal HBV infection and chronic HBV disease. More prospective studies are needed to investigate the specific HBV DNA threshold for treatment and the safety and comparative effectiveness of various antiviral therapies during pregnancy to further reduce the incidence of perinatal HBV infection in the infants of infected women.
Chronic hepatitis B virus (HBV) infection remains a significant public health burden in the United States and worldwide. Vaccination remains the most effective strategy to prevent transmission, especially in the high risk perinatal period when peak risk for chronic HBV infection occurs.
Perinatal transmission despite post exposure prophylaxis (PEP) and vaccination has been shown to occur in hepatitis B surface antigen (HBsAg) positive mothers with hepatitis B e antigen positivity and/or high maternal HBV DNA levels. Based on limited but ground-breaking international studies, practice guidelines have begun to suggest the use of antiviral therapy in the second or third trimester in woman with high HBV DNA level to reduce the risk of perinatal transmission. The certainty of evidence, the risk and benefit to both mother and infant, and the viral load threshold to recommend anti-viral therapy in the prenatal period remains under careful review.
This study is the largest single state sample size of perinatal transmission completed outside of Asia. In California, PEP and vaccination are widely used and highly effective to prevent perinatal HBV transmission with an overall perinatal transmission rate of only 1.1%. The incomplete post vaccination serologic testing (PVST) rate may underestimate the burden of pediatric chronic HBV infection in California and is a call to action for pediatricians caring for these at-risk infants. This study demonstrates that infants of mothers with high HBV DNA level of ≥ 2 × 107 IU/mL are clearly at an increased risk of perinatal transmission. Yet, the authors found very few pregnant women with maternal HBV DNA levels, limiting the ability for providers to risk stratify these women for possible anti-viral therapy to prevent perinatal transmission.
This study reveals a real-life assessment of the current HBV perinatal prevention strategies in California and identifies the opportunities for improvement. A comprehensive public health case managed program provides oversight to verify appropriate post-exposure prophylaxis and HBV vaccination for at-risk infants, and identify those with chronic HBV infection. A comprehensive prenatal screen including HBV DNA level has been recommended by ACOG for HBsAg positive pregnant woman. More research is crucial to determine the appropriate threshold for anti-viral therapy in mothers with high HBV DNA levels to safely advance the prevention strategies for chronic HBV infection.
The manuscript entitled “Perinatal transmission in Infants of mothers with chronic Hepatitis B in California” by Burgis et al is well written and well presented.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mihai C, Waheed Y S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1794] [Cited by in RCA: 1778] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 2. | Centers for Disease Control (CDC). Prevention of perinatal transmission of hepatitis B virus: prenatal screening of all pregnant women for hepatitis B surface antigen. MMWR Morb Mortal Wkly Rep. 1988;37:341-346, 351. [PubMed] |
| 3. | Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1-31. [PubMed] |
| 4. | Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, Maley MW, Ayres A, Locarnini SA, Levy MT. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190:489-492. [PubMed] |
| 5. | Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis. 1994;170:1418-1423. [PubMed] |
| 6. | Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19:e18-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 7. | Wen WH, Chang MH, Zhao LL, Ni YH, Hsu HY, Wu JF, Chen PJ, Chen DS, Chen HL. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol. 2013;59:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 8. | Hu Y, Chen J, Wen J, Xu C, Zhang S, Xu B, Zhou YH. Effect of elective cesarean section on the risk of mother-to-child transmission of hepatitis B virus. BMC Pregnancy Childbirth. 2013;13:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | López M, Coll O. Chronic viral infections and invasive procedures: risk of vertical transmission and current recommendations. Fetal Diagn Ther. 2010;28:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Shi Z, Yang Y, Wang H, Ma L, Schreiber A, Li X, Sun W, Zhao X, Yang X, Zhang L. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Shi Z, Yang Y, Ma L, Li X, Schreiber A. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, Yue X, Wang GJ, Tang X, Fang ZX. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 13. | Pan CQ, Mi LJ, Bunchorntavakul C, Karsdon J, Huang WM, Singhvi G, Ghany MG, Reddy KR. Tenofovir disoproxil fumarate for prevention of vertical transmission of hepatitis B virus infection by highly viremic pregnant women: a case series. Dig Dis Sci. 2012;57:2423-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Brown RS Jr, McMahon BJ, Lok AS, Wong JB, Ahmed AT, Mouchli MA, Wang Z, Prokop LJ, Murad MH, Mohammed K. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology. 2016;63:319-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 15. | Ko SC, Fan L, Smith EA, Fenlon N, Koneru AK, Murphy TV. Estimated Annual Perinatal Hepatitis B Virus Infections in the United States, 2000-2009. J Pediatric Infect Dis Soc. 2016;5:114-121. [PubMed] |
| 16. | Schillie S, Walker T, Veselsky S, Crowley S, Dusek C, Lazaroff J, Morris SA, Onye K, Ko S, Fenlon N. Outcomes of infants born to women infected with hepatitis B. Pediatrics. 2015;135:e1141-e1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 17. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1584] [Article Influence: 176.0] [Reference Citation Analysis (2)] |
| 18. | ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat. 2008;15:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Söderström A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis. 2003;35:814-819. [PubMed] |
| 20. | Lin HH, Chen PJ, Chen DS, Sung JL, Yang KH, Young YC, Liou YS, Chen YP, Lee TY. Postpartum subsidence of hepatitis B viral replication in HBeAg-positive carrier mothers. J Med Virol. 1989;29:1-6. [PubMed] |
| 21. | Kubo A, Shlager L, Marks AR, Lakritz D, Beaumont C, Gabellini K, Corley DA. Prevention of vertical transmission of hepatitis B: an observational study. Ann Intern Med. 2014;160:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Zhu YY, Mao YZ, Wu WL, Cai QX, Lin XH. Does hepatitis B virus prenatal transmission result in postnatal immunoprophylaxis failure? Clin Vaccine Immunol. 2010;17:1836-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Song YM, Sung J, Yang S, Choe YH, Chang YS, Park WS. Factors associated with immunoprophylaxis failure against vertical transmission of hepatitis B virus. Eur J Pediatr. 2007;166:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Hawkins AE, Gilson RJ, Beath SV, Boxall EH, Kelly DA, Tedder RS, Weller IV. Novel application of a point mutation assay: evidence for transmission of hepatitis B viruses with precore mutations and their detection in infants with fulminant hepatitis B. J Med Virol. 1994;44:13-21. [PubMed] |
| 25. | Kazim SN, Wakil SM, Khan LA, Hasnain SE, Sarin SK. Vertical transmission of hepatitis B virus despite maternal lamivudine therapy. Lancet. 2002;359:1488-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Screening Pregnant Women for Hepatitis B Virus (HBV) Infection and Screening and Referral Algorithm for Hepatitis B Virus (HBV) Infection among Pregnant Women - prenatalhbsagtesting. pdf [Internet]. Cited 2016-5-9. Available from: http://www.cdc.gov/hepatitis/hbv/pdfs/prenatalhbsagtesting.pdf. |
| 27. | Tran TT. Hepatitis B in Pregnancy. Clin Infect Dis. 2016;62 Suppl 4:S314-S317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Pan CQ, Lee HM. Antiviral therapy for chronic hepatitis B in pregnancy. Semin Liver Dis. 2013;33:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Fan L, Owusu-Edusei K Jr, Schillie SF, Murphy TV. Cost-effectiveness of testing hepatitis B-positive pregnant women for hepatitis B e antigen or viral load. Obstet Gynecol. 2014;123:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Brown RS Jr, Verna EC, Pereira MR, Tilson HH, Aguilar C, Leu CS, Buti M, Fagan EA. Hepatitis B virus and human immunodeficiency virus drugs in pregnancy: findings from the Antiretroviral Pregnancy Registry. J Hepatol. 2012;57:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Chotiyaputta W, Lok AS. Role of antiviral therapy in the prevention of perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2009;16:91-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Dusheiko G. Interruption of mother-to-infant transmission of hepatitis B: time to include selective antiviral prophylaxis? Lancet. 2012;379:2019-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |