Published online Jul 21, 2017. doi: 10.3748/wjg.v23.i27.4935
Peer-review started: February 1, 2017
First decision: March 16, 2017
Revised: April 1, 2017
Accepted: June 1, 2017
Article in press: June 1, 2017
Published online: July 21, 2017
Processing time: 175 Days and 17 Hours
To establish an inducible liver injury mouse model and transplant human hepatocytes to obtain liver-humanized mice.
We crossed three mouse strains, including albumin (Alb)-cre transgenic mice, inducible diphtheria toxin receptor (DTR) transgenic mice and severe combined immune deficient (SCID)-beige mice, to create Alb-cre/DTR/SCID-beige (ADSB) mice, which coincidentally harbor Alb-cre and DTR transgenes and are immunodeficient. As the Cre expression is driven by the liver-specific promoter Alb (encoding ALB), the DTR stop signal flanked by two loxP sites can be deleted in the ADSB mice, resulting in DTR expression in the liver. ADSB mice aged 8-10 wk were injected intraperitoneally (i.p.) with diphtheria toxin (DT) and liver damage was assessed by serum alanine aminotransferase (ALT) level. Two days later, mouse livers were sampled for histological analysis, and human hepatocytes were transplanted into the livers on the same day. A human ALB enzyme-linked immunosorbent assay was performed 7, 14, 21 and 28 d after transplantation. Human CD68 immunohistochemistry was performed 30 and 90 d after transplantation.
We crossed Alb-cre with DTR and SCID-beige mice to obtain ADSB mice. These mice were found to have liver damage 4 d after i.p. injection of 2.5 ng/g bodyweight DT. Bodyweight began to decrease on day 2, increased on day 7, and was lowest on day 4 (range, 10.5%-13.4%). Serum ALT activity began to increase on day 2 and reached a peak value of 289.7 ± 16.2 IU/mL on day 4, then returned to background values on day 7. After transplantation of human liver cells, peripheral blood human ALB level was 1580 ± 454.8 ng/mL (range, 750.2-3064.9 ng/mL) after 28 d and Kupffer cells were present in the liver at 30 d in ADSB mice.
Human hepatocytes were successfully repopulated in the livers of ADSB mice. The inducible mouse model of humanized liver in ADSB mice may have functional applications, such as hepatocyte transplantation, hepatic regeneration and drug metabolism.
Core tip: We established a novel liver chimeric mouse model following liver damage caused by intraperitoneal injection of diphtheria toxin (DT), and transplanted human hepatocytes to obtain liver-humanized mice. After 28 d, human albumin was detected in these mice. Human hepatocytes were successfully repopulated in the livers of triple-crossed albumin-cre transgenic mice, inducible DT receptor transgenic mice and severe combined immune deficient-beige mice [i.e., Alb-cre/DTR/SCID-beige (ADSB) mice]. Our inducible mouse model of humanized liver in ADSB mice may have functional applications, such as studies on hepatocyte transplantation, hepatic regeneration and drug metabolism.
- Citation: Ren XN, Ren RR, Yang H, Qin BY, Peng XH, Chen LX, Li S, Yuan MJ, Wang C, Zhou XH. Human liver chimeric mouse model based on diphtheria toxin-induced liver injury. World J Gastroenterol 2017; 23(27): 4935-4941
- URL: https://www.wjgnet.com/1007-9327/full/v23/i27/4935.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i27.4935
Liver diseases are a serious global health issue, particularly viral hepatitis infection and related diseases. The hepatitis B virus (HBV) and hepatitis C virus (HCV) are representative hepatotropic viruses. HBV is the prototype of the hepadnaviridae family of hepatotropic, partially double-stranded DNA viruses[1], while HCV is a single-stranded RNA virus[2]. Although different at the molecular level, they share many similarities as pathogens, and both infections can be acute or chronic[3]. Persistent HBV and HCV infections can lead to cirrhosis and/or hepatocellular carcinoma[4,5]. Despite the availability of vaccines and drugs, a huge number of patients suffer from the liver diseases related to these viral infections.
Animal models play a critical role in immunological or therapeutic drug development. The narrow spectrum of species that accommodate HBV and HCV infections restricts preclinical studies. Although chimpanzees have played an important role in studying HBV and HCV infections, there are few studies on chimpanzees due to high costs, ethics and their limited availability[6-8]. Other hepadnaviruses that infect woodchucks[9], ducks[10] and ground squirrels[11] harbor limitations due to genetic heterogeneity.
Fundamental questions regarding hepatotropic pathogen biology in vivo need to be addressed. However, this requires a suitable small animal model to guide the challenging and expensive studies. The transgenic 1.2 or 1.3 copy of the HBV genome in mice shows immunological tolerance to HBV antigens. Adenovirus-associated virus-based transduction or hydrodynamic transfection of mouse liver by the 1.2 or 1.3 copy of the HBV genome has also been used to study HBV immunobiology, but does not support viral replication for re-infection in the cycle. Human liver chimeric mouse models are useful in human liver disease research.
In this study, severe combined immune deficient (SCID)-beige mice were crossed with transgenic albumin (Alb)-cre mice which expressed cre enzyme[12] under the control of a liver cell-specific Alb promoter, and diphtheria toxin receptor (DTR)[13,14] transgenic mice, in which the DTR transgene is located in the ubiquitous gt(ROSA26)Sor(R26) locus after a loxp-flanked transcriptional stop sequence.
The resulting Alb-cre/DTR/SCID-beige (ADSB) mice specifically expressed DTR in the liver. Following administration of diphtheria toxin (DT), these mice developed liver injury. We further generated humanized liver in ADSB mice by the transplantation of human hepatocytes. The human hepatocytes were repopulated in the mouse liver, which were functional and secreted human albumin. Human Kupffer cells were also found to chimerize in the mouse liver.
Thus, we developed a novel animal model to investigate hepatocyte proliferation[15-17] and hepatotropic viruses.
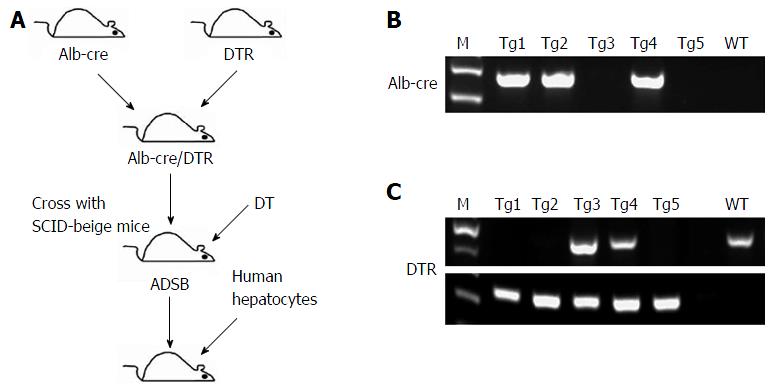
To generate the ADSB mice, we crossed Alb-cre mice (a gift from Dr. Qiang Deng, Institute Pasteur of Shanghai, Chinese of Academy Sciences, Shanghai, China) with DTR mice (a gift from Dr. Yue-Lei Shen, Beijing Biocytogen Co., Ltd, Beijing, China) to obtain Alb-cre/DTR mice. Transgenic mice were selected from the offspring by genomic PCR of tail DNA, and then Alb-cre/DTR transgenic mice were crossed with SCID-beige mice (purchased from the Shanghai SLAC Laboratory Animal Co., Ltd, Shanghai, China), and ADSB mice were selected by genomic PCR of tail DNA.
DT (Sigma-Aldrich, St. Louis, MO, United States) was intraperitoneally administered (2.5 ng/g) to 8-10-wk-old ADSB mice, and blood was collected from these mice at different time points after DT administration. Samples were centrifuged at 600 × g for 15 min to separate the serum. Serum alanine aminotransferase (ALT) activity was measured with a commercially-available kit according to the manufacturer’s instructions (Roche, Basel, Switzerland). Serum ALT activity levels in mice used for hepatocyte transplantation were measured 3 d after DT injection.
The livers were fixed with 4% formaldehyde for 24 h and stored in 75% ethanol. They were then embedded in paraffin and serial sections were cut and stained with hematoxylin and eosin (H and E).
We found that a single DT dose of 2.5 ng/g bodyweight was the maximum dose tolerated with a 100% survival. Using this dose, serum ALT activity levels were determined prior to cell transplantation. Human cryopreserved hepatocytes (Bioreclamation IVT, Baltimore, MD, United States) were thawed and the cryopreservation solution was removed by centrifugation at 100 × g for 5 min at 4 °C followed by resuspension in Dulbecco’s modified Eagle’s medium (DMEM). The resuspended hepatocytes were diluted 1:1 in trypan blue and then centrifuged again at 100 × g for 5 min at 4 °C and reconstituted in hepatocyte culture medium at 1 × 107 cells/mL, and 1 × 106 viable hepatocytes suspended in 100 μL DMEM were injected into the inferior splenic pole.
Starting 1 wk after transplantation, human ALB levels were monitored. Blood samples (10 μL) were collected and centrifuged at 600 × g for 15 min. Serum samples were assayed using the Quantitative Human Albumin ELISA Quantitation Kit (Bethyl Laboratory, Montgomery, TX, United States) according to the manufacturer’s protocol.
At the time of harvest, the liver was fixed in 4% formaldehyde for 24 h and stored in 75% ethanol. Sections were then prepared and incubated with primary human CD68 antibody (1:200 dilution; Servicebio, Shanghai, China) and were used to detect specific Kupffer cells in the chimeric mice, and then incubated with horseradish peroxidase-goat anti-rabbit secondary antibody (1:200; Servicebio).
Statistical analyses were performed using Prism 5.0 software (GraphPad Software, San Diego, CA, United States). A P value of < 0.05 was considered significant.
The experimental design is outlined in Figure 1. In this study, we crossed Alb-cre with DTR and SCID-beige mice to obtain ADSB mice. In PCR used to identify the Alb-cre gene, Tg1, Tg2 and Tg4 mice were cre-positive (Figure 1B) and in PCR for the DTR gene, Tg1, Tg2 and Tg5 were found in homozygous DTR mice, and Tg3 and Tg4 in heterozygous DTR mice (Figure 1C). Genotyping of SCID-beige mice was performed as previously described[18]. The mice were then injected intraperitoneally with DT to induce liver injury, and adult human hepatocytes were transplanted to obtain chimeric mice.
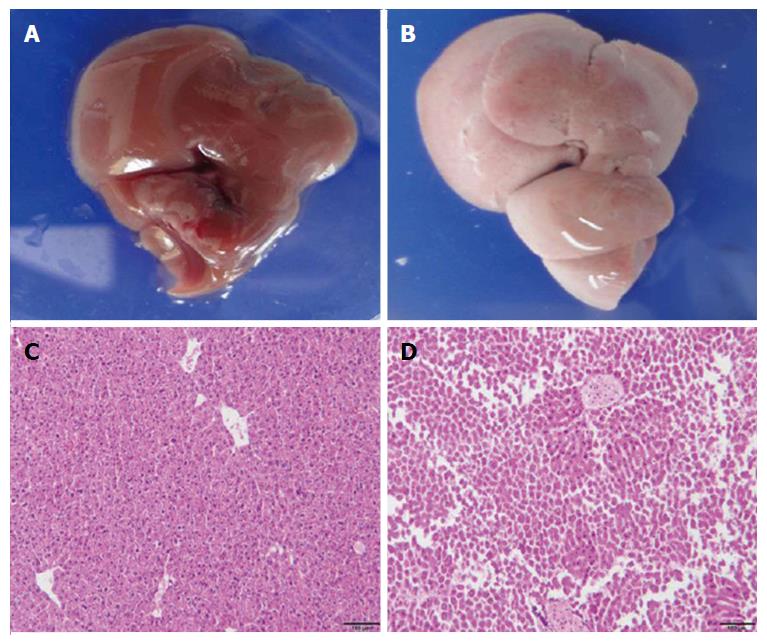
To examine the liver damage caused by DT, ADSB mice and non-transgenic mice (C57BL/6) were injected intraperitoneally with 2.5 ng/g bodyweight of DT in 200 μL phosphate-buffered saline. Both groups of mice were sacrificed 4 d later. The livers of non-transgenic mice appeared normal and dark red (Figure 2A), whereas the livers from ADSB mice were pale and almost white (Figure 2B). Liver sections from both types of mice were stained with H and E. Microscopically, the liver sections from non-transgenic mice were of normal histological appearance, the structure of the hepatic lobule was complete, the hepatic cord and hepatic sinusoid were appropriately arranged, and degeneration or necrosis of hepatocytes was not observed (Figure 2C). Hepatocyte nucleus fragmentation disappeared in ADSB mice, suggesting that ADSB mice had characteristic histological hepatocellular injury (Figure 2D).
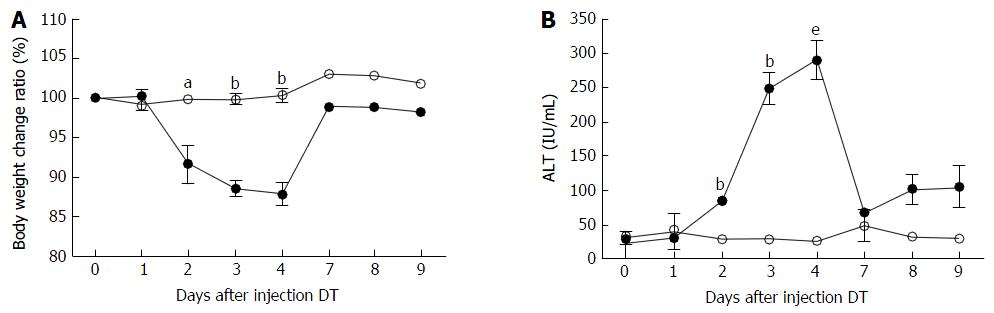
ADSB mice and non-transgenic mice were injected intraperitoneally with 2.5 ng/g bodyweight of DT. At different time points, bodyweight was recorded and blood samples were collected to determine ALT activity. In ADSB mice, after DT injection, bodyweight began to decrease on day 2, was regained on day 7, and was lowest on day 4 (range, 10.5%-13.4%). No weight reductions were found in non-transgenic mice (Figure 3A). Serum ALT activity in ADSB mice began to increase on day 2, reached a peak value of 289.7 ± 16.2 IU/mL on day 4, and then returned to background values on day 7 (Figure 3B). In non-transgenic mice, ALT activity remained at basal levels (< 50 IU/mL). Therefore, from day 2 to day 7 after DT injection liver damage occurred, demonstrating that proliferation of transplanted hepatocytes took place in this mouse model.
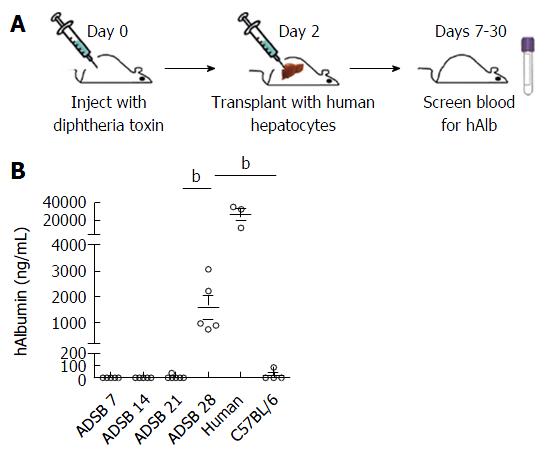
ADSB mice were transplanted 3 d after DT injection, and then peripheral blood ALB levels were determined on days 7, 14 and 21 after hepatocyte transplantation (Figure 4A). Serum levels of human ALB in ADSB mice are shown on days 7, 14, 21 and 28 after hepatocyte transplantation. Before 28 d, no human ALB was detectable either in ADSB mice or the non-transgenic mice. However, 28 d after transplantation we detected serum human ALB in ADSB mice at the level of 1580 ± 454.8 ng/mL (range, 750.2-3064.9 ng/mL), and no human ALB was detected in non-transgenic mice (Figure 4B). These results demonstrated that human ALB was expressed at least 4 wk after hepatocyte transplantation.
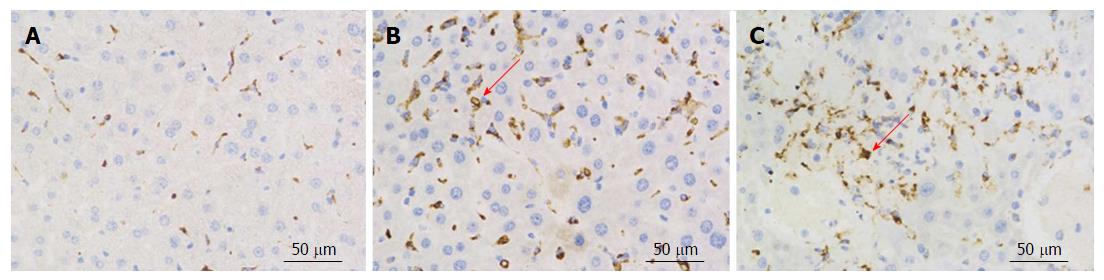
CD68 is considered a specific marker for activated Kupffer cells. Kupffer cells are essential for many hepatic functions and play a major role in inflammatory responses in this organ[19-21]. CD68 immunohistochemistry was used to measure CD68 expression in Kupffer cells. In ADSB mouse liver sections, CD68+ cells were present 4 wk and 12 wk after transplantation, and more CD68+ cells were found at 12 wk after transplantation than at 4 wk after transplantation (Figure 5).
Human liver chimeric mouse models are useful in human liver disease research. The urokinase-type plasminogen activator (uPA) transgenic mouse[22], was the first reported liver humanized mouse model; however, uPA mice have low breeding efficiency, are unhealthy and die due to hypofibrinogenemia; thus, the transplant time for uPA mice is limited. Two reports showed successful engraftment based on genetic knockout of the fumarylacetoacetate hydrolase (Fah) genes[23,24]. Fah is the last enzyme in the tyrosine breakdown pathway and its deficiency leads to lethal type I hypertyrosinemia in humans and liver failure in mice. However, Fah mice also have mouse health problems, and 2-(2-nitro-4-trifluoro-methylbenzoyl)-1,3-cyclohexanedione controls liver injury so that impacts its application in drug metabolism. More recently, two additional transgenic models have been developed, the TK-NOG[25] and the AFC8[26] models, which express active caspase 8 fused with the FK506 binding domain and has inducible suicidal activity in mouse liver under Alb promoter control, but its repopulation rate of human liver cells is only 30%. The FRG model was then developed. FRG[23] mice are immune-deficient, Fah knockout mice crossed with mice lacking the Rag-2 gene and the common gamma chain of the interleukin receptor.
We report here a novel ADSB mouse model which can be efficiently repopulated with human hepatocytes. The transplanted human hepatocytes can reside in the mouse host’s natural environment and maintain normal functions. Theoretically, these mouse models can be infected with HBV and HCV in a reproducible manner.
In this model, recipient mouse hepatocytes were destroyed by DT, and the transplanted human mature hepatocytes had a selective advantage in the mouse liver. We confirmed that these mice have the ability to engraft adult human hepatocytes, and the liver can harbor human Kupffer cells. Thus, this model provides a platform for basic biology in liver regeneration research and liver disease development.
Our mouse model has distinct advantages over the other chimeric models. First, ADSB mouse breeding is not as difficult as for uPA mice, making it possible to obtain sufficient ADSB mice for experiments. In addition, these mice are healthy and long-lived, and can be used for long-term transplantation studies. Second, the transplantation time points are flexible following DT injection to induce murine liver injury. Furthermore, we determined the appropriate dose of DT to be 2.5 ng/g bodyweight, which can sustain acute liver injuries with only one dose of DT, resulting in no death of mice, and can efficiently support the proliferation of transplanted hepatocytes.
In conclusion, this study introduced a new in vivo mouse model, which will serve as a promising tool for research into the interaction between host and virus in vivo, and in the development of new treatment approaches. This model is convenient for studies on hepatocyte transplantation, human drug metabolism research and drug-drug interactions[27,28]. Our model achieved the establishment of human liver without hemopoietic reconstitution. In a future study, we will attempt to establish human liver/immune dual chimeric mice in order to investigate HBV or HCV infections in these chimeric animals.
We appreciate Yue-Lei Shen from Biocytogen Co., Ltd, Beijing, China for kindly providing the DTR mice; Qiang Deng from Institut Pasteur of Shanghai, Chinese of Academy Sciences for kindly providing the Alb-cre mice; Professor Zheng-Hong Yuan from Fudan University, Shanghai, China for his intensive discussion. We thank the animal facilities in Shanghai Public Clinical Center, Fudan University, China for providing the platform and helping with animal experiments.
Hepatitis B virus and hepatitis C virus are hepatotropic viruses that represent a serious global health issue. Humanized mouse models are useful in human liver disease research. However, mouse models have disadvantages and need to be improved.
Recently, many humanized mouse models have been reported, such as the AFC8 mouse and Fah mouse models. However, these mouse models have disadvantages, such as low breeding efficiency, limited time window for transplantation and low repopulation rate.
In the present study, the authors developed a novel liver-chimeric mouse model. Liver failure was induced by diphtheria toxin (DT) and then human hepatocytes were transplanted and repopulated in the mice.
The results of this study suggest that the liver chimeric mouse model based on triple-crossed albumin -cre transgenic mice, inducible DT receptor (DTR) transgenic mice and severe combined immune deficient-beige mice (i.e., ADSB mice) may provide a more stable platform for human drug metabolism research and viral hepatitis infections.
A liver chimeric mouse is established using transgenic or knockout techniques to cause liver failure and human liver cells are transplanted to construct a chimeric mouse. In order to avoid host immune rejection following human hepatocyte transplantation, the mice used are usually immunodeficient.
The researchers provide a novel mouse model of human liver chimeric based on DTR transgenic mice, in which liver injury can be induced by DT injection. This model could serve as a promising tool for research on the interaction between host and hepatitis virus in vivo, and in the development of new treatment approaches against related liver diseases.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: De Ponti F S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51-68. [PubMed] |
| 2. | Wieland SF, Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79:9369-9380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 348] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 689] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 4. | Blumberg BS. Hepatitis B virus, the vaccine, and the control of primary cancer of the liver. Proc Natl Acad Sci USA. 1997;94:7121-7125. [PubMed] |
| 5. | Rogler CE, Chisari FV. Cellular and molecular mechanisms of hepatocarcinogenesis. Semin Liver Dis. 1992;12:265-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Barker LF, Chisari FV, McGrath PP, Dalgard DW, Kirschstein RL, Almeida JD, Edington TS, Sharp DG, Peterson MR. Transmission of type B viral hepatitis to chimpanzees. J Infect Dis. 1973;127:648-662. [PubMed] |
| 7. | Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825-829. [PubMed] |
| 8. | Abe K, Inchauspe G, Shikata T, Prince AM. Three different patterns of hepatitis C virus infection in chimpanzees. Hepatology. 1992;15:690-695. [PubMed] |
| 9. | Summers J, Smolec JM, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA. 1978;75:4533-4537. [PubMed] |
| 10. | Mason WS, Seal G, Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980;36:829-836. [PubMed] |
| 11. | Marion PL, Oshiro LS, Regnery DC, Scullard GH, Robinson WS. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci USA. 1980;77:2941-2945. [PubMed] |
| 12. | Austin S, Ziese M, Sternberg N. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981;25:729-736. [PubMed] |
| 13. | Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 708] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 14. | Roberts LM, Ledvina HE, Tuladhar S, Rana D, Steele SP, Sempowski GD, Frelinger JA. Depletion of alveolar macrophages in CD11c diphtheria toxin receptor mice produces an inflammatory response. Immun Inflamm Dis. 2015;3:71-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263:1149-1152. [PubMed] |
| 16. | Rhim JA, Sandgren EP, Palmiter RD, Brinster RL. Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proc Natl Acad Sci USA. 1995;92:4942-4946. [PubMed] |
| 17. | Dandri M, Burda MR, Török E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 321] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Araki R, Fujimori A, Hamatani K, Mita K, Saito T, Mori M, Fukumura R, Morimyo M, Muto M, Itoh M. Nonsense mutation at Tyr-4046 in the DNA-dependent protein kinase catalytic subunit of severe combined immune deficiency mice. Proc Natl Acad Sci USA. 1997;94:2438-2443. [PubMed] |
| 19. | Ajakaiye M, Jacob A, Wu R, Nicastro JM, Coppa GF, Wang P. Alcohol and hepatocyte-Kupffer cell interaction (review). Mol Med Rep. 2011;4:597-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Gregory SH, Wing EJ. Neutrophil-Kupffer cell interaction: a critical component of host defenses to systemic bacterial infections. J Leukoc Biol. 2002;72:239-248. [PubMed] |
| 21. | Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci. 2007;96:2-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Heckel JL, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Neonatal bleeding in transgenic mice expressing urokinase-type plasminogen activator. Cell. 1990;62:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25:903-910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 647] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 24. | Bissig KD, Le TT, Woods NB, Verma IM. Repopulation of adult and neonatal mice with human hepatocytes: a chimeric animal model. Proc Natl Acad Sci USA. 2007;104:20507-20511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Hasegawa M, Kawai K, Mitsui T, Taniguchi K, Monnai M, Wakui M, Ito M, Suematsu M, Peltz G, Nakamura M. The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochem Biophys Res Commun. 2011;405:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 26. | Washburn ML, Bility MT, Zhang L, Kovalev GI, Buntzman A, Frelinger JA, Barry W, Ploss A, Rice CM, Su L. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Nowak G, Ericzon BG, Nava S, Jaksch M, Westgren M, Sumitran-Holgersson S. Identification of expandable human hepatic progenitors which differentiate into mature hepatic cells in vivo. Gut. 2005;54:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Bateman TJ, Reddy VG, Kakuni M, Morikawa Y, Kumar S. Application of chimeric mice with humanized liver for study of human-specific drug metabolism. Drug Metab Dispos. 2014;42:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |