Published online Jul 21, 2017. doi: 10.3748/wjg.v23.i27.4879
Peer-review started: February 28, 2017
First decision: April 26, 2017
Revised: May 2, 2017
Accepted: June 9, 2017
Article in press: June 12, 2017
Published online: July 21, 2017
Processing time: 142 Days and 6.1 Hours
The red blood cell distribution width (RDW) is a routinely measured and automatically reported blood parameter, which reflects the degree of anisocytosis. Recently, the baseline RDW was found to have clinical significance for assessing clinical outcome and severity of various pathological conditions including cardiovascular diseases, sepsis, cancers, leukemia, renal dysfunction and respiratory diseases. A myriad of factors, most of which ill-defined, have an impact on the red cell population dynamics (i.e., production, maturation and turnover). A delay in the red blood cell clearance in pathological conditions represents one of the leading determinants of increased anisocytosis. Further study of RDW may reveal new insight into inflammation mechanisms. In this review, we specifically discuss the current literature about the association of RDW in various disease conditions involving the gastrointestinal and hepatobiliary systems. We also present some of the related measurements for their value in predicting clinical outcomes in such conditions. According to our data, RDW was found to be a valuable prognostic index in gastrointestinal disorders along with additional inflammatory biomarkers (i.e., C reactive protein, erythrocyte sedimentation rate, and platelet count) and current disease severity indices used in clinical practice.
Core tip: Mounting evidences show that red blood cell distribution width can be used as a prognostic marker in gastrointestinal disorders. A number of retrospective studies have been published about the use of this index of anisocytosis in prognostication of gastrointestinal disorders, especially inflammatory bowel disease and viral hepatitis among others. However, only a few have included confounding factors which could affect red blood cell distribution width. Our objective is to consolidate the current literature to better understand the use and further investigate the significance of red blood cell distribution width in gastrointestinal disorders.
- Citation: Goyal H, Lippi G, Gjymishka A, John B, Chhabra R, May E. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017; 23(27): 4879-4891
- URL: https://www.wjgnet.com/1007-9327/full/v23/i27/4879.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i27.4879
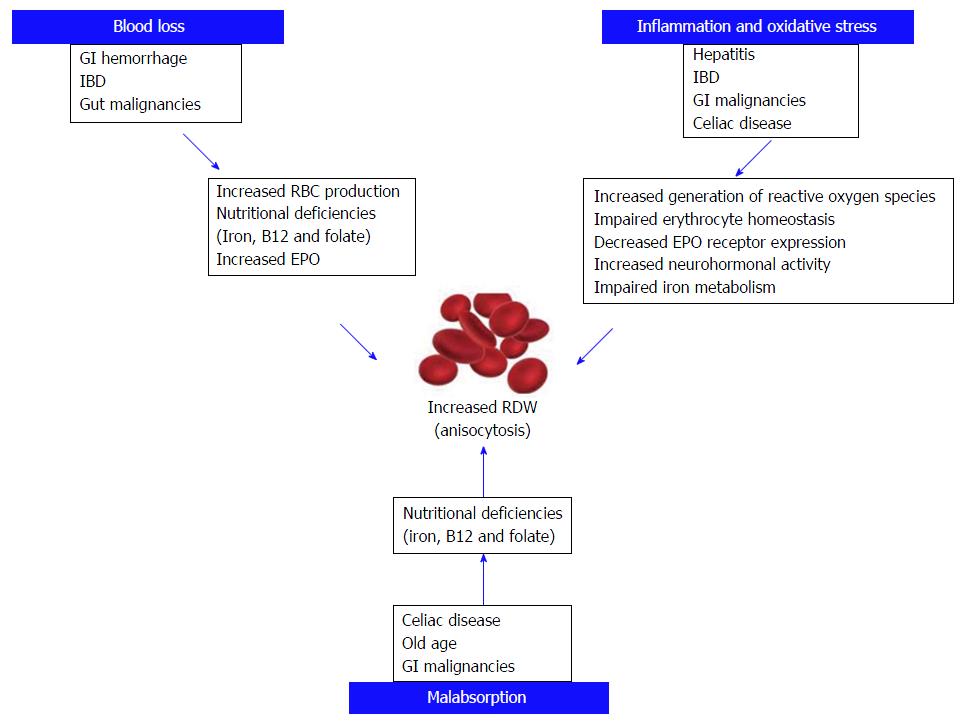
The red blood cell distribution width (RDW) is a measure of size variability and heterogeneity of erythrocytes in the peripheral blood (i.e., anisocytosis)[1]. RDW is a part of complete blood count and is a routinely measured and automatically generated blood parameter that has lately gained considerable interest due to its ability to help assessing the prognosis of various diseases. The value of RDW in assessing the severity of disease and clinical outcome has been proven in various conditions including, but not limited to, sepsis, renal dysfunction, cardiovascular and pulmonary diseases and malignancies. RDW was also proven useful in assessing mortality rates and survival of hospitalized patients, including those admitted to the intensive care unit (ICU)[2].
In a healthy adult, nearly 2 million red blood cells (RBCs) per second enter the peripheral circulation. The lifespan of a typical RBC is 100-120 d. During this period, a kaleidoscope of factors, many of which still unknown, may impact the RBC population dynamics (production, maturation, turnover and clearance). It is also known that RBCs, while in circulation, undergo a process of reduction (fast and slow phases) of volume (approximately 30%) and hemoglobin content (approximately 20%), leading to a slight increase in the relative hemoglobin concentration of RBCs (mean corpuscular hemoglobin; MCH) toward the end of their lifespan[3]. RDW increases with aging and this is clearly attributable to the gradual increased prevalence of comorbidities, which may actually contribute to derange erythrocyte biology and lead to release of a population of RBCs with heterogeneous size[4]. Hemoglobin concentration of RBCs is tightly controlled, because it is essential for adequate oxygen delivery to the tissues. In addition, recent studies showed that RBC clearance is another process under tight regulation, and that a delay in RBC clearance appears to be modulated in pathological conditions. RDW may increase in many diseases due to the impaired turnover of RBCs, which may both lead to increased permanence of aged cells in the circulation or release of immature and larger cells from the bone marrow due to increased turnover. Using a semi-mechanistic mathematical model of in vivo RBC population dynamics, Patel et al[3] showed that a delay in RBC clearance leads to a longer persistence of smaller volume RBCs in peripheral circulation, thereby increasing anisocytosis and consequently, RDW.
Most of the conditions affecting the gastrointestinal (GI) tract necessitate evaluation using minimally invasive and invasive procedures, such as endoscopy with or without biopsy. These procedures require specialized, expensive equipment and trained technical personnel. These may not be easily available in certain regions of the world, and may not be affordable to all the patients. Moreover, even with these evaluations, it is sometimes challenging to predict the clinical outcomes of GI conditions. The identification of a reliable and reproducible parameter that may provide important information on the evaluation of GI diseases is therefore of paramount importance.
In this manuscript, we review the most recent literature about the prognostic value of RDW for assessment of disease severity and clinical outcome in both benign and malignant conditions affecting the GI and pancreaticohepatobiliary systems.
The prognostic value of RDW in esophageal disorders has only been studied in esophageal cancer (EC). One of the first studies was conducted by Chen et al[5] who carried out a retrospective analysis of 277 esophageal squamous cell carcinoma (ESCC) patients undergoing radical esophagectomy without preoperative neoadjuvant therapy. Patients were followed every 3-6 mo for two years, and annually thereafter, with a median follow-up of 42.5 mo. The mean initial RDW was 14.5% ± 2.3%. The patients were divided in to two groups (i.e., RDW ≥ 14.5% and RDW < 14.5%). Patients with RDW < 14.5% had significantly better 5-year cancer-specific-survival than those with RDW ≥ 14.5%. Increased RDW was then found to be an independent prognostic factor for cancer specific survival, with mortality being nearly twice higher in patients with RDW ≥ 14.5% that in those with lower values[5]. Wan et al[6] studied 179 patients with EC (133 patients with squamous and 46 with adenocarcinomatous pathology) with median follow-up of 21 mo, and found that patients with high RDW > 15% exhibited a shorter disease-free survival (P = 0.043, HR = 1.907, 95%CI: 1.020-3.565) and unfavorable overall survival (P = 0.042, HR = 1.895, 95%CI: 1.023-3.508) independently from other cancer-related factors.
Hirahara et al[7] also investigated 144 patients undergoing esophagectomy for ESCC, observing that a high RDW value was independently associated with cancer-specific survival poor prognosis in patients aged 70 years or younger. Sun et al[8] proposed to calculate the ratio of hemoglobin (Hb) to RDW (i.e., Hb/RDW) as a novel prognostic factor in patients with ESCC. They observed that patients with a Hb/RDW ratio of < 0.989 had a 1.4-time higher risk of death during follow up compared to patients with Hb/RDW ratio > 0.989 (95%CI: 1.024-1.958, P = 0.035)[8]. Recently, Hu et al[9] studied 2396 patients (1822 men and 574 women) from a Fujian prospective cohort, who underwent three-field lymphadenectomy for ESCC and with median follow-up of 38.2 mo. Interestingly, RDW was found to be an independent predictor of ESCC mortality in the male gender only (adjusted HR = 1.5, 95%CI: 1.08-1.22, P < 0.001). In females, only lymphocyte count was marginally significant for predicting survival[9].
All of the above studies have consistently shown the existence of a relationship between increased RDW and poor disease-specific and overall survival in patients with EC, especially ESCC. However, these studies were mainly conducted on Asian population in China and Japan, where ESCC is more common than adenocarcinomatous EC in Western countries. This likely explains why all these studies were conducted mainly in patients with ESCC. At this time, it remains hence unclear whether or not the prognostic role of RDW can be extended to ESCC affecting western population.
The role of RDW in gastric disorders has not been extensively studied. Since one of the most common features of gastric disorders is anemia, which can also significantly impact RDW values, challenges remain to carry out studies for evaluating the relationship between RDW and gastric disorders. Tüzün et al[10] performed a retrospective study in 122 patients with autoimmune gastritis (AIG) who were compared with 101 patients with functional dyspepsia. RDW was found to be significantly increased in patients with AIG (16.11% ± 3.04% vs 13.41% ± 0.95%, P < 0.001). Receiver operating characteristics (ROC) curve analysis suggested 13.95% as the optimum cut-off point (AUC: 0.860)[10]. They further analyzed the same patients to define the potential role of RDW in AIG and gastric carcinoid tumor type I (94 AIG without gastric carcinoid and 28 AIG with gastric carcinoid), but failed to find a significant association[10]. However, the hemoglobin values were not included in a multivariate regression analysis to establish whether or not RDW was independent predictor of AIG compared to functional dyspepsia. This is a substantial drawback since the patients in the AIG cohort had significantly lower values of both vitamin B12 and ferritin.
In a retrospective study, Pietrzyk et al[11] studied RDW in gastric cancer and healthy individuals, concluding that gastric cancer patients had higher mean RDW values (14.9 ± 3.9) than healthy individuals (12.2 ± 0.7). It was hence suggested that elevated RDW, when combined with symptoms, can be used as an alert for upper endoscopy to early detect gastric cancer[11]. In another recent study in gastric cancer patients undergoing curative surgery, high preoperative RDW values were found to be significant predictors of 60-d mortality (17.9 ± 4.3 vs 16.0 ± 3.2; P = 0.015). The incidence of advanced gastric cancer was higher in patients with RDW ≥ 16% than in those with lower values (75% vs 51%, P = 0.002), whereas disease free and overall survival was found to be reduced (P = 0.04)[12].
Isik et al[13] conducted a retrospective study on 147 patients with upper gastrointestinal hemorrhage, and found that these patients had significantly higher median RDW value compared to the standard reference value (15.3% vs 14.5%). This was not surprising since anemia and anisocytosis are tightly linked. The eight patients who died during the study period had significantly higher median RDW value (18.9%) compared to those who did not (P = 0.02), so concluding that RDW could be used as a predictor of mortality in upper gastrointestinal hemorrhage[13]. In an interesting investigation, Fatemi et al[14] studied 6689 patients with myocardial infarction who underwent percutaneous coronary intervention (PCI). Higher RDW values were significantly associated with post-procedural in-hospital GI bleeding (P < 0.001), a risk increasing in parallel with RDW values[14]. Despite these results, the authors did not exclude confounding factors known to be associated with anisocytosis. The results of this large study suggest that physicians may use RDW for identifying patients at higher risk of GI bleeding post PCI.
Due to increasing incidence and prevalence, the need of simple laboratorial markers to diagnosing and monitoring adherence to gluten-free diet and the treatment of celiac disease have recently emerged. Brusco et al[15] studied 126 untreated histologically diagnosed celiac disease patients, and found that increased RDW was the most frequent hematological abnormality in these patients (73 of 126; 58%); followed by anemia (31%) and iron deficiency (29%). Forty three out of 73 patients with increased RDW were restudied after a median follow-up of 12 mo and RDW was found to be decreased to normal value except in five patients (P < 0.001) when on gluten-free diet[15]. Balaban et al[16] compared patients with newly diagnosed celiac disease, inflammatory bowel disease (IBD), and irritable bowel syndrome (IBS), and found that almost two-thirds of patients with celiac disease had elevated RDW as compared to only 9% of patients with IBS. However, the possible mechanisms of increased RDW in IBS were not explained. Importantly, they also found that a spleen diameter to RDW ratio < 6 had 0.73 sensitivity and 0.89 specificity for detecting celiac disease. The AUC for predicting celiac disease was 0.737 (95%CI: 0.597-0.877)[16]. Sategna Guidetti et al[17] also showed that increased RDW values may be reliable predictors of celiac disease even in the presence of normal hemoglobin value. They also observed that gluten-free diet was effective to significantly reduce RDW values within one year. Many other studies showed that RDW values significantly improved with gluten-free diet in patients with celiac disease[18-20]. Therefore, normalization of RDW in selected celiac disease patients can seemingly be used as a simple but indirect index for assessing patients’ response to gluten-free diet. Notably, no study in the current scientific literature has explored the role of RDW for predicting development of intestinal lymphoma or refractory sprue in patients with celiac disease.
Harmanci et al[19] retrospectively studied 49 newly diagnosed celiac disease patients and compared their RDW values according to the presence of intestinal atrophy. A RDW value of > 17.25% was found to be the most significant predictor of atrophy in these patients (P = 0.003). The authors also concluded that RDW values > 17.7% predict intestinal atrophy with 0.76 sensitivity, 1.00 specificity, 0.79 PPV and 1.00 NPV when combined with tissue transglutaminase antibody titers of > 200 U/L in patients with celiac disease[19].
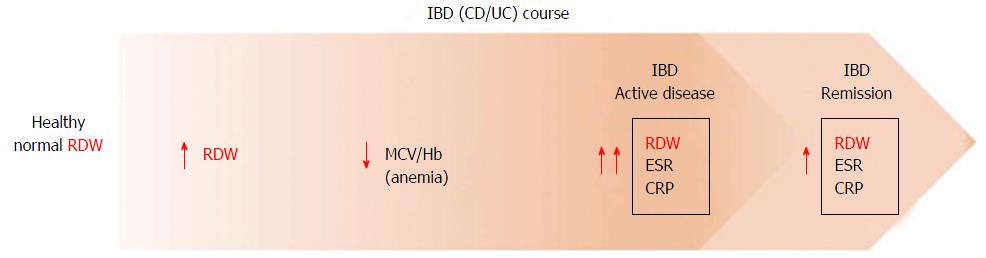
IBD is characterized by chronic relapsing and remitting inflammatory changes of bowel, which are increasing in incidence and prevalence in the general population. Multiple blood tests such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), interleukin (IL)-6, white blood cell count (WBC) (among others) have been evaluated as surrogate markers of disease activity in IBD, but showed variable diagnostic performance. Therefore, there is still wiggle room for searching an ideal, noninvasive, simple, inexpensive and highly specific laboratory test for monitoring disease activity. In a retrospective study, Clarke et al[21] found that mean RDW at diagnosis was significantly higher in Crohn’s disease (CD) compared to ulcerative colitis (UC) patients (14.9% vs 14.3%, P = 0.027). A higher prevalence of malabsorption-related anemia due to distribution of lesions in CD relative to UC has been brought to explain this finding, and it was finally suggested that RDW may be used as a marker for differentiating between the two distinct forms of IBD (CD and UC)[21]. However, the role of additional confounding factors such as hemoglobin, other causes of anemia (e.g., blood loss) and severity of disease at diagnosis was not explored.
Molnar et al[22] performed a retrospective study to investigate whether RDW value may be helpful in differentiating CD and UC in both active state and remission. It was hence observed that RDW value was increased in 53.2% of inactive CD vs 36.85% of inactive UC (mean RDW 14.3% vs 13.8%, P = 0.05), while no significant difference was found in the active state of both diseases (14.7% vs 14.4%, P = 0.393). Cakal et al[23] also showed that a cutoff 14% for RDW had 0.88 sensitivity and 0.71 specificity, so making this index the most sensitive and specific serologic inflammatory parameter (even better than fibrinogen, CRP, ESR and platelet count) for detecting active UC in the patient cohort. However, different results were obtained for active CD, since a value of 0.54 mg/dL for CRP had 0.92 sensitivity and 0.63 specificity, whereas a threshold of 14.1% for RDW was characterized by 0.78 sensitivity and 0.63 specificity. Authors concluded that this difference may be due to the variability in presentation of active UC and CD. More active UC patients have rectal bleeding and iron deficiency, whereas malabsorption of other micronutrients and vitamins in terminal ileitis (CD) may affect RDW value[23]. A study by Yeşil et al[24] yielded different results. In their study of patients with active CD, a RDW cut off of 14% had 0.79 sensitivity and 0.93 specificity (AUC = 0.935; P < 0.001), so making it the best overall parameter for identifying active CD. Interestingly, an increased RDW value was identified earlier than changes in hemoglobin and mean corpuscular volume (MCV), so supporting the notion that RDW may have a role as early indicator of active disease in both CD and UC[24]. Similarly, Ipek et al[25] showed that RDW may be a marker of disease activity in UC, with 0.41 sensitivity and 0.91 specificity (AUC = 0.65; P < 0.001). Song et al[26] investigated the relationship between RDW and disease activity in IBD patients with and without anemia, reporting that RDW was the best independent predictor of disease activity in both UC and CD irrespective of the presence of anemia. In another study by Oliveira et al[27] RDW was found to be associated with disease activity in CD (defined by CDAI ≥ 150); a RDW cutoff value of 16% had 0.88 specificity and 0.86 negative predictive value for active CD. These results support the role of RDW as an important inflammatory marker, as the positive correlation between anisocytosis and increase in systemic inflammation has been consistent throughout many studies (Table 1).
| Ref. | No. of subjects | Study period | Activity index | Outcome measures | RDW Value | Statistics | Other laboratory studies | Main findings | |
| Sensitivity (%) | Specificity (%) | ||||||||
| Clarke et al[21], 2008 | 156 CD | January 1st 2004 to December 31st 2005 | Differential diagnosis CD vs UC | CD 14.9 UC 14.3 | P = 0.027 | RDW value was significantly higher in CD relative to ulcerative colitis patients | |||
| 128 UC | |||||||||
| Cakal et al[23], 2009 | 74 UC, 22 CD, and 20 age/sex-matched controls | CDAI | CRP, ESR, Fibrinogen, PLT, WBC, Hb | RDW and CRP were the most significant indicators of active UC and active CD, respectively | |||||
| > 150 = active | Active UC or CD | UC 14 | 88 | 71 | |||||
| Truelove-Witts scale for UC moderate and severe = active | CD 14.1 | 78 | 63 | ||||||
| Oustamanolakis et al[28], 2011 | 51 CD | CDAI | Anemia (IDA/ACD) | 14 (cut off) | 93 | 81 | Ferritin, Tsat, sTfR | High RDW and low RSF values were the best markers for the diagnosis of IDA | |
| 49 UC | > 150 active | IDA | RDWR-C, RSF, IRF, | ||||||
| 102 age matched controls | < 150 inactive | Hb, ESR | |||||||
| Simple Clinical | CRP | ||||||||
| Colitis Activity Index for UC | |||||||||
| Active ≥ 3 | |||||||||
| Yeşil et al[24], 2011 | 56 CD | CDAI | Active UC/CD | 14 (cut off ) | 79 (CD) | 93 (CD) | CRP, ESR, PLT, Hb | RDW was elevated in IBD and markedly increased in active disease. RDW may be a marker of active CD, whereas ESR is for active UC | |
| 61 UC | > 150 = active | 17 (UC) | 84 (UC) | ||||||
| 44 age/sex matched controls | Truelove-Witts scale for UC | ||||||||
| Song et al[26], 2012 | 101 CD | January 2003 and December 2010 | CDAI | Active UC/CD | 14.1 CD without anemia (cut off) | 82 (CD) | 83 (CD) | CRP, ESR, PLT, WBC, Hb, | RDW was associated with active CD/UC in patients with or without anemia |
| 120 UC | remission < 150, mild 150-220, moderate to severe ≥ 220; Mayo score for UC | 13.8 UC without anemia (cut off ) | |||||||
| remission < 3, mild 3-6, moderate to severe ≥ 6 | 76 (UC) | 86 (UC) | |||||||
| Ipek et al[25], 2015 | 206 active UC | January 2009 to December 2011 | Endoscopic Rachmilewitz activity index > 4 = Active UC | Active UC 16.8 | CRP, ESR, PLT, WBC, Hb | RDW can be used as a marker for disease activity in ulcerative colitis, but not in the non-anemic group | |||
| 104 remission UC | Active UC vs remission | Remission UC 15.5 | P < 0.001 | ||||||
| Oliveira et al[27], 2016 | 20 Active CD | January 1st and September 30th 2013 | CDAI | RDW association with Active CD | 16 (Cut off ) | 30 | 88 | CRP, ESR, PLT, WBC, Hb, MCV | RDW was associated with the severity of CD. The RDW cutoff 16% showed possible clinical applicability |
| 99 remission CD | ≥ 150 = active CD | ||||||||
In an interesting study, Oustamanolakis et al[28] evaluated the value RBC indices for differentiating iron deficiency anemia (IDA) and anemia of chronic disease in IBD. They found that higher values of RDW in IBD performed better than ferritin values as markers of IDA, displaying 0.93 sensitivity and 0.81 specificity[28].
Therefore, RDW was shown to have good potential as indicator of disease severity and as a differentiating marker in IBD. This is particularly important considering that the use of this readily available hematological parameter would permit physicians to better evaluating and managing IBD patients (Figure 1).
Several lines of evidence attest that RDW may be useful for diagnosing and assessing the survival of patients with solid and hematological cancers. Riedl et al[29] investigated various RBC parameters in a prospective cohort of 1840 cancer patients, confirming that an increased RDW value was associated with enhanced risk of mortality (HR = 1.72, 95%CI: 1.39-2.12, P < 0.001). The association was virtually unaltered after adjustment for age, sex, hemoglobin, leukocyte and platelet count (HR = 1.34, 95%CI: 1.06-1.70, P = 0.016)[29]. In a retrospective case-control study, Spell et al[30] showed that the RDW had high sensitivity (0.84) and specificity (0.88) for identifying right-sided colon cancer, so concluding that this parameter may be seen as a cost-effective screening tool for colon cancer. In a more recent investigation, Ay et al[31] found that RDW values were significantly higher in patients with colon cancer compared to those with colonic polyps (P = 0.01). Patients with anemia and other hematological disorders were already excluded from the analysis, so enhancing the value of this parameter for differentiating neoplastic lesions of the colon[31].
An impressive amount of literature exists about the clinical significance of RDW in liver disorders. Many scientists have investigated the role of RDW for predicting severity, fibrosis, inflammation and monitoring therapy in liver disorders. The clinical usefulness of RDW has then been established in several studies in patients with liver disorders such as non-alcoholic fatty liver disease (NAFLD), viral hepatitis, hepatocellular carcinoma and primary biliary cirrhosis (PBC).
Cengiz et al[32] showed that patients with nonalcoholic steatohepatitis had higher RDW values compared to those with simple steatosis and healthy individuals. They also observed that RDW values were independently associated with severity of fibrosis, wherein more severe fibrosis was accompanied by higher RDW values[32]. Gao et al[33] showed that an increase in the viral load was accompanied by enhanced RDW in patients with hepatitis B, thereby suggesting that RDW may serve as an indicator of disease stage and treatment response. Xu et al[34] also compared RDW values in hepatitis B patients with moderate to severe liver fibrosis and those with absent or mild fibrosis, and found that RDW values were useful for predicting both liver fibrosis and necrotic inflammatory changes. Similarly, Lou et al[35] showed that RDW values could be used to assess the disease states in patients with hepatitis B virus infection. In patients with chronic hepatitis B (CHB) and liver cirrhosis related to CHB, Huang et al[36] observed that RDW values were high and correlated with the severity of cirrhosis in terms of Child-Pugh scores and model for end-stage liver disease (MELD) scores. Wang et al[37] confirmed these findings by demonstrating that RDW may be useful for predicting liver fibrosis in patients with chronic autoimmune hepatitis and PBC, while globulin value may help assessing liver inflammation (Table 2)[38,39]. Several investigators studied the RDW to platelet ratio (RPR) in liver disorders, and analyzed its efficiency for predicting severity of liver fibrosis and cirrhosis. Cengiz et al[40] reported that the RPR index has a good predictive value for significant and advanced liver fibrosis in NAFLD. Taefi et al[41] found that the RPR ratio was a stronger predictor of severity of fibrosis and cirrhosis in patients with chronic hepatitis having native liver compared to RDW and MELD score. No significant correlations of these variables were found in transplanted livers. Karagoz et al[42] showed that RPR and mean platelet volume (MPV) are associated with severity of fibrosis in patients with chronic hepatitis C, so allowing prognostic evaluation and minimizing the need for liver biopsy. These authors also confirmed the significance of MPV and RDW in prognostic evaluation of CHB[42]. Chen et al[43] also reported that RPR can predict significant fibrosis and cirrhosis in CHB patients. Lee et al[44] found that the RPR may be useful for assessing liver fibrosis in patients with CHB, so reducing the need for liver biopsy. On the other hand, a study by Thandassery et al[45] compared noninvasive scores with liver biopsy fibrosis stages in patients with chronic hepatitis C, and showed that MPV and RPR had low predictive accuracy for fibrosis stages. The role of RDW in liver disorders is double. First, it may represent a significant prognostic indicator of liver disease severity, fibrosis and inflammation. Then, its use in clinical practice could reduce the need for liver biopsy.
| Ref. | No. of subjects | Study period | Liver pathology | Outcome measures | RDW value (%) | Statistics | Other laboratory studies | Main findings |
| Lou et al[35], 2012 | 16 AHB | August 1st, 2010 | AHB, CHB, CHB-severe | RDW association with HBV related liver disease states and mortality | 14.38 ± 1.72 (AHB) | P < 0.05 | ALT, total bilirubin, total protein, albumin, WBC, Hb, MCV, INR, Creatinine, BUN, HBsAg HBeAg, HBcAb IgM, HBV DNA | RDW is significantly increased in HBV infected patients compared to controls, and RDW is an independent predicting factor for the 3 mo mortality rate in HBV infected patients. |
| 61 CHB | August 1st, 2011 | MELD score | 16.37 ± 2.43 (CHB) | P < 0.001 | ||||
| 46 CHB-severe | 18.3 ± 3.11 (CHB-severe) | P < 0.001 | ||||||
| 48 healthy controls | 13.03 ± 1.33 healthy controls | |||||||
| NASH (Brunt’s criteria) | P < 0.01 | Liver biopsy, | Patients with NASH had higher RDW relative to simple steatosis and healthy control groups. | |||||
| Cengiz et al[32], 2013 | 62 NASH | Jan-10 | Advanced fibrosis (2-4 points) | RDW association with NASH and fibrosis | NASH 14.28 ± 0.25 | P < 0.01 | Hb, platelets, MPV, WBC, lymphocytes, | RDW was higher in patients with advanced fibrosis compared to mild |
| 32 simple steatosis | May-13 | Mild fibrosis (0-1) | Simple steatosis 13.37 ± 0.12 | ALT, AST, GGT Albumin, BUN, Creatinine, alkaline phosphatase | ||||
| 30 healthy controls | Healthy controls 12.96 ± 0.14 | |||||||
| Advanced fibrosis 15.86 ± 0.4 | ||||||||
| Mild fibrosis 13.63 ± 0.67 | ||||||||
| Yang et al[38], 2013 | 1637 normal control | Individuals were initially enrolled during 2010 | NAFLD criteria presence of definite hepatic steatosis on US scan (grade 3), and exclusion of secondary hepatic steatosis. | RDW in NAFLD patients | 12.96 ± 1.08 (control) | P = 0.000 | Total cholesterol, TG, Fasting glucose, Hb | RDW was increased in NAFLD patients |
| 619 NALFD | 13.23 ± 1.01 (NAFLD) | |||||||
| Kim et al[55], 2013 | 24547 NAFLD patients | Individuals were enrolled in 2010 (January 1st to December 30th) | NAFLD diagnosis by US and questionnaires about alcohol consumption. Degree of liver fibrosis by BARD and FIB-4 scores | RDW and the level of fibrosis in NAFLD | 12.59±0.62 BARD score (0,1) | P < 0.001 | Hb, MCV, LDL, TG, HDL, HbA1C, high sensitivity CRP, ferritin, Platelet | Increased RDW is independently associated with advanced fibrosis in NAFLD |
| 12.99 ± 0.85 (BARD score 2-4) | P < 0.001 | |||||||
| 12.61±0.77 (FIB-4 score < 1.3) | ||||||||
| 12.89 ± 0.71(FIB-4 score ≥ 1.3) | ||||||||
| Karagoz et al[42], 2014 | 229 biopsy proven naïve chronic hepatitis B (CHB) patients | January 2010 and November 2013 | Fibrosis in CHB (Ishak score) | Relationship of RDW and MPV with the severity of fibrosis in CHB patients | 12.6 (cut off) | 91.50% | Liver biopsy, WBC, Hb, Ht, platelets, MPV, PDW, AST, ALT, total bilirubin, albumin, | RDW and MPV are significantly higher in HBV infected patients with severe fibrosis |
| Sensitivity | ||||||||
| 42.50% | ||||||||
| Specificity | ||||||||
| Huang et al[36], 2014 | 69 CHB | January 2011 and October 2013 | HBV related liver cirrhosis | Correlation of RDW with HBV cirrhosis, CHB; Child-Pugh and MELD scores | 16.07 ± 2.41 (HBV cirrhosis) | P < 0.01 | AST, ALT, total bilirubin, albumin, WBC, Hb, platelets, INR, Creatinine, BUN. HBeAg, HBV DNA | RDW was elevated in HBV related cirrhosis and CHB relative to control, and was positively correlated with severity of HBV related cirrhosis |
| 61 HBV liver cirrhosis | Child-Pugh and MELD scores | 13.29 ± 1.09 (CHB) | ||||||
| 41 controls | 12.75 ± 0.7 (controls) | |||||||
| Dogan et al[39], 2015 | 54 NASH | Dec-10 | NASH (NAFLD activity score) | Inflammation in NASH | 13.3 (cut off) | 79.50% | Liver biopsy, | RDW is a specific and sensitive method to assess inflammation in NASH patients |
| 39 controls | Mar-12 | Fibrosis, 0 not significant (F0-F1); 1 significant (F2-F4) | Sensitivity | Ht, MCV platelets, ALT, AST, GGT LDL, HDL, TG, Fasting glucose, insulin, | ||||
| Steatosis, 0 mild (grade 1); 1 moderate to severe (grade 2-3) | 73.30% | Alkaline phosphatase | ||||||
| 0 lobular inflammation (0-1); | Specificity | |||||||
| 1 moderate-severe (2-3) | ||||||||
| Xu et al[34], 2015 | 446 HBV infected patients who underwent liver biopsy | January 2010 and December 2011 | Liver fibrosis (no significant S0-S2, fibrosis vs advanced, S3-S4) | RDW in liver fibrosis and inflammation | 13.3 (S0-S2) | P = 0.01 | Liver biopsy, AST, ALT, total bilirubin, albumin, WBC, Hb, platelets, MCV, MPV, HBeAg, HBV DNA | RDW, together with other serum markers, could be useful in predicting liver fibrosis and necroinflammation in HBV infected patients |
| Inflammation (no significant (G0-G2) vs significant (G3-G4) | 13.6 (S3-S4) | P < 0.001 | ||||||
| 13.2 (G0-G2) | ||||||||
| 13.7 (G3-G4) | ||||||||
| Wang et al[37], 2016 | 116 CHB | January 2010 to January 2015 | Liver fibrosis and inflammation: absent-mild (S0-S1, G0-G1) vs moderate-severe (S2-S4, G2-G4) | RDW association with liver fibrosis and inflammation in chronic hepatitis | 13.4 (S0-S1) | P < 0.001 | AST, ALT, alkaline phosphatase, GGT, globulin, total bilirubin, total bilirubin acid, total protein, albumin, WBC, RBC, Hb, MCV, platelets | RDW and globulin could be useful predictors of liver fibrosis and inflammation in chronic hepatitis patients, respectively. |
| 65 PBC | 14.5 (S2-S4) | |||||||
| 37 AIH | 13.0 (G0-G1) | |||||||
| 14.2 (G2-G4) |
RPR was also shown to be related to histologic severity in treatment-naïve PBC. Seventy three patients were divided in two groups: Early stage (stage I) and advanced stage (Stage II, III and IV) of liver fibrosis as per Ludwig and Scheuer criteria. RPR was found to have 0.47 sensitivity and 0.96 specificity, performing better than Fibrosis-4score for predicting the severity of liver fibrosis[46]. In another study, RPR was not associated with histologic severity in PBC. However, in this investigation patients were divided in two groups, early (I and II) and late (III and IV) stage, as per histologic criteria[47] and did not consider the impact of confounding factors on results. In another study, 194 patients with biliary obstruction were studied. A RDW value of 14.8% was found to predict malignant obstruction with 0.72 sensitivity and 0.69 specificity. Both the hemoglobin value and MCV were not significantly different in either group[48].
The significance of RDW has also been evaluated in HCC. Smirne et al[49] performed a retrospective study in a training cohort (n = 208) and in an independently prospectively collected validated cohort (n = 106) of patients with HCC. In both cohorts, median survival time was significantly lower in patients with RDW ≥ 14.6% at the time of diagnosis, and RDW remained independently associated with survival in multivariate analysis[49]. In another study, Wei et al[50] retrospectively evaluated 110 treatment-naïve HCC patients, reporting that the RDW admission value was significantly higher in HCC patients than in healthy controls. RDW was also found to be correlated with liver function tests but not with tumor staging at the time of diagnosis[50]. Zhao et al[51] investigated the significance of RDW in patients undergoing curative radical resection of HCC, and found that patients with high preoperative RDW value (> 14.5%) had significantly worse survival than those with lower values. RDW remained independently associated with overall survival in multivariate analysis[51].
In a recent retrospective study, Caire et al[52] investigated the utility of RDW for predicting mortality in post-liver transplant patients. They found that at-transplant RDW values was a prognostic factor of 1-year mortality in liver transplant patients. Notably, RDW outperformed all other liver pre-transplant prognostic laboratory value, including serum total bilirubin, prothrombin time, bicarbonate, WBC count and MELD score[52].
Despite these promising results, some authors raised concerns about the use of RDW alone in the prognostic evaluation of liver disorders. Gulcan Kurt et al[53] and Balta et al[54] argued about the specificity of RDW for predicting the extent of fibrosis in chronic liver diseases, pointing out that other confounding factors (e.g., inflammatory biomarkers) should also be considered. Kim et al[55] showed that RDW was an independent predictor of nonalcoholic fatty liver disease. However, Kang and Kim highlighted that this index should be used in combination with other inflammatory markers for reaching more efficient diagnostic efficiency[56].
Acute pancreatitis (AP) is one of the most frequent GI causes of hospital admissions in the United States, with an annual incidence of 13 to 45/100000 persons[57]. Despite most of patients with AP have mild and self-limited disease, nearly 20% of them develop severe disease, which is in turn associated with a mortality rate of 7%-42%. Due to high mortality and high rate of complications, patients with severe pancreatitis need an early diagnosis. Multiple scoring systems, including Ranson’s criteria, Acute Physiology and Chronic Health Evaluation, Glasgow scores, Blathazar score, BISAP score, Revised Atlanta criteria and classification, have been developed and evaluated for predicting severity of pancreatitis in early disease course, but these are not easily usable by physicians due to the cumbersome calculation, especially in emergency settings[58]. Recently, Blood Urea Nitrogen has emerged as a promising prognostic marker in AP, but its value has not been extensively evaluated in patients with chronic kidney disease. Therefore, a simple, reproducible, cost-effective and specific prognostic marker in AP is still lacking.
Uçar Karabulut et al[59] studied 104 patients with AP and found that the mean admission RDW value was significantly higher during the acute phase compared to after recovery (P < 0.01). Although patients with severe pancreatitis were excluded and the severity of disease was not analyzed in this study, an increased RDW was identified as a reliable marker of AP, so suggesting that this parameter can be used for early detection and prognostic evaluation[59]. Cetinkaya et al[60] performed both univariate and multivariate analyses in a retrospective cohort study of 102 patients, identifying both RDW and RPR on admission were independent and significant factors for predicting the risk of in-hospital mortality in APs (P = 0.001). The association between RDW and mortality in AP was also confirmed by Yao et al[61] in a cross-sectional study, showing that non-survivors had higher values than survivors. The RDW displayed 0.75 sensitivity and 0.90 specificity for predicting mortality in AP[61]. Şenol et al[62] found that RDW was the only admission variable predicting AP mortality in multivariate analysis. A RDW value of 14.8% was found to predict mortality in 77% cases[62]. Wang et al[63] also showed that AP patients with RDW > 13.4% had significantly higher mortality rate than those with lower RDW values. A RDW value of 14.3% was characterized by 0.88 sensitivity and 0.92 specificity for predicting mortality in AP[63]. In another study based on the Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC II) database showed that higher RDW value predicts mortality in AP with an AUC of 0.66 (95%CI: 0.52-0.81)[64]. However, authors could not comment on confounding factors causing elevation in RDW[65]. Despite the differences in the cut-off values used in the various studies and relative heterogeneity of sensitivity and specificity values associated with these cut-offs, the available data supports the conclusions that RDW may be a sensitive predictor of mortality in patients with AP. Only one study failed to show an association between RDW and mortality in AP. However, patients with anemia, malignancy, kidney and hepatic diseases were also included in the final analysis, so potentially flawing the outcome[66]. Notably, Peng et al[67] also showed that a high RDW at admission was an independent risk factor for acute pancreatitis-associated lung injury (OR = 2.671, 95%CI: 1.145-6.138; P = 0.026).
The significance of RDW has been evaluated in only one study in pancreatic cancer. Yilmaz et al[68] studied 104 patients undergoing pancreatic cancer surgery, who were divided them in two groups with high (> 14%) and low (< 14%) RDW values. A positive correlation was hence observed between pancreatic cancer staging and RDW, but no association was noticed between RDW and postoperative complications or morbidity[68].
Huang et al[69] studied 35 patients with intestinal tuberculosis (ITB) who were compared with healthy controls (n = 22). Patients with ITB had higher RDW, which overall displayed a better diagnostic efficiency (AUC = 0.812) than CRP (AUC = 0.176) and ESR (AUC = 0.804) in diagnosing ITB[68]. In an interesting study, Yazıcı et al[70] showed that patients with acute cholecystitis undergoing surgery had a significant decrease in RDW compared to those subjected to conservative management.
Acute Mesenteric ischemia (AMI) is a relatively uncommon, but life-threatening, condition. Early recognition and management is imperative to prevent the related complications. Kisaoglu et al[71] carried a cross-sectional study by comparing AMI patients (n = 49) with patients with abdominal pain who did not undergo surgery (n = 110). A RDW value of 15.4% had 0.41 sensitivity and 0.81 specificity for identifying AMI patients after adjustment for anemia. However, no correlation was found between RDW and size of ischemia or mortality[71]. Bilgiç et al[72] investigated the preoperative RDW value in a retrospective cohort of 61 patients with AMI, and found that increased RDW predicted both the extent of necrosis and mortality. A cut-off values of 14.8% predicted mortality in nearly 70% of cases[72].
Several lines of evidences, summarized in this article, attest that RDW may be a useful prognostic factor in a variety of GI conditions, including IBD, celiac disease, cancer of colon and esophagus, liver disorders including hepatitis and liver cancer[73], pancreatic disorders, especially AP (Figure 2).
Since RDW can be easily measured with routine blood tests, without additional technical requirements and at a rather affordable cost (i.e., that of a complete blood cell count), this parameter may be considered a valuable perspective for prognostic assessment of patients with GI disorders. Along with RDW, other related parameters such as the RPR and the hemoglobin to RDW ratio were found have a prognostic value in various conditions. Due to its efficiency as an inflammatory biomarker, as a measure of disease activity and as a prognostic indictor in GI, RDW offers several advantages over other additional tests. RDW measurement is noninvasive, So that widespread use may help minimizing the use of invasive procedure such as endoscopy and biopsy to assess the prognosis in various clinical conditions. This is especially significant in certain regions where medical facilities may be limited or inaccessible. Nevertheless, despite a large number of studies showing utility and benefit from measuring and monitoring RDW values, some factors still make the use of results challenging. These encompass the lack of prospective studies, the heterogeneity of the diagnostic and prognostic cut-offs as well as the poor generalizability of outcomes due to unmet standardization of the available techniques for measuring RDW[74]. The cut-offs of RDW may vary according to the technique used for its measurement. This may explain why some subjects may display higher or lower values when RDW is measured with different analyzers. Moreover, some people in the general population may display values higher than the reference range due to the presence of undiagnosed conditions, which may ultimately lead to increase anisocytosis. However, a single cut-off for RDW cannot be identified so far, since the various analyzers use different techniques. Therefore, the application of a universal cut-off is unfeasible until a major degree of standardization can be reached[74]. In modern haematological analysers, the RDW is conventionally calculated from the histogram of erythrocyte volumes. It is, hence, predictable that further studies aimed to more deeply investigate the full distribution graph (i.e., identifying extraordinarily large or small cells) may provide more meaningful information than the simple numerical value of RDW. Although, increased RDW seems to be a marker for severe GI disease, perhaps, but possibly not for functional GI disorders such as irritable bowel syndrome. This is probably due to the fact that functional disorders have a much lower impact on erythrocyte biology, so that RBC still display a normal turnover. Then, the role of the many confounding factors which may have an impact on RBC biology is still incompletely understood and evaluated. Therefore, due to the mounting evidence regarding the usefulness and the considerable diagnostic potential of RDW,further prospective investigations are needed to validate its effectiveness and concrete steps should be undertaken to define standards that can serve as guidelines for effectively using RDW as a tool for diagnostic and prognostic assessment.
The authors thank Dr. Roxana Coman MD for critical final review of the manuscript and Dr. Edwin Grimsley MD MACP for final review and developing audio core tip about the manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Adams JB S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Daland GA, Heath CW, Minot GR. Differentiation of pernicious anemia and certain other macrocytic anemias by the distribution of red blood cell diameters. Blood. 1946;1:67-75. [PubMed] |
| 2. | Lippi G, Mattiuzzi C, Cervellin G. Learning more and spending less with neglected laboratory parameters: the paradigmatic case of red blood cell distribution width. Acta Biomed. 2017;87:323-328. [PubMed] |
| 3. | Patel HH, Patel HR, Higgins JM. Modulation of red blood cell population dynamics is a fundamental homeostatic response to disease. Am J Hematol. 2015;90:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med. 2014;52:e197-e199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Chen GP, Huang Y, Yang X, Feng JF. A Nomogram to Predict Prognostic Value of Red Cell Distribution Width in Patients with Esophageal Cancer. Mediators Inflamm. 2015;2015:854670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Wan GX, Chen P, Cai XJ, Li LJ, Yu XJ, Pan DF, Wang XH, Wang XB, Cao FJ. Elevated red cell distribution width contributes to a poor prognosis in patients with esophageal carcinoma. Clin Chim Acta. 2016;452:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Hirahara N, Matsubara T, Kawahara D, Mizota Y, Ishibashi S, Tajima Y. Prognostic value of hematological parameters in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Int J Clin Oncol. 2016;21:909-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Sun P, Zhang F, Chen C, Bi X, Yang H, An X, Wang F, Jiang W. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: a retrospective study from southern China. Oncotarget. 2016;7:42650-42660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Hu D, Lin X, Chen Y, Chang Q, Chen G, Li C, Zhang H, Cui Z, Liang B, Jiang W. Preoperative blood-routine markers and prognosis of esophageal squamous cell carcinoma: The Fujian prospective investigation of cancer (FIESTA) study. Oncotarget. 2017;8:23841-23850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Tüzün A, Keskin O, Yakut M, Kalkan C, Soykan I. The predictive value of mean platelet volume, plateletcrit and red cell distribution width in the differentiation of autoimmune gastritis patients with and without type I gastric carcinoid tumors. Platelets. 2014;25:363-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Pietrzyk L, Plewa Z, Denisow-Pietrzyk M, Zebrowski R, Torres K. Diagnostic Power of Blood Parameters as Screening Markers in Gastric Cancer Patients. Asian Pac J Cancer Prev. 2016;17:4433-4437. [PubMed] |
| 12. | Yazici P, Demir U, Bozkurt E, Isil GR, Mihmanli M. The role of red cell distribution width in the prognosis of patients with gastric cancer. Cancer Biomark. 2017;18:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Isik B, Yilmaz MS, Yel C, Kavalci C, Solakoglu GA, Ozdemir M, Ongar M, Demirci B. Importance of red blood cell distribution width (RDW) in patients with upper gastrointestinal haemorrhage. J Pak Med Assoc. 2016;66:151-154. [PubMed] |
| 14. | Fatemi O, Torguson R, Chen F, Ahmad S, Badr S, Satler LF, Pichard AD, Kleiman NS, Waksman R. Red cell distribution width as a bleeding predictor after percutaneous coronary intervention. Am Heart J. 2013;166:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Brusco G, Di Stefano M, Corazza GR. Increased red cell distribution width and coeliac disease. Dig Liver Dis. 2000;32:128-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Balaban DV, Popp A, Lungu AM, Costache RS, Anca IA, Jinga M. Ratio of spleen diameter to red blood cell distribution width: a novel indicator for celiac disease. Medicine (Baltimore). 2015;94:e726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 17. | Sategna Guidetti C, Scaglione N, Martini S. Red cell distribution width as a marker of coeliac disease: a prospective study. Eur J Gastroenterol Hepatol. 2002;14:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Mitchell RM, Robinson TJ. Monitoring dietary compliance in coeliac disease using red cell distribution width. Int J Clin Pract. 2002;56:249-250. [PubMed] |
| 19. | Harmanci O, Kav T, Sivri B. Red cell distribution width can predict intestinal atrophy in selected patients with celiac disease. J Clin Lab Anal. 2012;26:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Guglielmi V, Manchisi M, Pellegrini V, Tutino M, Guerra V. [RDW: new screening test for coeliac disease?]. Minerva Med. 2002;93:419-421. [PubMed] |
| 21. | Clarke K, Sagunarthy R, Kansal S. RDW as an additional marker in inflammatory bowel disease/undifferentiated colitis. Dig Dis Sci. 2008;53:2521-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Molnar T, Farkas K, Szepes Z, Nagy F, Nyari T, Wittmann T. RDW can be a useful additional marker in diagnosing Crohn’s disease and ulcerative colitis. Dig Dis Sci. 2008;53:2828-2829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Cakal B, Akoz AG, Ustundag Y, Yalinkilic M, Ulker A, Ankarali H. Red cell distribution width for assessment of activity of inflammatory bowel disease. Dig Dis Sci. 2009;54:842-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Yeşil A, Senateş E, Bayoğlu IV, Erdem ED, Demirtunç R, Kurdaş Övünç AO. Red cell distribution width: a novel marker of activity in inflammatory bowel disease. Gut Liver. 2011;5:460-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Ipek S, Cekic C, Alper E, Coban E, Eliacik E, Arabul M, Aslan F, Vatansever S, Yalcin H, Unsal B. Can red cell distribution width be a marker of disease activity in ulcerative colitis? Int J Clin Exp Med. 2015;8:13848-13853. [PubMed] |
| 26. | Song CS, Park DI, Yoon MY, Seok HS, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. Association between red cell distribution width and disease activity in patients with inflammatory bowel disease. Dig Dis Sci. 2012;57:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Oliveira AM, Cardoso FS, Rodrigues CG, Santos L, Martins A, Ramos de Deus J, Reis J. Can Red Cell Distribution Width Be Used as a Marker of Crohn’s Disease Activity? GE Port J Gastroenterol. 2016;23:6-12. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Oustamanolakis P, Koutroubakis IE, Messaritakis I, Kefalogiannis G, Niniraki M, Kouroumalis EA. Measurement of reticulocyte and red blood cell indices in the evaluation of anemia in inflammatory bowel disease. J Crohns Colitis. 2011;5:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Riedl J, Posch F, Königsbrügge O, Lötsch F, Reitter EM, Eigenbauer E, Marosi C, Schwarzinger I, Zielinski C, Pabinger I. Red cell distribution width and other red blood cell parameters in patients with cancer: association with risk of venous thromboembolism and mortality. PLoS One. 2014;9:e111440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Spell DW, Jones DV Jr, Harper WF, David Bessman J. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prev. 2004;28:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Ay S, Eryilmaz MA, Aksoy N, Okus A, Unlu Y, Sevinc B. Is early detection of colon cancer possible with red blood cell distribution width? Asian Pac J Cancer Prev. 2015;16:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Cengiz M, Candır BA, Yılmaz G, Akyol G, Ozenirler S. Is increased red cell distribution width an indicating marker of nonalcoholic steatohepatitis and fibrotic stage? World J Gastroenterol. 2013;19:7412-7418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Gao P, Xiao P, Yang YL, Chen QF, Mao XR, Zhao ZB, Shi L, Yang LZ, Zhou W. [Effects and clinical significance of virus load on red blood cell parameters in different stage of hepatitis B]. Beijing Daxue Xuebao. 2014;46:941-944. [PubMed] |
| 34. | Xu WS, Qiu XM, Ou QS, Liu C, Lin JP, Chen HJ, Lin S, Wang WH, Lin SR, Chen J. Red blood cell distribution width levels correlate with liver fibrosis and inflammation: a noninvasive serum marker panel to predict the severity of fibrosis and inflammation in patients with hepatitis B. Medicine (Baltimore). 2015;94:e612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Lou Y, Wang M, Mao W. Clinical usefulness of measuring red blood cell distribution width in patients with hepatitis B. PLoS One. 2012;7:e37644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Huang R, Yang C, Wu K, Cao S, Liu Y, Su R, Xiong Y, Huang A, Wu C. Red cell distribution width as a potential index to assess the severity of hepatitis B virus-related liver diseases. Hepatol Res. 2014;44:E464-E470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Wang H, Xu H, Qu L, Wang X, Wu R, Gao X, Jin Q, Niu J. Red blood cell distribution width and globulin, noninvasive indicators of fibrosis and inflammation in chronic hepatitis patients. Eur J Gastroenterol Hepatol. 2016;28:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Yang W, Huang H, Wang Y, Yu X, Yang Z. High red blood cell distribution width is closely associated with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2014;26:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Dogan S, Celikbilek M, Zararsiz G, Deniz K, Sivgin S, Guven K, Gursoy S, Ozbakir O, Yucesoy M. Red blood cell distribution width as a non-invasive marker for the assessment of inflammation in non-alcoholic steatohepatitis. Hepatogastroenterology. 2015;62:393-398. [PubMed] |
| 40. | Cengiz M, Ozenirler S. Comparative diagnostic accuracy of red cell distribution width-to-platelet ratio versus noninvasive fibrosis scores for the diagnosis of liver fibrosis in biopsy-proven nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Taefi A, Huang CC, Kolli K, Ebrahimi S, Patel M. Red cell distribution width to platelet ratio, a useful indicator of liver fibrosis in chronic hepatitis patients. Hepatol Int. 2015;9:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Karagoz E, Ulcay A, Tanoglu A, Kara M, Turhan V, Erdem H, Oncul O, Gorenek L. Clinical usefulness of mean platelet volume and red blood cell distribution width to platelet ratio for predicting the severity of hepatic fibrosis in chronic hepatitis B virus patients. Eur J Gastroenterol Hepatol. 2014;26:1320-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Chen B, Ye B, Zhang J, Ying L, Chen Y. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One. 2013;8:e68780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 44. | Lee HW, Kang W, Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, Park YN, Han KH. Red cell volume distribution width-to-platelet ratio in assessment of liver fibrosis in patients with chronic hepatitis B. Liver Int. 2016;36:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Thandassery RB, Al Kaabi S, Soofi ME, Mohiuddin SA, John AK, Al Mohannadi M, Al Ejji K, Yakoob R, Derbala MF, Wani H. Mean Platelet Volume, Red Cell Distribution Width to Platelet Count Ratio, Globulin Platelet Index, and 16 Other Indirect Noninvasive Fibrosis Scores: How Much Do Routine Blood Tests Tell About Liver Fibrosis in Chronic Hepatitis C? J Clin Gastroenterol. 2016;50:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Wang H, Xu H, Wang X, Wu R, Gao X, Jin Q, Niu J. Red Blood Cell Distribution Width to Platelet Ratio is Related to Histologic Severity of Primary Biliary Cirrhosis. Medicine (Baltimore). 2016;95:e3114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Tahtaci M, Yurekli OT, Bolat AD, Balci S, Akin FE, Buyukasik NS, Ersoy O. Increased mean platelet volume is related to histologic severity of primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2015;27:1382-1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Beyazit Y, Kekilli M, Ibis M, Kurt M, Sayilir A, Onal IK, Purnak T, Oztas E, Tas A, Yesil Y. Can red cell distribution width help to discriminate benign from malignant biliary obstruction? A retrospective single center analysis. Hepatogastroenterology. 2012;59:1469-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 49. | Smirne C, Grossi G, Pinato DJ, Burlone ME, Mauri FA, Januszewski A, Oldani A, Minisini R, Sharma R, Pirisi M. Evaluation of the red cell distribution width as a biomarker of early mortality in hepatocellular carcinoma. Dig Liver Dis. 2015;47:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Wei TT, Tang QQ, Qin BD, Ma N, Wang LL, Zhou L, Zhong RQ. Elevated red blood cell distribution width is associated with liver function tests in patients with primary hepatocellular carcinoma. Clin Hemorheol Microcirc. 2016;64:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Zhao T, Cui L, Li A. The significance of RDW in patients with hepatocellular carcinoma after radical resection. Cancer Biomark. 2016;16:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Caire MT, Kumar A, Stravitz RT, Kemmer N. Preliver transplant red cell distribution width predicts postliver transplant mortality. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Gulcan Kurt Y, Cayci T, Aydin FN, Agilli M. Red cell distribution width and nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:16387-16388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Balta S, Demir M, Kurtoglu E, Demirkol S. Red cell distribution width in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2014;26:361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Kim HM, Kim BS, Cho YK, Kim BI, Sohn CI, Jeon WK, Kim HJ, Park DI, Park JH, Joo KJ. Elevated red cell distribution width is associated with advanced fibrosis in NAFLD. Clin Mol Hepatol. 2013;19:258-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Kang W, Kim SU. Chasing after novel non-invasive markers to identify advanced fibrosis in NAFLD. Clin Mol Hepatol. 2013;19:255-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1347] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 58. | Goyal H, Smith B, Bayer C, Rutherford C, Shelnut D. Differences in Severity and Outcomes Between Hypertriglyceridemia and Alcohol-Induced Pancreatitis. N Am J Med Sci. 2016;8:82-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Uçar Karabulut K, Narcı H, Uçar Y, Uyar M. Association between red blood cell distribution width and acute pancreatitis. Med Sci Monit. 2014;20:2448-2452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Cetinkaya E, Senol K, Saylam B, Tez M. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol. 2014;20:14450-14454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Yao J, Lv G. Association between red cell distribution width and acute pancreatitis: a cross-sectional study. BMJ Open. 2014;4:e004721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Şenol K, Saylam B, Kocaay F, Tez M. Red cell distribution width as a predictor of mortality in acute pancreatitis. Am J Emerg Med. 2013;31:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 63. | Wang D, Yang J, Zhang J, Zhang S, Wang B, Wang R, Liu M. Red cell distribution width predicts deaths in patients with acute pancreatitis. J Res Med Sci. 2015;20:424-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Hu ZD, Wei TT, Tang QQ, Fu HT, Yang M, Ma N, Wang LL, Qin BD, Zhou L, Zhong RQ. Prognostic value of red blood cell distribution width in acute pancreatitis patients admitted to intensive care units: an analysis of a publicly accessible clinical database MIMIC II. Clin Chem Lab Med. 2016;54:e195-e197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Goyal H, Gupta S, Singla U. Level of red cell distribution width is affected by various factors. Clin Chem Lab Med. 2016;54:e387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Gülen B, Sonmez E, Yaylaci S, Serinken M, Eken C, Dur A, Turkdogan FT, Söğüt Ö. Effect of harmless acute pancreatitis score, red cell distribution width and neutrophil/lymphocyte ratio on the mortality of patients with nontraumatic acute pancreatitis at the emergency department. World J Emerg Med. 2015;6:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Peng YF, Zhang ZX, Cao W, Meng CR, Xu SS, Zhang Q. The association between red blood cell distribution width and acute pancreatitis associated lung injury in patients with acute pancreatitis. Open Med (Wars). 2015;10:176-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Yilmaz A, Malya F, Ozturk G, Citgez B, Ozdenkaya Y, Ersavas C, Agan A, Senturk H, Karatepe O. Effect of pre-operative red blood cell distribution on cancer stage and morbidity rate in patients with pancreatic cancer. Int J Clin Exp Med. 2014;7:3072-3075. [PubMed] |
| 69. | Huang S, Yi FM, Zhou R, Chen M, Lei Y, Zhao JZ, Zhang H, Xia B. The utility of platelet, mean platelet volume, and red cell distribution width in the diagnosis of active Crohn’s disease and intestinal tuberculosis. Saudi Med J. 2013;34:1161-1166. [PubMed] |
| 70. | Yazıcı P, Demir U, Bozdağ E, Bozkurt E, Işıl G, Bostancı Ö, Mihmanlı M. What is the effect of treatment modality on red blood cell distribution width in patients with acute cholecystitis? Ulus Cerrahi Derg. 2015;31:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Kisaoglu A, Bayramoglu A, Ozogul B, Atac K, Emet M, Atamanalp SS. Sensitivity and specificity of red cell distribution width in diagnosing acute mesenteric ischemia in patients with abdominal pain. World J Surg. 2014;38:2770-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Bilgiç I, Dolu F, Şenol K, Tez M. Prognostic significance of red cell distribution width in acute mesenteric ischemia. Perfusion. 2015;30:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 73. | Goyal H, Hu ZD. Prognostic value of red blood cell distribution width in hepatocellular carcinoma. Ann Transl Med. 2017; Epub ahead of print. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Lippi G, Pavesi F, Bardi M, Pipitone S. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem. 2014;47:1100-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |