Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4839
Peer-review started: March 7, 2017
First decision: March 30, 2017
Revised: April 12, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: July 14, 2017
Processing time: 126 Days and 21.9 Hours
To determine the diagnostic accuracy of two-dimensional shear wave elastography (2D-SWE) for the non-invasive assessment of liver fibrosis in patients with autoimmune liver diseases (AILD) using liver biopsy as the reference standard.
Patients with AILD who underwent liver biopsy and 2D-SWE were consecutively enrolled. Receiver operating characteristic (ROC) curves were constructed to assess the overall accuracy and to identify optimal cut-off values.
The characteristics of the diagnostic performance were determined for 114 patients with AILD. The areas under the ROC curves for significant fibrosis, severe fibrosis, and cirrhosis were 0.85, 0.85, and 0.86, respectively, and the optimal cut-off values associated with significant fibrosis (≥ F2), severe fibrosis (≥ F3), and cirrhosis (F4) were 9.7 kPa, 13.2 kPa and 16.3 kPa, respectively. 2D-SWE showed sensitivity values of 81.7% for significant fibrosis, 83.0% for severe fibrosis, and 87.0% for cirrhosis, and the respective specificity values were 81.3%, 74.6%, and 80.2%. The overall concordance rate of the liver stiffness measurements obtained using 2D-SWE vs fibrosis stages was 53.5%.
2D-SWE showed promising diagnostic performance for assessing liver fibrosis stages and exhibited high cut-off values in patients with AILD. Low overall concordance rate was observed in the liver stiffness measurements obtained using 2D-SWE vs fibrosis stages.
Core tip: The study determined the diagnostic accuracy of two-dimensional shear wave elastography (2D-SWE) for the non-invasive assessment of liver fibrosis in patients with autoimmune liver diseases (AILD) using liver biopsy as the reference standard. The characteristics of the diagnostic performance were determined for 114 patients with AILD. The areas under the receiver operating characteristic curves for significant fibrosis, severe fibrosis, and cirrhosis were 0.85, 0.85 and 0.86, respectively, and the optimal cut-off values were 9.7 kPa, 13.2 kPa and 16.3 kPa, respectively. 2D-SWE showed promising diagnostic performance in assessing liver fibrosis stages and exhibited high cut-off values in patients with AILD.
- Citation: Zeng J, Huang ZP, Zheng J, Wu T, Zheng RQ. Non-invasive assessment of liver fibrosis using two-dimensional shear wave elastography in patients with autoimmune liver diseases. World J Gastroenterol 2017; 23(26): 4839-4846
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4839.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4839
Autoimmune liver diseases (AILD) are a group of diseases characterized by an anomalous immune response that is directed at hepatocytes or bile ducts along with the presence of serum antimitochondrial antibodies and a potential tendency to progress to cirrhosis. Autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC) are the three major forms of AILD, which differ according to the focus of the autoimmune injury, the pattern of inflammation, and the clinical phenotype[1,2]. These diseases are important because they may result in chronic liver damage with fibrosis and cirrhosis. An evaluation of the degree of liver fibrosis is important for determining medical management and prognosis. Liver biopsy (LB) is still considered the reference standard for the evaluation of liver fibrosis[3]. However, LB is an invasive procedure, limiting its use for screening and frequent follow-up. The demand for a non-invasive and reliable test to evaluate liver fibrosis in patients with AILD has increased.
Two-dimensional shear wave elastography (2D-SWE), an ultrasound elastography technique based on shear waves that is available on a clinical diagnostic ultrasound scanner, has been used to non-invasively measure liver fibrosis[4]. The 2D-SWE technique creates a real-time, two-dimensional quantitative map of liver tissue stiffness under the guidance of very-high-frame-rate B mode imaging[5]. This method has good diagnostic accuracy for the staging of liver fibrosis in patients with chronic liver diseases[6-11]. However, studies on the use of 2D-SWE to assess histologically confirmed liver fibrosis in patients with AILD have not been published. AILD are considered a relatively uncommon etiology in the Asia-Pacific region, where viral hepatitis is the primary diagnosis in the majority of patients with chronic liver diseases. However, based on recent findings, the prevalence of both AIH and PBC is increasing worldwide[12]. Despite advances in the understanding and treatment of AILD, areas of unmet need remain.
Therefore, the goal of this study was to assess the diagnostic accuracy of 2D-SWE for the non-invasive staging of liver fibrosis in patients with AILD. LB samples that were scored with the histology-based METAVIR staging system were used as the diagnostic reference standard.
Informed consent was obtained from all patients, and the study including the 2D-SWE examination was approved by the clinical medical research ethics committee of our hospital. One hundred and thirty-nine patients with AILD who underwent LB and 2D-SWE examinations were enrolled consecutively between April 2011 and March 2016. The diagnoses of AIH, PBC and PSC in all patients were confirmed by histological evidence[13-18]. All patients were not under immunosuppressive treatment at time of the LB and 2D-SWE examinations. The time intervals between LB and 2D-SWE were less than three days. The exclusion criteria included: patients younger than 18 years; a lack of consent for the 2D-SWE examination or LB; chronic liver disease accompanied by hepatitis virus infection or another disease; and LB samples less than 15 mm long or with fewer than 6 portal tracts under the microscopic examination. The following data were collected from all patients: liver stiffness measurements (LSM) obtained using 2D-SWE; fibrosis stages; necroinflammatory activity grades; age; gender; weight; height; alanine aminotransferase, aspartate aminotransferase, serum alkaline phosphatase (ALP), gamma-glutamyl transpeptidase, total bilirubin, and serum albumin concentrations; platelet count; and prothrombin activity. Body mass index was calculated as body weight (kg)/[height (m2)].
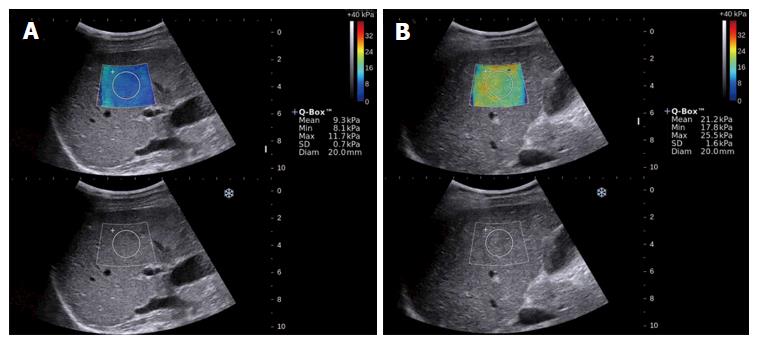
2D-SWE was performed within three days of LB. Two radiologists (Zeng J and Zheng J) performed the 2D-SWE examinations. Both radiologists had at least 6 mo of experience in performing 2D-SWE examinations. The radiologists were blinded to the patients’ clinical information and pathology results. 2D-SWE was performed using the Aixplorer US system (SuperSonic Imagine, France) with a convex broadband probe (SC6-1, 1-6 MHz). All patients had fasted for at least 6 h prior to the examination. 2D-SWE measurements were obtained from the right lobe of the liver through the intercostal spaces when the patient was lying in the supine position and the right arm was positioned in maximal abduction. The operator positioned the target area of the liver under the guidance of conventional, real-time B-mode imaging. When the target area was located, SWE was launched, and the patient was asked to hold his or her breath for approximately 5 s during a quiet breathing period. The elasticity image box, which was approximately 4 cm × 3 cm, was set 1-2 cm deeper than Glisson’s capsule of the liver and in an area of the liver parenchyma that was free of large vessels.
A circular region of interest with a 2-cm diameter was drawn inside the elasticity image box, and the mean, minimum and maximum liver stiffness and SD were calculated (Figure 1). The mean value was used to represent the LSM for each 2D-SWE image. Five consecutive 2D-SWE images were obtained for each patient. Each measurement was performed during a separate breath hold. The mean value of the five 2D SWE measurements was calculated for statistical analysis. The entire 2D-SWE examination lasted three to five minutes for each patient. Five consecutive 2D-SWE images were obtained from each patient. Measurements were considered failures when no or little signal was obtained in the SWE box for any of the acquisitions[8].
Ultrasonography-assisted percutaneous LB was performed in the right liver lobe using a 16-gauge Magnum needle (Bard, Tempe, AZ). The LB specimens were fixed in formalin and embedded in paraffin. The biopsy specimens were analyzed by two expert liver pathologists with more than 10 and 20 years of experience, respectively, and who were blinded to the results of 2D-SWE but not to the clinical and biochemical data for each patient. Liver fibrosis and necroinflammatory activity were semiquantitatively evaluated using the METAVIR scoring system[19]. Liver fibrosis was staged on a scale from 0 to 4 according to the METAVIR scoring system: stage F0: no fibrosis; F1: portal fibrosis without septa; F2: portal fibrosis and few septa; F3: numerous septa without cirrhosis; and F4: cirrhosis[19]. Significant fibrosis was defined as stage F2 or higher, and severe fibrosis was defined as stage F3 or higher. Necroinflammatory activity was graded as follows: A0 = none; A1 = mild; A2 = moderate; and A3 = severe[19].
The demographic, clinical, and laboratory values are summarized using descriptive statistics. The data were first tested for normality using the one-sample Kolmogorov-Smirnov test. Spearman’s rank coefficient was used to determine the correlation between two study variables. Receiver operating characteristic (ROC) curves were constructed to assess the overall accuracy of the LSMs and to identify optimal cut-off values. Areas under the ROC curves (AUROCs) were compared using the method described by DeLong et al[20] for correlated data. The optimal cut-off values were the points with the highest Youden’s index[21]. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated from the same data. The positive likelihood ratio (LR+) and negative likelihood ratio (LR-) were calculated from the respective sensitivity and specificity values. All factors collected from patients with AILD were entered in a multivariate logistic model to analyze the disagreement between LSMs obtained using 2D-SWE and significant fibrosis or cirrhosis. ORs were estimated from the model and are presented with their 95%CIs. Continuous variables, such as age, were dichotomized around the median (46 years), unless a cut-off was considered clinically relevant.
All statistical tests were two-sided, and the alpha value was set at 0.05. Statistical analyses were performed using SPSS software for Windows, version 13.0 (SPSS, Chicago, IL, United States), and MedCalc software, version 12.7.0 (MedCalc Software, Mariakerke, Belgium).
One hundred and thirty-nine patients were enrolled in the study. Twenty-two patients were not included based on the exclusion criteria, including 2 patients who were younger than 18 years, 14 patients with biopsy samples less than 15 mm long or with fewer than six portal tracts under the microscope, 1 patient with a hepatitis A virus coinfection, 3 patients with hepatitis B virus coinfections, and 2 patients with hepatitis C virus coinfections. Therefore, 117 patients with AILD defined by a reliable reference standard were consecutively enrolled in the study. The 2D-SWE examination failed in three patients. Therefore, 114 patients with reliable LSMs obtained using 2D-SWE were used to assess diagnostic accuracy. The characteristics of the 114 patients are summarized in Table 1. Fibrosis conditions that were scored as significant fibrosis (≥ F2), severe fibrosis (≥ F3), and cirrhosis (F4) were detected in 71.9%, 41.2%, and 20.2% of patients, respectively. The numbers of patients with different fibrosis stages of AILDs are reported in Table 2.
| Characteristic | n = 114 |
| Age, yr (SD; range) | 45.6 (12.5; 18-74) |
| Gender, male, n (%) | 21 (18.4) |
| BMI, kg/m2 (SD; range) | 21.6 (3.0; 15.4-36.4) |
| AST, IU/L (IQR; range) | 84.0 (51.3-148.0;16.0-473.0) |
| ALT, IU/L (IQR; range) | 78.5 (49.5-142.0; 9.0-920.0) |
| Alkaline phosphatase, IU/L (IQR; range) | 208 (110.0-353.0; 47.0-873.0) |
| Gamma-glutamyl transferase, IU/L (IQR; range) | 252.0 (111.0-523.8; 16.0-1535.0) |
| Total bilirubin, umol/L (IQR; range) | 20.3 (13.6-43.6; 3.6-375.8) |
| Serum albumin, g/L (SD; range) | 37.6 (5.0; 25.9-47.0) |
| Platelets count, 109/L ((SD; range) | 188.2 (69.5; 28.0-414.0) |
| Prothrombin activity, % (SD; range) | 106.6 (22.6; 50.0-158.0) |
| METAVIR fibrosis stage1 | |
| F0 | 4 (0.04) |
| F1 | 28 (24.6) |
| F2 | 35 (30.7) |
| F3 | 24 (21.1) |
| F4 | 23 (20.2) |
| METAVIR activity grade2 | |
| A0 | 2 (0.02) |
| A1 | 17 (14.9) |
| A2 | 48 (42.1) |
| A3 | 47(41.2) |
| F0-F1 (n) | F2 (n) | F3 (n) | F4 (n) | |
| AIH | 19 | 18 | 9 | 16 |
| PBC | 8 | 10 | 8 | 4 |
| PSC | 1 | 1 | 0 | 1 |
| PBC-AIH | 4 | 6 | 7 | 2 |
| Total | 32 | 35 | 24 | 23 |
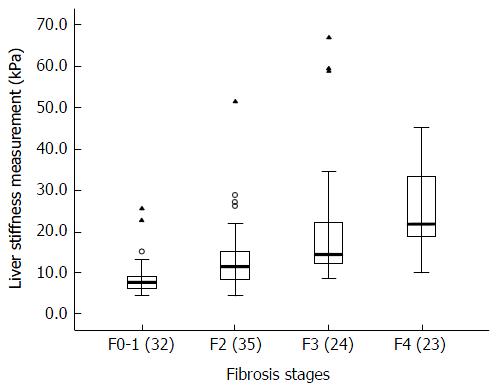
The median values, interquartile ranges, ranges, and P values of the measurements obtained for each fibrosis stage with 2D-SWE are shown in Table 3. As the fibrosis stage progressed, the median LSM of the fibrosis stage increased on 2D-SWE (Figure 2). The LSMs of patients by 2D-SWE at a given fibrosis stage had significantly higher median LSMs obtained using 2D-SWE than patients with less fibrosis (P < 0.05). Spearman’s correlation coefficients for LSMs and fibrosis stages were 0.68 (P < 0.001) (95%CI: 0.57-0.77).
| METAVIR stage | F0-F1 | F2 | F3 | F4 |
| Median value, kPa | 7.7 | 11.7 | 14.7 | 22.0 |
| IQR | 6.5-9.2 | 8.3-15.4 | 12.3-22.3 | 18.8-33.3 |
| Range | 4.9-25.8 | 4.6-51.7 | 8.7-67.2 | 10.1-45.3 |
| P value1 | < 0.001 | 0.009 | 0.011 |
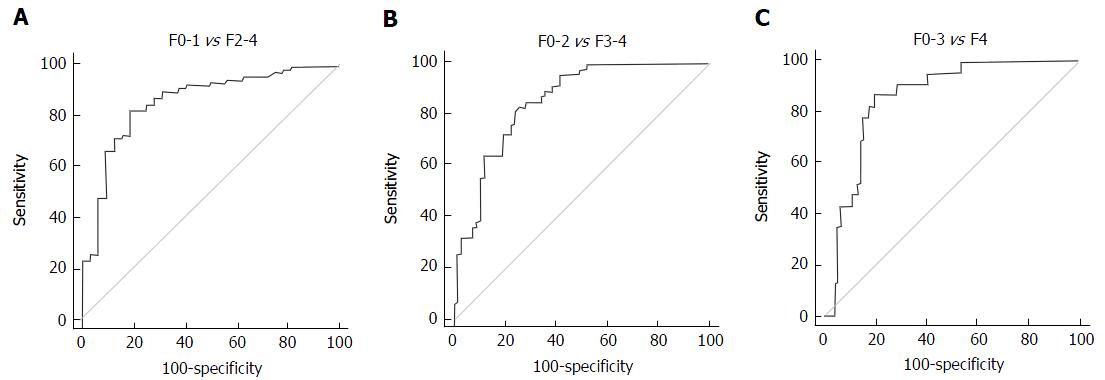
The AUROCs of 2D-SWE for significant fibrosis, severe fibrosis, and cirrhosis were 0.85 (95%CI: 0.77-0.91), 0.85 (95%CI: 0.77-0.92), and 0.86 (95%CI: 0.78-0.92), respectively (Figure 3). The optimal cut-off values for the different stages of fibrosis were determined by analyzing the ROCs. The optimal cut-off values associated with significant fibrosis (≥ F2), severe fibrosis (≥ F3), and cirrhosis (F4) were 9.7 kPa, 13.2 kPa and 16.3 kPa, respectively.
The sensitivity, specificity, PPV, NPV, LR+, and LR- for each METAVIR stage are shown in Table 4. Using the optimal cut-off values, 2D-SWE showed sensitivity and specificity values of 81.7% and 81.3% for significant fibrosis, 83.0% and 74.6% for severe fibrosis, and 87.0% and 80.2% for cirrhosis, respectively. The corresponding optimal cut-off values for patients with chronic hepatitis B (CHB) were 7.2 kPa, 9.1 kPa and 11.7 kPa, and the corresponding values for patients with chronic hepatitis C (CHC) were 7.1 kPa, 8.7 kPa and 10.4 kPa, respectively[8,22]. Using the cut-off values for CHB and CHC, the sensitivity values for assessing the stages of fibrosis were greater than 90%. However, trade-offs were observed: the accompanying specificity values for predicting significant fibrosis were less than 50%, and the accompanying specificity values for predicting severe fibrosis and cirrhosis fibrosis were approximately 50%.
| Cutoff, kPa | Se, % (95%CI) | Sp, % (95%CI) | PPV, % (95%CI) | NPV, % (95%CI) | LR+, ratio (95%CI) | LR-, ratio (95%CI) | |
| ≥ F2 | 9.71 | 81.7 (71.6-89.4) | 81.3 (63.6-92.8) | 91.8 (83.0-96.9) | 63.4 (46.9-77.9) | 4.4 (3.6-5.3) | 0.23 (0.10-0.5) |
| 7.22 | 93.9 (86.3-98.0) | 40.6 (23.7-59.4) | 80.2 (70.8-87.6) | 72.2 (46.5-90.3) | 1.6 (1.0-2.4) | 0.15 (0.06-0.4) | |
| 7.13 | 93.9 (86.3-98.0) | 37.5 (21.1-56.3) | 79.4 (70.0-86.9) | 70.6 (44.0-89.7) | 1.5 (1.0-2.4) | 0.16 (0.07-0.4) | |
| ≥ F3 | 13.21 | 83.0 (69.2-92.4) | 74.6 (62.5-84.5) | 69.6 (55.8-81.3) | 86.2 (74.6-93.9) | 3.3 (2.7-4.0) | 0.23 (0.1-0.5) |
| 9.12 | 95.7 (85.5-9.5) | 52.2 (39.7-64.6) | 58.4 (46.6-69.6) | 94.6 (81.5-99.4) | 2.0 (1.6-2.5) | 0.08 (0.02-0.3) | |
| 8.73 | 97.9 (88.7-99.9) | 49.3 (36.8-61.8) | 57.5 (45.9-68.5) | 97.1 (84.7-99.9) | 1.9 (1.5-2.5) | 0.04 (0.01-0.3) | |
| F4 | 16.31 | 87.0 (66.4-97.2) | 80.2 (70.6-87.8) | 52.6 (35.6-69.2) | 96.1 (88.9-99.2) | 4.4 (3.6-5.3) | 0.16 (0.05-0.5) |
| 11.72 | 95.7 (78.1-99.9) | 57.1 (46.3-67.5) | 36.1 (24.2-49.4) | 98.1 (89.8-100.0) | 2.2 (1.8-2.7) | 0.08 (0.01-0.5) | |
| 10.43 | 95.7 (78.1-99.9) | 48.4 (37.7-59.1) | 31.9 (21.2-44.2) | 97.8 (88.2-99.9) | 1.9 (1.5-2.3) | 0.09 (0.01-0.6) |
Table 5 shows the concordance rates of the LSMs obtained using 2D-SWE vs METAVIR stages. Overall, 2D-SWE correctly classified 61 of 114 (53.5%) patients. 2D-SWE returned the highest rates of correctly classified patients at the F0-1 stage at 63.4%. 2D-SWE showed lower rates of correctly classified patients at other stages, particularly the F2 and F3 stages. The concordance rates of the F2 and F3 stages were less than 50%.
| METAVIR stage | Concordance rate | |||||
| F0-F1 | F2 | F3 | F4 | Total | ||
| F0-F1, ≤ 9.7kPa | 26 | 13 | 2 | 0 | 41 | 63.4% |
| F2, > 9.7 to ≤ 13.2 kPa | 3 | 7 | 4 | 2 | 16 | 43.8% |
| F3, > 13.2 to ≤ 16.3 kPa | 1 | 9 | 8 | 1 | 19 | 42.1% |
| F4, > 16.3 kPa | 2 | 6 | 10 | 20 | 38 | 52.6% |
| Cumulative concordance | 53.5% | |||||
According to the multivariate logistic regression analysis, the disagreement between LSMs obtained using 2D-SWE and cirrhosis was independently associated with the following factors: age greater than 46 years (OR = 7.5, 95%CI: 1.7-33.3, P = 0.008), abnormal ALP levels (OR = 14.0, 95%CI: 1.5-129.1, P = 0.02) and abnormal serum albumin levels (OR = 11.6, 95%CI: 3.2-42.0, P < 0.001). No factors were significantly associated with the disagreement between LSMs obtained using 2D-SWE and significant fibrosis.
This study included a cohort of patients with AILD and aimed to evaluate the diagnostic accuracy of 2D-SWE for the non-invasive staging of hepatic fibrosis using LB as the reference standard. AILD are divided into two groups; the first group is predominantly characterized by hepatocellular damage and its prototype is AIH. The second group is characterized by cholestasis and includes PBC and PSC[23]. AIH, PBC and PSC represent complex disorders, as they result from interactions between genetic and environmental factors. In AIH, autoimmune injury affects hepatocytes, leading to the histological manifestation of interface hepatitis. In PBC, autoimmune injury affects the small, interlobular bile ducts, causing the typical appearance of non-suppurative, destructive cholangitis. In PSC, autoimmune or immune-mediated injury affects the medium-sized intra- and extrahepatic bile ducts, causing concentric and obliterative fibrosis and multifocal bile duct structuring[1]. However, AIH, PBC and PSC share pathophysiologic mechanisms[24,25]. Overlap syndromes encompass a small group of patients within the spectrum of AILD that may have characteristics of cholestasis (PBC or PSC) in combination with AIH[17]. In our study, 62 patients were diagnosed with AIH and 30 patients diagnosed with PBC. Overlap syndromes of AIH and PBC were diagnosed in 19 (16.7%) of 114 patients. Therefore, we used the patients with AILD as the study cohort.
Although 2D-SWE has been widely recognized as a reliable method to assess liver fibrosis in recent years, the diagnostic performance of 2D-SWE for assessing liver fibrosis stages in patients with AILD remains unclear. The 2D-SWE examinations performed in our study were successful in all but 3 of the 117 patients. We concluded that 2D-SWE provides a very high percentage of interpretable test results. Our results regarding the rate of successful 2D-SWE measurement in patients with AILD were similar to those reported in a previous study using 2D-SWE in patients with CHB[22].
Previous studies reported the diagnostic accuracy of 2D-SWE for the detection of fibrosis stage in patients with CHB or CHC when the histology-based METAVIR staging system was used as the diagnostic reference standard[8,11]. The METAVIR staging system is simple and practical and is widely used for liver fibrosis staging in patients with chronic liver diseases. In our study, we also used the METAVIR staging system as the diagnostic reference standard. AUROCs for the identification of significant fibrosis and cirrhosis were approximately 0.85 and 0.86, respectively. Stage F2 or greater indicates the beginning of progressive liver disease and therefore suggests a stronger indication to initiate treatment; thus, 2D-SWE may serve as a screening tool to differentiate patients with significant fibrosis from patients without. Cirrhosis is the end stage of chronic liver disease. 2D-SWE may also be used to differentiate patients with cirrhosis from those without. According to previous studies, AUROCs of 2D-SWE for predicting significant fibrosis, severe fibrosis, and cirrhosis were 0.88, 0.93 and 0.98 in patients with CHB and 0.92, 0.98 and 0.98 in patients with CHC, respectively[8,11]. In our study, moderate diagnostic performance for assessing liver fibrosis stages was observed in patients with AILD, with AUROCs of approximately 0.85. The AUROCs in patients with AILD were lower than those in patients with CHB or CHC. In our series, 2D-SWE correctly classified 53.5% of patients with AILD. The concordance rates were also lower for 2D-SWE vs METAVIR stages in patients with CHB or CHC[8,22].
The optimal cut-off values associated with significant fibrosis, severe fibrosis and cirrhosis in patients with AILD determined by the ROC analysis were 9.7 kPa, 13.2 kPa and 16.3 kPa, respectively. The corresponding optimal cut-off values in patients with CHB were 7.2 kPa, 9.1 kPa and 11.7 kPa, and the corresponding values for patients with CHC were 7.1 kPa, 8.7 kPa and 10.4 kPa, respectively[8,22]. Thus, the optimal cut-off values in patients with AILD were markedly higher than the values in patients with CHB or CHC. Using the optimal cut-off values calculated from the ROC curves in our study, 2D-SWE showed sensitivity values of greater than 80% and the corresponding specificity values were approximately 80%. Using the cut-off values for CHB or CHC, the sensitivity values for assessing the stages of fibrosis were greater than 90%, but the accompanying specificity values were very low. Fibrosis stages would be overestimated using the cut-off values for CHB or CHC in patients with AILD. Therefore, the cut-off values for CHB and CHC were not appropriate for patients with AILD. The lower diagnostic accuracy and higher cut-off values might be characteristics of 2D-SWE for assessing liver fibrosis in patients with AILD, and differences in patient populations and etiology might explain the differences in these results.
This study has certain limitations that warrant discussion. First, our study used patients with AILD as its cohort. Patients with AIH, PBC and PSC were not separately analyzed. Second, the distribution of patients across all fibrosis stages was not uniform, as a very low percentage of enrolled patients were at stage F0. Third, the LB did not meet the American Society for the Study of Liver Diseases criteria of being at least 2-3 cm in length with at least 11 portal tracts[26]; also, agreement between the readers of the liver biopsies was not assessed in our study. Fourth, although other ultrasound techniques have been used to evaluate liver fibrosis, such as transient elastography (FibroScan; Echosens) and acoustic radiation force impulse (ARFI; Siemens Healthcare), we only evaluated 2D-SWE.
In conclusion, 2D-SWE showed promising diagnostic performance for assessing the liver fibrosis stages and exhibited high cut-off values in patients with AILD; however, the concordance rates of the LSMs obtained using 2D-SWE vs METAVIR stages were low. Our study opens possibilities for a non-invasive assessment of fibrosis progression in patients suffering from ALID.
Autoimmune liver diseases (AILD) are a group of diseases characterized by an anomalous immune response that is directed at hepatocytes or bile ducts along with the presence of serum antimitochondrial antibodies and a potential tendency to progress to cirrhosis. These diseases are important because they may result in chronic liver damage with fibrosis and cirrhosis. An evaluation of the degree of liver fibrosis is important for determining medical management and prognosis. The demand for a non-invasive and reliable test to evaluate liver fibrosis in patients with AILD has increased.
Two-dimensional shear wave elastography (2D-SWE) has been used to non-invasively measure liver fibrosis. This method has good diagnostic accuracy for the staging of liver fibrosis in patients with chronic liver diseases. However, studies on the use of 2D-SWE to assess histologically confirmed liver fibrosis in patients with AILD have not been published.
2D-SWE showed promising diagnostic performance for assessing liver fibrosis stages and exhibited high cut-off values in patients with AILD; however, the concordance rates of the liver stiffness measurements obtained using 2D-SWE vs METAVIR stages were low.
2D-SWE is beneficial for assessing liver fibrosis stages in patients with AILD as this method is non-invasive and reproducible in the clinical setting. The optimal cut-off values are high but appropriate for patients with AILD.
2D-SWE, an ultrasound elastography technique based on shear waves that is available on a clinical diagnostic ultrasound scanner, has been used to non-invasively measure liver fibrosis. The 2D-SWE technique creates a real-time, two-dimensional quantitative map of liver tissue stiffness under the guidance of very-high-frame-rate B mode imaging.
A well-written manuscript dealing with an interesting topic. Authors could ameliorate the description of results and give some important additional information to apply these data into clinical practice.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: La Mura V S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Li D
| 1. | Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014;60:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Hepatology. 2015;62:1620-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1736] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 4. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 772] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 5. | Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 6. | Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, Cosgrove D, Dietrich CF, Amy D, Bamber JC. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 482] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 7. | Jeong JY, Kim TY, Sohn JH, Kim Y, Jeong WK, Oh YH, Yoo KS. Real time shear wave elastography in chronic liver diseases: accuracy for predicting liver fibrosis, in comparison with serum markers. World J Gastroenterol. 2014;20:13920-13929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 8. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C; Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 507] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 9. | Poynard T, Munteanu M, Luckina E, Perazzo H, Ngo Y, Royer L, Fedchuk L, Sattonnet F, Pais R, Lebray P. Liver fibrosis evaluation using real-time shear wave elastography: applicability and diagnostic performance using methods without a gold standard. J Hepatol. 2013;58:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Yoon JH, Lee JM, Joo I, Lee ES, Sohn JY, Jang SK, Lee KB, Han JK, Choi BI. Hepatic fibrosis: prospective comparison of MR elastography and US shear-wave elastography for evaluation. Radiology. 2014;273:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, Chu SH, Chan AW, Choi PC, Ahuja AT. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 12. | Tanaka A, Ma X, Yokosuka O, Weltman M, You H, Amarapurkar DN, Kim YJ, Abbas Z, Payawal DA, Chang ML. Autoimmune liver diseases in the Asia-Pacific region: Proceedings of APASL symposium on AIH and PBC 2016. Hepatol Int. 2016;10:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1252] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 14. | Qiu D, Wang Q, Wang H, Xie Q, Zang G, Jiang H, Tu C, Guo J, Zhang S, Wang J. Validation of the simplified criteria for diagnosis of autoimmune hepatitis in Chinese patients. J Hepatol. 2011;54:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1200] [Article Influence: 75.0] [Reference Citation Analysis (1)] |
| 16. | Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 896] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 17. | Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E; International Autoimmune Hepatitis Group. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 347] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 18. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 19. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 20. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] |
| 22. | Zeng J, Liu GJ, Huang ZP, Zheng J, Wu T, Zheng RQ, Lu MD. Diagnostic accuracy of two-dimensional shear wave elastography for the non-invasive staging of hepatic fibrosis in chronic hepatitis B: a cohort study with internal validation. Eur Radiol. 2014;24:2572-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Aguilar-Nájera O, Velasco-Zamora JA, Torre A. Overlap syndromes of autoimmune hepatitis: diagnosis and treatment. Rev Gastroenterol Mex. 2015;80:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Trivedi PJ, Hirschfield GM. Review article: overlap syndromes and autoimmune liver disease. Aliment Pharmacol Ther. 2012;36:517-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Czaja AJ. Diagnosis and management of the overlap syndromes of autoimmune hepatitis. Can J Gastroenterol. 2013;27:417-423. [PubMed] |
| 26. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1580] [Article Influence: 98.8] [Reference Citation Analysis (1)] |