Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4831
Peer-review started: December 16, 2016
First decision: February 23, 2017
Revised: March 14, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: July 14, 2017
Processing time: 210 Days and 1.5 Hours
To characterize the expression of toll-like receptors (TLR) 2 and 4 in colorectal cancer (CRC) and in normal colorectal mucosa.
We analysed tissue samples from a prospective series of 118 unselected surgically treated patients with CRC. Sections from formalin fixed, paraffin embedded specimens were analysed for TLR2 and TLR4 expression by immunohistochemistry. Two independent assessors evaluated separately expression at the normal mucosa, at the invasive front and the bulk of the carcinoma, and in the lymph node metastases when present. Expression levels in different locations were compared and their associations with clinicopathological features including TNM-stage and the grade of the tumour and 5-year follow-up observations were analysed.
Normal colorectal epithelium showed a gradient of expression of both TLR2 and TLR4 with low levels in the crypt bases and high levels in the surface. In CRC, expression of both TLRs was present in all cases and in the major proportion of tumour cells. Compared to normal epithelium, TLR4 expression was significantly weaker but TLR2 expression stronger in carcinoma cells. Weak TLR4 expression in the invasive front was associated with distant metastases and worse cancer-specific survival at 5 years. In tumours of the proximal colon the cancer-specific survival at 5 years was 36.9% better with strong TLR4 expression as compared with those with weak expression (P = 0.044). In contrast, TLR2 expression levels were not associated with prognosis. Tumour cells in the lymph node metastases showed higher TLR4 expression and lower TLR2 expression than cells in primary tumours.
Tumour cells in CRC show downregulation of TLR4 and upregulation of TLR2. Low expression of TLR4 in the invasive front predicts poor prognosis and metastatic disease.
Core tip: Toll-like receptor 4 (TLR4) is downregulated in colorectal cancer (CRC), suggesting a role as a tumour suppressor. Low expression of TLR4 in the invasive front marks poor prognosis and is associated with metastatic disease. The most significant finding was the association between weak TLR4 expression in the tumour front and 36.9% (P = 0.044) lower cancer-specific survival in cancer of the proximal colon as compared with strong TLR4 expression. Weak TLR4 staining in tumour front was present in 50% (11/22) of the cases with metastases and only in 17% (16/94) of those without metastases (P = 0.001). TLR2 is upregulated in CRC but not associated with prognosis.
- Citation: Paarnio K, Väyrynen S, Klintrup K, Ohtonen P, Mäkinen MJ, Mäkelä J, Karttunen TJ. Divergent expression of bacterial wall sensing Toll-like receptors 2 and 4 in colorectal cancer. World J Gastroenterol 2017; 23(26): 4831-4838
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4831.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4831
Colorectal cancer (CRC) is the third most common cancer in both Europe[1] and the United States[2]. It evolves through stepwise accumulation of genetic mutations and the effects of environmental factors. Recent research has focused on the significance of tumour-associated inflammation in the development and progression of carcinoma. Inflammation and immunity are related to the pathogenesis of cancer by several mechanisms, including alterations in the intestinal microbiome, oncogene activation-induced immune reaction, and a role of local inflammation in tumour progression[3]. An elevated systemic inflammatory response is associated with poor outcome[4], but local inflammation at the invasive front of the tumour is linked to better survival[5].
Toll-like receptors (TLRs) are a family of receptors with a significant role in defence against pathogens. They recognize microorganisms, and ligand binding to TLRs initiates signalling cascades leading to the inflammatory response[6]. TLR4 detects lipopolysaccharide from Gram-negative bacteria[7], and previous studies concerning its role in CRC are controversial[8-10]. TLR2 recognizes several bacterial, fungal, and viral proteins[11], and in CRC, higher TLR2 expression has been found compared to normal mucosa but with no link to prognosis[8]. Both are expressed in normal colorectal epithelium and in immune system cells[12].
We have characterized TLR2 and TLR4 tissue expression in a series of colorectal carcinomas and their metastases, evaluating tumour properties that affect expression and the influence of expression on prognosis. Our hypothesis was that TLR2 and TLR4 expression levels would change with tumour progression and show an association with prognosis.
A case series consisting of patients with CRC was collected prospectively between April 2006 and January 2010 at Oulu University Hospital. The prospective study (Kantola et al[13]) was explained to all newly diagnosed CRC patients who underwent surgery in our hospital during that time (n = 344). A total of 149 patients who were both eligible for the study and had signed informed consent to participate were included in the study. The Regional Ethical Committee of North Ostrobothnia Hospital District has accepted both the original study design and the follow-up study (58/2005, 184/2009, 60/2012). The study was conducted in collaboration between the Department of Surgery and the Department of Pathology.
The clinical details and follow-up information for the patients were obtained from clinical records and information about time and cause of death from Statistics Finland. The preoperative staging of CRC was made by whole body computed tomography scan and local staging for rectal cancer by magnetic resonance imaging scan. The patients with T3 or T4 rectal cancer received preoperative neoadjuvant radiation or chemoradiation therapy (n = 31) and were excluded from the final analysis, leaving a total of 118 patients. The histological features of the tumours were obtained from pathological records. The classifications used were TNM6[14] for staging and the WHO 2010 classification[15] for grading. In addition, serrated adenocarcinomas were diagnosed according to WHO 2010 criteria, as described in more detail[15-17]. All these histopathological analyses were performed by experienced pathologist.
We defined disease-free survival as the time interval between the primary operation and detection of recurrence. Disease-free survival analysis was based on the data for patients whose primary operation was curative and who did not die for a cause other than CRC during the 5-year follow-up period (83 patients).
Immunohistochemistry was performed on formalin fixed, paraffin-embedded tissue sections. Tissue microarrays were conducted using the primary tumour specimens[18], and a human kidney tissue specimen was included in the multi-tissue block as a positive control for TLR2 staining[19]. Macrophages served as positive control for TLR4 staining. Samples representing the normal mucosa and lymph node metastases were stained and analysed separately.
For antigen retrieval, sections were treated at a high temperature in Tris-EDTA buffer for 15 min. Immunostaining was performed with Dako Autostainer (Dako Copenhagen, Denmark) using mouse monoclonal antibodies (Abnova MAB0066 Clone 1030A5.138 for TLR2 and Abnova H00007099-M02 Clone 3B6 for TLR4; Abnova, Taoyuan City, Taiwan), at dilutions of 1:50 and 1:1000, respectively. For detection, we used Dako Envision kit (Dako) and diaminobenzidine (Dako basic DAB-kit) as a chromogen. For negative controls, we omitted the primary antibody and replaced the primary antibody with a mouse primary antibody isotype control.
The two independent researchers (Paarnio K and Väyrynen S) analysed the expression of TLR2 and TLR4 with a close guidance by an experienced gastrointestinal pathologist (Karttunen TJ). The assessors were blinded to the clinical data and the results of assessment of other patient specimens, such as normal mucosa or metastases. We assessed separately the intensity and extent of the staining. Carcinoma cells at the invasive front and the bulk of the primary tumour, lymph node metastases if present, and epithelial cells in the normal mucosa were separately assessed. The intensity of the staining was assigned using a four-point scale [negative (0), weak (1), moderate (2), and strong (3)], and the extent of staining was assigned as a percentage of positive cells (0%-100%). If there was more than one step difference in the intensity score or over 30% difference in the percentages between the two assessors, a consensus was reached after joint re-evaluation[20]. Otherwise, we used the mean values of the two assessors. Histoscore (0-300) for tissue samples was obtained by multiplying the intensity score by the percentage of positive cells.
The statistical methods of this study were reviewed by biostatistician Pasi Ohtonen, MSc, Division of Operative Care, Oulu University Hospital. Summary statistics are presented as medians with 25th-75th percentiles unless stated otherwise. The χ2 test or Fisher’s exact test was used to analyse categorical data, the Student’s t-test for continuous data, and the paired samples t-test to compare mean differences between different sample sites. The Cox proportional hazards model was used to estimate the impact of TLR2 and TLR4 on 5-year survival. In the Cox model, age and sex, along with tumour size, location (distal/proximal), and stage (0-II/III-IV) were used as adjusting factors and left in the model only if their P < 0.05 or the impact on the -2*Log Likelihood function was significant. Interaction terms with TLR2 and TLR4 were calculated and found to be non-significant. HRs with 95%CIs are presented as results of the Cox models. Two-tailed P-values are presented. Analyses were performed using SPSS for Windows (Released 2012. IBM SPSS Statistics for Windows, Version 21.0. IBM Corp., Armonk, NY, United States).
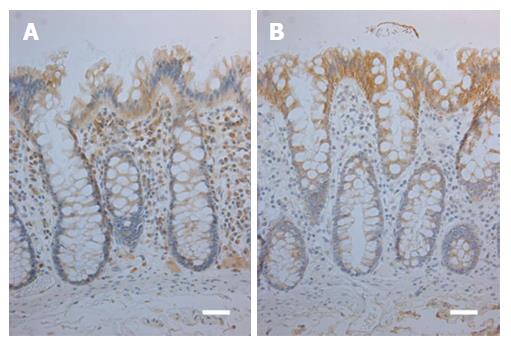
Patient characteristics and tumour features are presented in Tables 1 and 2. In the normal mucosa, the expression of TLR2 and -4 was cytoplasmic and present in all epithelial cells and in lymphocytes of the lamina propria (Figure 1). There was a constant gradient of the expression of both, with the crypt base cells showing weaker expression than cells in the upper parts of the crypts and surface.
| Whole study group | Survival in 5-yr follow-up | |
| Gender | ||
| Male | 56 (47.5) | 35 (62.5) |
| Female | 62 (52.5) | 36 (58.1) |
| Age, median (range) | 69 (36-89) | |
| Other morbidities | ||
| No | 27 (22.9) | 16 (59.3) |
| Yes | 91 (77.1) | 55 (60.4) |
| Other neoplasm | ||
| No | 109 (92.4) | 62 (56.9) |
| Sex organs or breast cancer | 2 (1.7) | 2 (100.0) |
| Other cancer | 3 (2.5) | 3 (100.0) |
| Colon adenoma | 4 (3.4) | 4 (100.0) |
| Cancer in family | ||
| No/not known | 104 (88.1) | 59 (56.7) |
| CRC | 8 (6.8) | 7 (87.5) |
| HNPCC family | 2 (1.7) | 2 (100.0) |
| Other HNPCC-associated cancer | 3 (2.5) | 2 (66.7) |
| Other cancer | 1 (0.8) | 1 (100.0) |
| Type of operation | ||
| Radical1 | 94 (80.3) | 69 (73.4) |
| Palliative2 | 23 (19.7) | 2 (8.7) |
| Location of tumour | ||
| Proximal colon | 46 (39.0) | 27 (58.7) |
| Distal colon | 39 (33.1) | 25 (64.1) |
| Rectum | 32 (27.1) | 18 (56.3) |
| Multiple tumours | 1 (0.8) | 1 (100.0) |
| Whole study group | Survival in 5-yr follow-up | |
| Stage | ||
| I | 18 (15.3) | 15 (83.3) |
| II | 48 (40.7) | 37 (77.1) |
| III | 30 (25.4) | 16 (53.3) |
| IV | 22 (18.6) | 3 (13.6) |
| Grade | ||
| I | 17 (14.4) | 13 (76.5) |
| II | 86 (72.9) | 53 (61.6) |
| III | 14 (11.9) | 5 (35.7) |
| Data missing | 1 (0.8) | 0 (0) |
| Lymph node metastasis | ||
| No | 72 (61.0) | 53 (73.6) |
| Yes | 46 (39.0) | 18 (39.1) |
| Distant metastasis | ||
| No | 96 (81.4) | 68 (70.8) |
| Yes | 22 (18.6) | 3 (13.6) |
| Mucinous carcinoma | ||
| No | 99 (83.9) | 59 (59.6) |
| Yes | 10 (8.5) | 6 (60.0) |
| Data missing | 9 (7.6) |
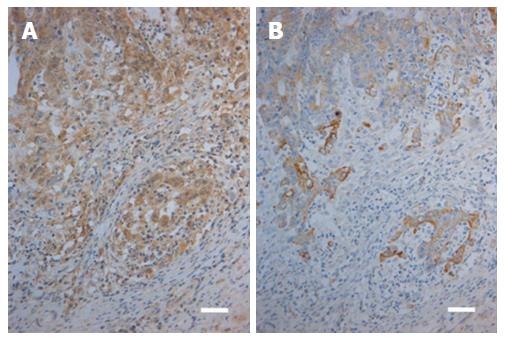
In carcinomas, both TLR2 and TLR4 showed cytoplasmic expression (Figure 2), and the expression was present in all cases. In the majority of the cases, the expression was present in most tumour cells.
TLR2 expression was increased in carcinoma cells compared with normal colon epithelium (Table 3). Expression did not differ between the tumour bulk and the front, but levels were lower in lymph node metastases than in the primary tumours.
| TLR2 intensity, mean ± SD | P value | TLR2 histoscore, mean ± SD | P value | TLR4 intensity, mean ± SD | P value | TLR4 histoscore, mean ± SD | P value | |
| Invasive front | 2.3 ± 0.6 | < 0.001 | 224 ± 67 | < 0.001 | 2.2 ± 0.6 | < 0.001 | 223 ± 66 | 0.024 |
| Normal mucosa | 1.4 ± 0.7 | 129 ± 72 | 2.6 ± 0.6 | 243 ± 77 | ||||
| Invasive front | 2.2 ± 0.7 | 0.006 | 210 ± 77 | 0.054 | 2.2 ± 0.6 | 0.060 | 216 ± 68 | 0.048 |
| Lymph node | 1.8 ± 0.7 | 180 ± 72 | 2.4 ± 0.7 | 241 ± 70 | ||||
| Tumour bulk | 2.3 ± 0.6 | < 0.001 | 224 ± 65 | < 0.001 | 2.0 ± 0.7 | < 0.001 | 197 ± 73 | < 0.001 |
| Normal mucosa | 1.4 ± 0.7 | 129 ± 72 | 2.6 ± 0.6 | 244 ± 78 | ||||
| Tumour bulk | 2.2 ± 0.6 | 0.007 | 216 ± 65 | 0.011 | 2.0 ± 0.6 | 0.001 | 194 ± 69 | 0.001 |
| Lymph node | 1.8 ± 0.7 | 180 ± 72 | 2.4 ± 0.7 | 241 ± 70 | ||||
| Lymph node | 1.8 ± 0.7 | 0.011 | 179 ± 70 | 0.003 | 2.4 ± 0.7 | 0.017 | 238 ± 70 | 0.40 |
| Normal mucosa | 1.4 ± 0.7 | 127 ± 71 | 2.7 ± 0.5 | 251 ± 72 | ||||
| Invasive front | 2.3 ± 0.6 | 0.55 | 225 ± 66 | > 0.9 | 2.3 ± 0.6 | < 0.001 | 223 ± 66 | < 0.001 |
| Tumour bulk | 2.3 ± 0.6 | 225 ± 64 | 2.0 ± 0.7 | 197 ± 73 |
The TLR4 expression pattern was distinct from that of TLR2 and showed the highest levels in the normal mucosa; in cancer, the tumour bulk had lower TLR4 expression levels than the tumour front and lymph node metastases (Table 3).
The comparison between TLR2 and TLR4 expression levels is shown in Table 4. The intensity of staining did not differ in the invasive front, but TLR2 staining was significantly stronger in the tumour bulk, as was TLR4 staining in both the lymph node metastasis and the normal mucosa.
| Intensity, mean ± SD | P value | Histoscore, mean ± SD | P value | n | |
| Tumour bulk | |||||
| TLR2 | 2.3 ± 0.6 | < 0.001 | 225 ± 64 | < 0.001 | 117 |
| TLR4 | 2.0 ± 0.7 | 198 ± 73 | 117 | ||
| Invasive front | |||||
| TLR2 | 2.3 ± 0.6 | 0.55 | 225 ± 66 | 0.83 | 116 |
| TLR4 | 2.3 ± 0.6 | 223 ± 66 | 116 | ||
| Lymph node | |||||
| TLR2 | 1.8 ± 0.7 | < 0.001 | 180 ± 71 | < 0.001 | 43 |
| TLR4 | 2.4 ± 0.7 | 241 ± 70 | 43 | ||
| Normal mucosa | |||||
| TLR2 | 1.4 ± 0.7 | < 0.001 | 129 ± 72 | < 0.001 | 114 |
| TLR4 | 2.6 ± 0.6 | 243 ± 79 | 114 |
Weak intensity (less than 2) of TLR4 staining in the invasive front was associated with metastasis and higher TNM stage. Weak staining was present in 50% (11/22) of the cases with metastases, 17% (16/94) of those without metastases (P = 0.001), 23.5% (4/17) in stage I, 19.1% (9/47) in stage II, 10.0% (3/30) in stage III, and 50.0% (11/22) in stage IV (P = 0.009). Weak staining in lymph node metastases also was associated with advanced disease: weak intensity was present in 0% (0/1) of lymph node metastases in stage II, 7.4% (2/27) in stage III, and 40.0% (6/15) in stage IV (P = 0.028).
TLR2 and TLR4 expression was similar in conventional and serrated carcinomas (data not shown). There also was no association with the presence of a mucinous component. However, tumours with a low WHO grade showed a tendency to strong TLR2 expression in the tumour front and lymph node metastases. In the invasive front, TLR2 expression was strong in 80% of grade I, 87.2% of grade 2, and 64.3% of grade 3 tumours (P = 0.078); in the lymph node metastases, strong TLR2 expression was seen in 100% of grade 1, 63.6% of grade 2, and 25% of grade 3 tumours (P = 0.063).
To assess the effect of TLR2 and TLR4 expression on overall survival, cancer-specific survival, and disease-free survival, we grouped cases according to the intensity of staining into two categories: weak staining, with an intensity score less than 2, and strong staining, with an intensity score of 2 or higher.
The most significant finding was the association between strong TLR4 expression in the tumour front and 36.9% (P = 0.044) better cancer-specific survival with cancer of the proximal colon compared with weak TLR4 expression (Table 5). Strong TLR4 expression in the invasive front also was linked to 14.8% (62.9% vs 48.1%, P = 0.19) better overall survival compared with weak expression; in the tumour bulk, the difference in survival was 18.4% (65.8% vs 47.4%, P = 0.071). The same tendency was seen even more distinctly in the proximal colon: strong expression in both the front and bulk was linked to 28.3% (64.7% vs 36.4%, P = 0.16) better survival; in lymph node metastases, the difference in survival was 50% (50% vs 0%, P = 0.23).
| Intensity of staining | Whole colon | P value1 | Proximal colon | P valu1 | Distal colon and rectum | P value1 | ||
| TLR2 | Tumour bulk | Weak | 14 (63.6) | 0.43 | 4 (66.7) | 0.65 | 9 (60.0) | 0.33 |
| Strong | 71 (74.7) | 29 (74.4) | 42 (75.0) | |||||
| Invasive front | Weak | 14 (73.7) | > 0.9 | 6 (66.7) | 0.68 | 8 (80.0) | 0.71 | |
| Strong | 70 (72.2) | 27 (75.0) | 42 (70.0) | |||||
| Lymph node | Weak | 9 (50.0) | 0.55 | 3 (37.5) | > 0.9 | 6 (60.0) | 0.26 | |
| Strong | 10 (40.0) | 3 (42.9) | 6 (35.3) | |||||
| TLR4 | Tumour bulk | Weak | 25 (65.8) | 0.27 | 7 (63.6) | 0.45 | 18 (66.7) | 0.59 |
| Strong | 60 (75.9) | 26 (76.5) | 33 (75.0) | |||||
| Invasive front | Weak | 17 (63.0) | 0.33 | 5 (45.5) | 0.044 | 12 (75.0) | > 0.9 | |
| Strong | 67 (75.3) | 28 (82.4) | 38 (70.4) | |||||
| Lymph node | Weak | 2 (25.0) | 0.27 | 0 (0.0) | 0.23 | 2 (40.0) | > 0.9 | |
| Strong | 17 (48.6) | 6 (50.0) | 10 (45.5) | |||||
In a stage-adjusted Cox model, strong TLR4 expression showed a trend to association with a better prognosis; in the tumour front, the HR for overall survival was 1.8 (P = 0.074, 95%CI: 0.9-3.3), and in the tumour bulk, it was 1.7 (P = 0.059, 95%CI: 1.0-3.1). There was no significant association between TLR expression and disease-free survival.
TLR2 expression did not associate with overall, cancer-specific, or disease-free survival. In the tumour front, weak TLR2 expression showed some non-significant trend to an association with better disease-free survival than strong expression (92.3% vs 75%, P = 0.28); for expression in lymph node metastases, the difference was even higher (66.7% vs 36.8%, P = 0.23). Similarly, there was a tendency to a better outcome with weak TLR2 expression in lymph node metastasis in the cancer of the distal colon or rectum; the overall survival was 30.6% (60% vs 29.4%, P = 0.22), and cancer-specific survival was 24.7% (60% vs 35.3%, P = 0.26) higher than with strong TLR2 expression.
In this study, we found that weak TLR4 expression in the invasive front was associated with poor prognosis in CRC, linked to distant metastases and worse cancer-specific survival. Loss of TLR4 in carcinomas in comparison to normal mucosa supports the idea of a potential tumour suppressor role for the protein. In contrast, TLR2 expression was increased in carcinomas independent of stage, which is in agreement with previous reports[8]. The association with weak TLR2 expression in lymph nodes and better prognosis did not reach significance at the 5% level, even though 30.6% better overall survival seems clinically notable for subgroup analysis; however, a bigger sample size would be needed to make any conclusions. Considering that both TLR2 and TLR4 receptors activate the same cascade[21], TLR2 upregulation and TLR4 downregulation during progression from normal mucosa to carcinoma indicate the occurrence of a distinct set of alterations in the innate immune response during the development of CRC.
TLR4 expression was significantly higher in normal mucosa compared to any cancer samples. Reports on inflammatory bowel disease-associated cancers have yielded contrasting results, and higher TLR4 expression in CRC compared to normal mucosa has been found[9,22]. The increased TLR4 expression compared to normal tissue also has been reported in sporadic CRC[23], and another study demonstrated a correlation between high expression of TLR4 and advanced disease[10]. Yet results also have been published showing that the loss of TLR4 correlates with increased metastatic status[24], and TLR4 expression by tumour cells has been significantly associated with a lower rate of tumour recurrence[25]. Nihon-Yanagi et al[8] showed equally weak TLR4 expression in both cancerous and noncancerous tissue in general, but higher expression in the cancer of the proximal colon.
In addition to variable detection methods used, the differences in the analysis schemes are likely explanations for the conflicting results. Previous studies have not examined expression separately in the tumour bulk and invasive front, which we considered to be crucial because tumour infiltration and progression occur in the invasive front. Furthermore, the peritumoural immune reaction and tumour budding at the invasive front have a profound effect on survival in CRC. Functional polymorphisms of TLR2 and -4 show association with the risk and features of CRC, such as differentiation, advanced stage, lymph node status, and metastasis, and also affect expression levels[26,27]. Accordingly, occurrence of TLR polymorphisms may contribute to contradictory results of expression analyses and warrant further studies.
In normal colorectal mucosa in the current work, gradual accumulation of TLR2 and TLR4 were associated with the location of the epithelial cells, with the highest expression in the surface. In addition to possibly being linked to maturation of epithelial cells, this gradient might reflect the abundance of ligands originating from the luminal flora in this region. Also in CRCs, there was a distinct variation in expression in specific tumour compartments. We found that TLR4 expression in the invasive front was significantly stronger than in the tumour bulk and that lymph node metastases expressed TLR4 more strongly than either the bulk or the front. These findings suggest that areas of tumour with active invasion have higher TLR4 expression, which we speculate might be related to induction by endogenous ligands released from dead or damaged cells and matrix[28].
Again, TLR2 was different, showing similarity between the tumour bulk and the invasive front, and lymph node metastases showed less intensive expression than the primary lesion. However, our findings suggest (P = NS) that if TLR2 expression in lymph node metastases is strong, the prognosis weakens. Because intratumoural or intranodal differences in the supply of microbiological ligands to these TLRs do not likely differ, the observed location-related differences might be related to other factors regulating TLR expression, like mediators from the cells in the microenvironment, including endogenous TLR ligands released along with invasion[29]. We speculate that the sum effect of these factors is different in terms of expression of TLR4 and TLR2. Our finding of increased TLR4 expression and decreased TLR2 expression in metastatic carcinoma cells suggests either some selection based on TLR expression levels during the metastatic cascade or that regulatory signals from the lymph node parenchyma cells have opposite effects on TLR2 and TLR4 expression.
High TLR4 expression in the invasive front was related to better prognosis. In the proximal colon, the benefit in terms of cancer-specific survival was 36.9% (P = 0.044), and in overall survival, it was 28.3% (P = 0.16). Tumour bulk showed the same pattern, and in lymph node metastasis, the overall survival benefit was as high as 50%, but likely because of our small sample size, these clinically highly notable differences did not reach statistical significance. In line with our findings, Simiantonaki et al[24] showed that a loss of TLR4 correlated with the presence of metastases, and Eiró et al[25] found that TLR4 expression was associated with a lower rate of recurrence.
Mechanisms linking high TLR4 expression with better survival and rarity of metastases remain speculative. Our earlier studies[30] have shown that high-grade peritumoural inflammation is associated with good prognosis, and this finding has also been used in Klintrup-Mäkinen grading; thus, it would be plausible that stronger TLR4 expression would contribute to a stronger inflammatory cell response, eventually protecting against cancer progression. Simiantonaki et al[24] suggested that during CRC development, immune-mediated signals downregulate the level of TLR4 expression and that epithelial cells consequently do not respond to lipopolysaccharide with an inflammatory reaction. Thus, the lack of immune response in the tumour would lead to tumour progression. Additionally, they suggested that the loss of TLR4 is linked to the lack of an anti-tumoural immune response[24]. Considering the complexity of the relationship among inflammation, immunity, and cancer, it is not surprising that other studies have reported contrasting findings. In the tumour microenvironment, the inflammatory component may either destroy neoplastic cells or potentiate tumour progression depending on the molecular combinations and expression levels.
Both TRL2 and TLR4 recognize bacteria, and TLR2 also fungal and viral proteins[7,11]. Recent findings suggest, that gut microbes may play a role in CRC development[31], but a lot of questions remain, and need further investigation. When it comes to viruses, a recent meta-analysis supports the association of cytomegalovirus infection with colorectal tumour formation[32]. Supporting possible role of Cytomegalovirus, levels of Cytomegalovirus protein were reported to correlate with those of TLR2 and TLR4 in CRC, and Cytomegaloovirus infection in a colorectal carcinoma cell line induced TLR2 production[33].
In conclusion, our study shows that TLR2 is upregulated and TLR4 downregulated in CRC. Low expression of TLR4 at the invasive front associates with short survival, but for TLR2, possible value as prognostic markers could not be established. Further studies with larger study groups are needed to clarify the role of these receptors in the development and prognosis of CRC.
We would like to thank Erja Tomperi and Riitta Vuento for the preparation of the immunohistochemical stainings.
Colorectal cancer (CRC) is a very common disease all over the world. Peritumoural inflammation is linked to CRC development and prognosis. Toll-like receptor 2 (TLR2) and TLR4 are receptors detecting bacteria and activating the signalling cascade leading to inflammation response.
The previous knowledge of the role of TLR2 and TLR4 in CRC was scarce or conflicting. However, there were some results suggesting TLR2 upregulation in CRC, but the studies concerning the role of TLR4 in CRC were controversial.
This study shows TLR4 downregulation and TLR2 upregulation in CRC, and low expression of TLR4 in the invasive front of the tumour predicts poor prognosis and metastatic disease.
These findings give new information of the role of TLR2 and TLR4 in CRC development and prognosis, and can serve as a ground for further studies aiming for better understanding of the factors affecting the course of CRC and more effective and individualized treatment strategies.
In this study the expression of TLR2 and TLR4 in colorectal carcinoma have been investigated. It is observed that TLR4 expression was significantly weaker but TLR2 expression stronger in carcinoma cells when compared to normal mucosa. Moreover, down-regulation of TLR4 in the invasive front of CRC predicted poor prognosis and metastatic disease. Basically this is a well-written study of an interesting topic.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Finland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Elpek GO S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Steliarova-Foucher E, O’Callaghan M, Ferlay J, Masuyer E, Forman D, Comber H, Bray F (2012) European Cancer Observatory: Cancer Incidence, Mortality, Prevalence and Survival in Europe. Version 1.0 (September 2012) European Network of Cancer Registries, International Agency for Research on Cancer. Available from: http://eco.iarc.fr. |
| 2. | American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society 2017; . |
| 3. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2082] [Article Influence: 130.1] [Reference Citation Analysis (1)] |
| 4. | Maeda K, Shibutani M, Otani H, Nagahara H, Ikeya T, Iseki Y, Tanaka H, Muguruma K, Hirakawa K. Inflammation-based factors and prognosis in patients with colorectal cancer. World J Gastrointest Oncol. 2015;7:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Richards CH, Flegg KM, Roxburgh CS, Going JJ, Mohammed Z, Horgan PG, McMillan DC. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br J Cancer. 2012;106:2010-2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Shibolet O, Podolsky DK. TLRs in the Gut. IV. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1469-G1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Hsu RY, Chan CH, Spicer JD, Rousseau MC, Giannias B, Rousseau S, Ferri LE. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71:1989-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Nihon-Yanagi Y, Terai K, Murano T, Matsumoto T, Okazumi S. Tissue expression of Toll-like receptors 2 and 4 in sporadic human colorectal cancer. Cancer Immunol Immunother. 2012;61:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869-1881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 498] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 10. | Wang EL, Qian ZR, Nakasono M, Tanahashi T, Yoshimoto K, Bando Y, Kudo E, Shimada M, Sano T. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br J Cancer. 2010;102:908-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240-273, Table of Contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1862] [Cited by in RCA: 2187] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 12. | Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 370] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 13. | Kantola T, Klintrup K, Väyrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig KH, Näpänkangas J, Mäkelä J, Karttunen TJ. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. West Sussex: Wiley-Blackwell 2010; 100-105. |
| 15. | Hamilton S, Aaltonen L. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press 2000; . |
| 16. | Tuppurainen K, Mäkinen JM, Junttila O, Liakka A, Kyllönen AP, Tuominen H, Karttunen TJ, Mäkinen MJ. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J Pathol. 2005;207:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. Geneva: WHO Press 2010; . |
| 18. | Väyrynen JP, Tuomisto A, Klintrup K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer. 2013;109:1839-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23:86-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 20. | Huhta H, Helminen O, Kauppila JH, Takala H, Metsikkö K, Lehenkari P, Saarnio J, Karttunen T. Toll-like receptor 9 expression in the natural history of Barrett mucosa. Virchows Arch. 2015;467:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Moossavi S, Rezaei N. Toll-like receptor signalling and their therapeutic targeting in colorectal cancer. Int Immunopharmacol. 2013;16:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Cammarota R, Bertolini V, Pennesi G, Bucci EO, Gottardi O, Garlanda C, Laghi L, Barberis MC, Sessa F, Noonan DM. The tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic marker. J Transl Med. 2010;8:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Doan HQ, Bowen KA, Jackson LA, Evers BM. Toll-like receptor 4 activation increases Akt phosphorylation in colon cancer cells. Anticancer Res. 2009;29:2473-2478. [PubMed] |
| 24. | Simiantonaki N, Kurzik-Dumke U, Karyofylli G, Jayasinghe C, Michel-Schmidt R, Kirkpatrick CJ. Reduced expression of TLR4 is associated with the metastatic status of human colorectal cancer. Int J Mol Med. 2007;20:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Eiró N, González L, González LO, Fernandez-Garcia B, Andicoechea A, Barbón E, García-Muñiz JL, Vizoso FJ. Toll-like receptor-4 expression by stromal fibroblasts is associated with poor prognosis in colorectal cancer. J Immunother. 2013;36:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Omrane I, Baroudi O, Kourda N, Bignon YJ, Uhrhammer N, Desrichard A, Medimegh I, Ayari H, Stambouli N, Mezlini A. Positive link between variant Toll-like receptor 4 (Asp299Gly and Thr399Ile) and colorectal cancer patients with advanced stage and lymph node metastasis. Tumour Biol. 2014;35:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Pimentel-Nunes P, Teixeira AL, Pereira C, Gomes M, Brandão C, Rodrigues C, Gonçalves N, Boal-Carvalho I, Roncon-Albuquerque R, Moreira-Dias L. Functional polymorphisms of Toll-like receptors 2 and 4 alter the risk for colorectal carcinoma in Europeans. Dig Liver Dis. 2013;45:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Ran S. The Role of TLR4 in Chemotherapy-Driven Metastasis. Cancer Res. 2015;75:2405-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 422] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 30. | Klintrup K, Mäkinen JM, Kauppila S, Väre PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 31. | Peters BA, Dominianni C, Shapiro JA, Church TR, Wu J, Miller G, Yuen E, Freiman H, Lustbader I, Salik J. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. 2016;4:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 32. | Bai B, Wang X, Chen E, Zhu H. Human cytomegalovirus infection and colorectal cancer risk: a meta-analysis. Oncotarget. 2016;7:76735-76742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Li X, Qian D, Ju F, Wang B. Upregulation of Toll-like receptor 2 expression in colorectal cancer infected by human cytomegalovirus. Oncol Lett. 2015;9:365-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |