Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4815
Peer-review started: February 8, 2017
First decision: March 16, 2017
Revised: May 3, 2017
Accepted: June 9, 2017
Article in press: June 12, 2017
Published online: July 14, 2017
Processing time: 164 Days and 0.1 Hours
To evaluate safety and outcomes of a new technique for extreme hepatic resections with preservation of segment 4 only.
The new method of extreme liver resection consists of a two-stage hepatectomy. The first stage involves a right hepatectomy with middle hepatic vein preservation and induction of left lobe congestion; the second stage involves a left lobectomy. Thus, the remnant liver is represented by the segment 4 only (with or without segment 1, ± S1). Five patients underwent the new two-stage hepatectomy (congestion group). Data from volumetric assessment made before the second stage was compared with that of 10 matched patients (comparison group) that underwent a single-stage right hepatectomy with middle hepatic vein preservation.
The two stages of the procedure were successfully carried out on all 5 patients. For the congestion group, the overall volume of the left hemiliver had increased 103% (mean increase from 438 mL to 890 mL) at 4 wk after the first stage of the procedure. Hypertrophy of the future liver remnant (i.e., segment 4 ± S1) was higher than that of segments 2 and 3 (144% vs 54%, respectively, P < 0.05). The median remnant liver volume-to-body weight ratio was 0.3 (range, 0.28-0.40) before the first stage and 0.8 (range, 0.45-0.97) before the second stage. For the comparison group, the rate of hypertrophy of the left liver after right hepatectomy with middle hepatic vein preservation was 116% ± 34%. Hypertrophy rates of segments 2 and 3 (123% ± 47%) and of segment 4 (108% ± 60%, P > 0.05) were proportional. The mean preoperative volume of segments 2 and 3 was 256 ± 64 cc and increased to 572 ± 257 cc after right hepatectomy. Mean preoperative volume of segment 4 increased from 211 ± 75 cc to 439 ± 180 cc after surgery.
The proposed method for extreme hepatectomy with preservation of segment 4 only represents a technique that could allow complete resection of multiple bilateral liver metastases.
Core tip: Extreme hepatic resections with preservation of one segment only may be required for complete resection of multiple bilobar liver metastases. We evaluated a new technique of two-stage hepatectomy with preservation of segment 4 only. Stage one involves a right hepatectomy with middle hepatic vein preservation and associated left lobe congestion through reduction of the left hepatic vein diameter. This combination optimizes segment 4 regeneration while allowing the left lobe (to be resected) to maintain function with reduced hypertrophy. Stage two involves a left lobectomy. Hypertrophy rates of non-congested segment 4 were significantly greater than in congested left lobe.
- Citation: Balzan SMP, Gava VG, Magalhães MA, Dotto ML. Extreme liver resections with preservation of segment 4 only. World J Gastroenterol 2017; 23(26): 4815-4822
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4815
Complete surgical resection of colorectal liver metastases (CLM) leads to long-term survival for selected patients with isolated hepatic disease or resectable associated extrahepatic disease[1-5]. Technical improvements, mainly in blood flow modulation (such as portal vein embolization, portal vein ligation, associating liver partition and portal vein ligation (ALPPS), and simultaneous portal and hepatic vein embolization), have extended the limits of resection for CLM[6-8]. Stimulation of hepatic regeneration has also made liver resection feasible in patients who would otherwise have a small future liver remnant (FLR). However, these methods are not adequate for cases requiring bilateral anatomical liver resection with preservation of only a small central liver segment, as they cannot provide sufficient selective FLR hypertrophy.
Extensive bilateral hepatic involvement by CLM that requires complete resection of the right hemiliver and of the left lateral section, but which shows minimal or no involvement of segment 4, is an unusual as well as challenging clinical situation. Usually, the volume of liver segment 4 is insufficient to meet the minimal requirements for safe resection of all other segments. Additionally, the commonly applied methods for stimulating liver regeneration were designed to induce hypertrophy in lateral segments of the liver. Deprivation of portal flow to the entire right liver plus to the left lateral section has not yet been reported but would be expected to lead to liver failure if the FLR was very small.
Right portal vein embolization (with or without hepatic vein deprivation) induces parenchymal hypertrophy of both FLR (segment 4) and the left lateral section[9-12]. The ALPPS method was developed to induce hypertrophy of the left lateral section, in particular[13,14]. Recently, our group published a technical alternative option that optimizes hypertrophy of segment 4, while avoiding hypertrophy of the left lateral section and maintaining its function[15]. In this study, we aimed to target hypertrophy to segment 4 by generating a congestion of segments 2 and 3 after performance of a right hepatectomy. The safety and efficacy outcomes of this method are presented herein.
From January 2013 to December 2015, patients (Table 1) with multiple bilateral CLM who underwent a two-stage hepatectomy with preservation of segment 4 [± segment 1 (S1)] only, using a technique previously described by our team[15], were selected for study inclusion and designated as the “congestion group”. For comparative analysis, patients that underwent right hepatectomy were selected to fit a 2: 1 case-matched proportion and included as the “comparison group”. Inter-group comparisons were made for the hypertrophy rates of each left side hepatic segment after a right hepatectomy only (in the comparison group) and after a right hepatectomy associated with left lobe congestion (i.e., the first-stage procedure in the congestion group). Pre- and postoperative hepatic triple-phase contrast-enhanced computed tomography (CT) images were used for comparative volumetric assessment of all patients.
| Sex | Age, yr | Weight, kg | Preoperative chemotherapy | Comorbidities | CLM | |
| Patient 1 | Female | 52 | 55 | Oxaliplatin | None | Metachronous |
| Patient 2 | Female | 61 | 63 | Oxaliplatin | SAH | Metachronous |
| Patient 3 | Female | 39 | 98 | Oxaliplatin + bevacizumab | None | Synchronous |
| Patient 4 | Female | 54 | 66 | Oxaliplatin | None | Metachronous |
| Patient 5 | Female | 65 | 46 | Oxaliplatin | DM, SAH | Metachronous |
The congestion group included 5 patients with multiple bilateral CLM who had been selected for resection according to fulfillment of the following criteria: (1) hepatic segment 4 (± S1) without lesions or with only superficial lesions appropriate for atypical resection; (2) right hemiliver and left lateral sector both considered ineligible for surgical preservation due to the presence of multiple deep and/or sectorial or segmental hepatic pedicle tumor involvement; and (3) absence of detectable extrahepatic metastases (Table 1).
Abdominal and thoracic contrast-enhanced CT, abdominal magnetic resonance imaging (MRI), and positron emission tomography (PET)-CT were used to detect hepatic and extrahepatic disease. Hepatic metastases were defined as metachronous if they were diagnosed at least 6 mo after colorectal tumor resection. All patients received chemotherapy with oxaliplatin-based standard protocols and showed no hepatic disease progression prior to the surgical resection.
At the time of presentation of the metastatic liver disease, none of the patients were considered candidates for surgery as their FRLs were too small. Individual case discussion was carried out by the multidisciplinary tumor board, with alternatives for ensuring achievement of complete hepatic resection of the metastatic disease being considered. In this scenario, simultaneous embolization of the right portal vein and left lateral sector portal branches was deemed to be too risky because of the very small FLR (segment 4 ± S1). Of note, the left lateral lobe was evaluated for preservation but complete cleanup was deemed impossible, even with application of local ablation methods.
The comparison group consisted of 10 patients that had been randomly selected from a large database so that each patient was matched according to the following criteria: age (classified according to decades), sex, American Society of Anesthesiologists (commonly known as ASA) score, CLM diagnoses, preoperative chemotherapy (chemotherapeutic agents and duration), and type of resection (right hepatectomy with middle hepatic vein preservation).
Extended hepatic resection with preservation of segment 4 only represents a significant clinical challenge. The usual options for resection in patients with multiple bilateral metastases include methods of portal vein privation with or without local ablation techniques. However, when segment 4 is the only one to be preserved, performance of isolated right portal vein embolization (or even in conjunction with right hepatic vein occlusion) induces hypertrophy of both segment 4 and the left lateral sector, namely of both the segments to be preserved and the segments to be respected. Thus, the increase of segment 4 is not optimal, and it might not be sufficient for successful outcome. On the other hand, coincident embolization of the left lateral sector portal branches (in addition to the right portal vein embolization) is a risky procedure since the non-embolized segment is too small.
Thus, to optimize hypertrophy of the FLR (i.e., segment 4), our team conceived of a two-stage procedure (Figure 1) in which the initial surgery consists of a right hepatectomy with middle hepatic vein preservation and induction of congestion of the left lobe, and the second stage is a left lateral sectionectomy.
Since parenchymal resection provides the highest expected rate of remnant liver hypertrophy, a major hepatic resection (right hepatectomy) is performed in the first stage. Additionally, to promote future hypertrophy of segment 4 and to avoid hypertrophy of the left lateral sector, congestion of left lobe is induced. To induce the desired segmental congestion, a silicone tube is wrapped around the left hepatic vein, outside of the liver. The diameter of the looping silicone is progressively diminished around the vein. When macroscopic signs of congestion become evident in segments 2 and 3, a Doppler study is performed to ensure that hepatopetal flow is still present in these segments. Finally, the silicone tube is tied-off in order to maintain the diameter of the vessel according to the size of the loop. The loop is left in situ until the second stage is performed. It is important to ensure the hepatopetal flow despite congestion, as this will help maintain the function of the congested segments but will limit their hypertrophy rates.
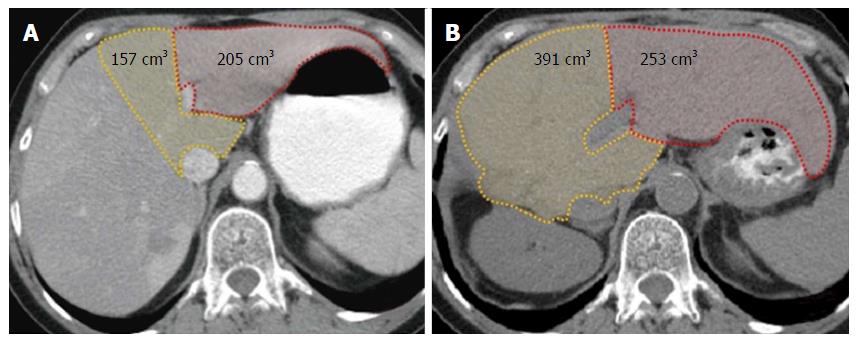
At 4 wk after the first stage, a CT with volumetric assessment is performed to evaluate parenchymal hypertrophy (Figure 2), after which a left lateral sectionectomy is performed if the FLR is considered sufficient. When lesions are also present on segments 4 and/or 1, the tumors are resected during the first stage, thereby “cleaning” the FLR for the subsequent procedure.
Both the first and second stages were performed with a J-shaped incision. Intraoperative ultrasound was used to verify the transection plane and assure preservation and permeability of the middle hepatic vein. The left hepatic vein was dissected and encircled by the standard technique. Parenchymal transection was performed using a saline-irrigated bipolar device, and all vascular and biliary structures sized ≥ 3 mm were divided between ligatures. Intermittent Pringle maneuver (15 min of clamping with 5 min intervals) was used as needed.
Volumetry of segments 4 and 1 (or of only segment 4 if S1 should be resected) and of segments 2 and 3 (left lobe) was performed preoperatively and at 4 wk after the right hepatectomy (Figure 2). The regeneration rate (percent hypertrophy) was calculated with the following formula: [(segmental volume after surgery - segmental volume before surgery) × 100]/segmental volume before surgery. Volumetries were determined by contrast-enhanced CT acquisitions and by use of the Osirix for IOS medical imaging viewer software (Pixmeo SARL, Bernex, Switzerland, 2012).
Data are expressed as mean ± SE of the mean or median. A t-test was used to compare means. P value < 0.05 indicated statistical significance. All statistical analyses were performed by the SPSS statistical software package, version 10.1 (SPSS Inc, Chicago, IL, United States).
The overall left liver increased by 103% (mean increase from 438 mL to 890 mL) in the 4 wk after performance of the first stage of the procedure. Hypertrophy of the FLR (i.e., segment 4 ± S1) was greater than that of segments 2 and 3 (144% vs 54%, respectively, P < 0.05). Table 2 shows the individual volumes and hypertrophy rates for each patient. The median remnant liver volume-to-body weight ratio was 0.3 (range, 0.28-0.40) before the first stage and 0.8 (range, 0.45-0.97) before the second stage.
| Segments 2/3 | Segment 4 (± S1) | P vaule | |||||
| 1st stage, in cc | 2nd stage, in cc | Hypertrophy rate | 1st stage, in cc | 2nd stage, in cc | Hypertrophy rate | ||
| Case 1 | 205 | 253 | 24% | 157 | 391 | 149% | |
| Case 2 | 228 | 331 | 45% | 192 | 505 | 163% | |
| Case 3 | 309 | 679 | 120% | 272 | 823 | 203% | |
| Case 4 | 248 | 407 | 64% | 225 | 641 | 185% | |
| Case 5 | 186 | 214 | 15% | 167 | 206 | 23% | |
| mean ± SD | 235 ± 42 | 377 ± 165 | 54 ± 37 | 203 ± 42 | 513 ± 211 | 144 ± 63 | < 0.05 |
The overall hypertrophy rate of the left liver at 4-6 wk after right hepatectomy with middle hepatic vein preservation in this group was 116% ± 34%. The hypertrophy rates were proportional for segments 2 and 3 and segment 4 (123% ± 47% vs 108% ± 60%, P > 0.05). The mean preoperative volume of segments 2 and 3 was 256 ± 64 cc, which increased to 572 ± 257 cc after the right hepatectomy. The mean preoperative volume of segment 4 increased from 211 ± 75 cc to 439 ± 180 cc after the surgery.
Both of the two stages of the novel procedure were successfully completed in all 5 patients. One patient (case #3) developed postoperative ascites after the first stage, requiring prolonged hospital stay. Another patient (case 5, a 65-year-old female) died at 30 d after the second stage, due to liver failure that likely resulted from small-for-size syndrome. In this patient, ascites and jaundice developed on postoperative day 7 and increased progressively with no evidence of biliary obstruction. She had been receiving systemic oxaliplatin-based chemotherapy for 8 mo prior to performance of the first stage. Her hypertrophy rate at 4 wk after the first stage was below expected, for both congested and non-congested segments (15% and 23%, respectively).
All other patients experienced uneventful postoperative recovery (i.e., no Dindo-Clavien complication of grade 2 or more) after the first and second stages.
The present study demonstrates that modulation of outflow allows for targeted liver regeneration. The method reported is particularly useful for patients in whom only segment 4 (± S1) can be preserved. In such cases, as described herein, the hypertrophy rate of segment 4 (± S1) after right hepatectomy was optimized when partial outflow deprivation of segments 2/3 was employed.
The classical paradigm of liver resectability comprises the preservation of at least two contiguous functional liver segments, with appropriate portal and arterial inflow, as well as venous outflow and biliary drainage. Right and left trisectionectomies are the most extensive liver resections routinely performed[16]. Moreover, two-stage hepatectomy for bilateral CLM may provide long-term outcomes that are comparable to those in patients treated with a planned single-stage hepatectomy[16-20]. However, extreme resections with preservation of only a single liver segment have been reported rarely[21-23]. Two cases of hepatic resection with only segment 4 preservation were reported by Adam et al[21], and both used staged procedures. For these, the first stage involved a major hepatectomy (right hepatectomy) in one of the patients and a minor hepatectomy (left lobectomy) in the other one. Schadde et al[19] reported 6 cases with left lobectomy performed during the first stage, in the context of ALPPS, followed by right hepatectomy in the second stage.
Our technique comprises a right hepatectomy (with or without wedge resections on segment 4) during the first stage of the procedure and a left lobectomy as the second stage. The potential disadvantages of performing a major liver resection first include: (1) submitting the patient to a higher-risk procedure initially, although this is offset by the chance that the patient may eventually not be a candidate for the second stage due to disease progression; and (2) subjecting the surgeon of the second stage to a procedure with increased technical difficulty if adhesions involving the diaphragm have formed. However, other positive aspects should be considered: (1) a major resection performed as the initial procedure induces a greater extent of hepatic regeneration than a minor hepatectomy; (2) anatomical right hepatectomy is a standardized procedure with acceptable morbidity; and (3) lysis of adhesions from the diaphragm to the cut section of the liver is not routinely required to perform the second stage procedure (left lobectomy). Besides these, no oncological benefit has been demonstrated for one strategy over another.
Extensive parenchymal resection results in the highest rate of regeneration of the remaining liver, as compared to other reported methods that induce FLR hypertrophy. In our study, the overall volume increase of the left liver after the first stage was 103%, and the FLR (i.e., segment 4) increased 144% before the second stage. In ALPPS, the reported hypertrophy rates of FLR before the second stage have ranged from 47% to 110%[13,24-26]. Likewise, in portal vein embolization or ligation alone, or when associated with ipsilateral hepatic vein occlusion, even more modest rates of hypertrophy have been reported (from as low as 8.2% and up to 46%)[27-31].
Hypertrophy of the remaining liver after major parenchymal hepatic resection is fast and powerful, and involves a complex process of signaling pathways[32]. The role of portal flow in the process of regeneration after major hepatectomy is unclear, but induction of regeneration seems to occur due to: (1) the increase of portal flow per unit mass itself; and (2) the large incoming amounts (per hepatocyte) of signaling molecules usually present in portal blood[33]. According to these concepts, the temporary increase of portal flow to some hepatic territories would strongly stimulate the regenerative process of these hepatocytes, while the maintenance of a usual portal flow would prevent regeneration.
This phenomenon explains the disproportional rate of hypertrophy that is seen between the left lobe and segment 4 after right hepatectomy, with or without preservation of the middle hepatic vein. It is known that after right hepatectomy with middle hepatic vein resection, the rate of regeneration of segment 4 is lower than that of segments 2 and 3. On the other hand, after right hepatectomy with middle hepatic vein preservation, all remnant segments show a similar hypertrophy[33-35]. In our technique, this knowledge was applied in a reverse manner, specifically a reduction of outflow from the left lobe after the right hepatectomy with preservation of middle hepatic vein. In fact, the induced congestion of the left lobe prevents the expected increase of portal flow to this hepatic area, maintaining its function while avoiding hypertrophy. In the meantime, the portal flow is redirected to non-congested segments (segments 4 and 1), i.e., the FLR, optimizing its regeneration.
Thus, induced congestion of the left lobe (which is to be resected) during the first stage of the procedure aims to prevent its hypertrophy while optimizing that of segment 4 (which is to be preserved). Our results confirm a significant disproportional hypertrophy rate of the congested left lobe (54%) compared to the non-congested segment 4 (144%), allowing for safe resection of the left lobe in the second stage of the procedure. The consistency of this strategy is evidenced by the hypertrophy rate of the matched comparison group of patients that underwent a right hepatectomy with middle hepatic vein preservation. In this comparison group, in the context of single-stage hepatectomy for CLM, a proportional hypertrophy rate was observed for left lobe and segment 4.
The interval between performance of the first and second stages ranged from 6 wk to 8 wk, and all patients underwent the second stage of the procedure. One patient died following the second stage. This patient presented an unexpected low rate of hypertrophy after the first stage (15% for the left lobe and 23% for segment 4) and underwent the second stage with a FRL of 206 cc (0.45% of the body weight). The restricted rate of hypertrophy could have been related to the long period of preoperative chemotherapy (8 mo) that the patient had undergone. The postoperative clinical findings were compatible with small-for-size syndrome and started to develop after postoperative day 7, with progressive hyperbilirubinemia and voluminous ascites. The patient died of liver failure on postoperative day 30.
Future studies should take into account both the expected rate of hypertrophy and the kinetic rate of hypertrophy. These rates should probably be considered as contraindications for the second procedure, as in other strategies used to induce liver hypertrophy. Also, the ideal interval between the two stages remains to be determined, as well as the benefit of chemotherapy during the interval period. To evaluate the viability of performing the second stage early, our group recently initiated a prospective protocol that includes volumetric assessment on day 7 after performance of the first stage.
In summary, this novel method for extreme hepatectomy with preservation of only segment 4 represents a new understanding of the clinical treatment that could refine the assignment of patients with multiple bilateral liver metastases to achieve a complete resection.
Complete resection is the only potential curative treatment for colorectal liver metastases (CLM). Resection of multiple bilateral lesions represents a technical challenge and a real risk of postoperative hepatic failure when segment 4 is the only hepatic segment to be preserved.
The usual technical options for resection of multiple bilateral CLM are preoperative portal vein embolization, associating liver partition and portal vein ligation, and two-stage hepatectomy. Unfortunately, these are rarely useful for extreme resection with preservation of segment 4 only, due to the common very-small future liver remnant. Thus, a method allowing safe hepatic resection with preservation of only segment 4 will benefit a significant number of patients.
A two-stage procedure that can overcome the risk of postoperative hepatic failure among patients with preservation of segment 4 only is presented. The first stage involves a right hepatectomy (to accomplish the higher expected hypertrophy rate) and a reduction of the left hepatic vein diameter (venous congestion to preclude proper hypertrophy). This strategy allows for an extraordinary hypertrophy rate of segment 4 (future liver remnant), while the left lobe maintains its function with a much less extent of increase in size. Then, the second stage can be safely accomplished through a left lobectomy. Compared to conventional right hepatectomy, the use of outflow modulation optimizes the hypertrophy rate of the target area.
This surgical technique represents a useful and safe method to perform extreme resections when the only hepatic segment to be preserved is segment 4.
This is a retrospective study, limited by the small number of patients. However, the use of this innovative technique allows extensive liver resection in patients with multiple bilateral liver metastases in a scenario that cannot be treated by conventional approaches. The comparison group in this paper clearly shows that venous congestion is a useful tool to optimize liver hypertrophy of the future liver remnant. The spreading of such technique should encourage other centers to perform it. Its benefit in a larger number of patients shall become apparent with further series.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Rezaee-Zavareh MS, Zhu X S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Abbas S, Lam V, Hollands M. Ten-year survival after liver resection for colorectal metastases: systematic review and meta-analysis. ISRN Oncol. 2011;2011:763245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Marudanayagam R, Ramkumar K, Shanmugam V, Langman G, Rajesh P, Coldham C, Bramhall SR, Mayer D, Buckels J, Mirza DF. Long-term outcome after sequential resections of liver and lung metastases from colorectal carcinoma. HPB (Oxford). 2009;11:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Sakamoto Y, Sakaguchi Y, Oki E, Minami K, Toh Y, Okamura T. Surgical outcomes after resection of both hepatic and pulmonary metastases from colorectal cancer. World J Surg. 2012;36:2708-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | McNally SJ, Parks RW. Surgery for colorectal liver metastases. Dig Surg. 2013;30:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Leung U, Gönen M, Allen PJ, Kingham TP, DeMatteo RP, Jarnagin WR, D’Angelica MI. Colorectal Cancer Liver Metastases and Concurrent Extrahepatic Disease Treated With Resection. Ann Surg. 2017;265:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | May BJ, Talenfeld AD, Madoff DC. Update on portal vein embolization: evidence-based outcomes, controversies, and novel strategies. J Vasc Interv Radiol. 2013;24:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Spelt L, Sparrelid E, Isaksson B, Andersson RG, Sturesson C. Tumour growth after portal vein embolization with pre-procedural chemotherapy for colorectal liver metastases. HPB (Oxford). 2015;17:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet P, Fabre JM, Quenet F, Herrero A, Panaro F. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. 2016;26:4259-4267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Abdalla EK. Portal vein embolization (prior to major hepatectomy) effects on regeneration, resectability, and outcome. J Surg Oncol. 2010;102:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 11. | Hwang S, Lee SG, Ko GY, Kim BS, Sung KB, Kim MH, Lee SK, Hong HN. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. 2009;249:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Donati M, Stavrou GA, Oldhafer KJ. Current position of ALPPS in the surgical landscape of CRLM treatment proposals. World J Gastroenterol. 2013;19:6548-6554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, Soubrane O, Schnitzbauer AA, Raptis D, Tschuor C. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260:829-836; discussion 836-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 14. | Hasselgren K, Malagò M, Vyas S, Campos RR, Brusadin R, Linecker M, Petrowsky H, Clavien PA, Machado MA, Hernandez-Alejandro R. Neoadjuvant chemotherapy does not affect future liver remnant growth and outcomes of associating liver partition and portal vein ligation for staged hepatectomy. Surgery. 2017;161:1255-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Balzan SM, Gava VG, Magalhaes MA, Dotto ML. Outflow modulation to target liver regeneration: something old, something new. Eur J Surg Oncol. 2014;40:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037-1049; discussion 1049-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 364] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 17. | Tsai S, Marques HP, de Jong MC, Mira P, Ribeiro V, Choti MA, Schulick RD, Barroso E, Pawlik TM. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB (Oxford). 2010;12:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Tsim N, Healey AJ, Frampton AE, Habib NA, Bansi DS, Wasan H, Cleator SJ, Stebbing J, Lowdell CP, Jackson JE. Two-stage resection for bilobar colorectal liver metastases: R0 resection is the key. Ann Surg Oncol. 2011;18:1939-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Schadde E, Malagó M, Hernandez-Alejandro R, Li J, Abdalla E, Ardiles V, Lurje G, Vyas S, Machado MA, de Santibañes E. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery. 2015;157:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Makuuchi M, Hasegawa H, Yamazaki S, Takayasu K. Four new hepatectomy procedures for resection of the right hepatic vein and preservation of the inferior right hepatic vein. Surg Gynecol Obstet. 1987;164:68-72. [PubMed] |
| 21. | Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 536] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 22. | Lau WY, Lai EC, Lau SH. Associating liver partition and portal vein ligation for staged hepatectomy: the current role and development. Hepatobiliary Pancreat Dis Int. 2017;16:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Edmondson MJ, Sodergren MH, Pucher PH, Darzi A, Li J, Petrowsky H, Campos RR, Serrablo A, Jiao LR. Variations and adaptations of associated liver partition and portal vein ligation for staged hepatectomy (ALPPS): Many routes to the summit. Surgery. 2016;159:1058-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Tanaka K, Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, Endo I, Ichikawa Y, Taguri M, Tanabe M. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): short-term outcome, functional changes in the future liver remnant, and tumor growth activity. Eur J Surg Oncol. 2015;41:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Mise Y, Passot G, Wang X, Chen HC, Wei S, Brudvik KW, Aloia TA, Conrad C, Huang SY, Vauthey JN. A Nomogram to Predict Hypertrophy of Liver Segments 2 and 3 After Right Portal Vein Embolization. J Gastrointest Surg. 2016;20:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Pandanaboyana S, Bell R, Hidalgo E, Toogood G, Prasad KR, Bartlett A, Lodge JP. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery. 2015;157:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Zeile M, Bakal A, Volkmer JE, Stavrou GA, Dautel P, Hoeltje J, Stang A, Oldhafer KJ, Brüning R. Identification of cofactors influencing hypertrophy of the future liver remnant after portal vein embolization-the effect of collaterals on embolized liver volume. Br J Radiol. 2016;89:20160306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 473] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 29. | Hwang S, Ha TY, Ko GY, Kwon DI, Song GW, Jung DH, Kim MH, Lee SK, Lee SG. Preoperative Sequential Portal and Hepatic Vein Embolization in Patients with Hepatobiliary Malignancy. World J Surg. 2015;39:2990-2998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 569] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 31. | Hata S, Sugawara Y, Kishi Y, Niiya T, Kaneko J, Sano K, Imamura H, Kokudo N, Makuuchi M. Volume regeneration after right liver donation. Liver Transpl. 2004;10:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Zappa M, Dondero F, Sibert A, Vullierme MP, Belghiti J, Vilgrain V. Liver regeneration at day 7 after right hepatectomy: global and segmental volumetric analysis by using CT. Radiology. 2009;252:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Scatton O, Plasse M, Dondero F, Vilgrain V, Sauvanet A, Belghiti J. Impact of localized congestion related to venous deprivation after hepatectomy. Surgery. 2008;143:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Wicherts DA, Miller R, de Haas RJ, Bitsakou G, Vibert E, Veilhan LA, Azoulay D, Bismuth H, Castaing D, Adam R. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 35. | Viganò L, Torzilli G, Cimino M, Imai K, Vibert E, Donadon M, Castaing D, Adam R. Drop-out between the two liver resections of two-stage hepatectomy. Patient selection or loss of chance? Eur J Surg Oncol. 2016;42:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |