Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4735
Peer-review started: February 8, 2017
First decision: March 16, 2017
Revised: March 30, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: July 14, 2017
Processing time: 156 Days and 6.7 Hours
To assess the therapeutic potential of Lactobacillus acidophilus (LA) for the treatment of pouchitis in a rat model.
Sprague Dawley rats underwent proctocolectomy and ileal pouch-anal anastomosis followed by administration of dextran sulfate sodium (DSS) to induce pouchitis. Rats with pouchitis were randomly divided into three groups: no intervention (NI), normal saline (NS, 3 mL/d normal saline for 7 d), and LA (3 mL/d LA at 1× 1010 colony-forming units for 7 d). General body condition was recorded and pouch specimens were obtained for histological examination. mRNA expression levels of interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor-α were determined by RT-PCR. Zonula occludens protein 1 (ZO-1) levels were measured by immunohistochemistry.
LA reduced weight loss associated with pouchitis (P < 0.05) and improved the symptoms of pouchitis in rats. Compared with the NI and NS groups, rats in the LA group showed earlier disappearance of hematochezia (6.17 ± 0.75, 6.50 ± 0.55, 3.17 ± 0.75, P < 0.05) and higher fecal scores (2.67 ± 0.48, 2.50 ± 0.51, 4.42 ± 0.50, respectively, P < 0.05). Histological scores were also lower in the LA group compared with the other two groups (7.17 ± 0.98, 8.00 ± 0.89, 4.00 ± 0.89, respectively, P < 0.05). mRNA expression levels of IL-1β, IL-6, and tumor necrosis factor-α were significantly reduced, while IL-10 mRNA levels were significantly increased in the LA group (P < 0.05, respectively). ZO-1 protein levels were also significantly increased after administration of LA (P < 0.05).
LA alleviates pouchitis induced by DSS after ileal pouch-anal anastomosis by decreasing pro-inflammatory factors and increasing anti-inflammatory factors, and restoring ZO-1 expression in the mucosa.
Core tip: This study aimed to assess the therapeutic potential of Lactobacillus acidophilus (LA) for the treatment of pouchitis in a rat model. Rats with pouchitis were randomly divided into three groups: no intervention, normal saline (NS, 3 mL/d normal saline for 7 d), and LA (3 mL/d LA at 1 × 1010 colony-forming units for 7 d). General body condition was recorded and pouch specimens were obtained for histological examination. mRNA expression levels of interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor-α were determined by RT-PCR. Zonula occludens protein 1 levels were measured by immunohistochemistry.
- Citation: Xu YY, Zhang YY, He AQ, Li KY, Gao SY, Liu G. Lactobacillus acidophilus alleviates pouchitis after ileal pouch-anal anastomosis in rats. World J Gastroenterol 2017; 23(26): 4735-4743
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4735.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4735
Ulcerative colitis (UC) is defined as a chronic nonspecific inflammatory disorder with recurrent symptoms, involving the mucosa and submucosa of the colon and rectum[1]. Ileal pouch-anal anastomosis (IPAA) is an ideal surgical treatment for UC, allowing complete removal of the colorectal lesion while retaining the anus and avoiding the need for a permanent ileostomy. Ileal pouchitis is a common complication after IPAA in patients with UC, and occurs in approximately 50% of patients[2]. However, the pathogenesis of pouchitis is unclear and basic studies regarding this complication are lacking.
Lactobacillus acidophilus (LA) is a gram-positive bacterium that can form a protein crystal layer on the surface of intestinal cells[3], thus conferring a protective effect on the intestinal barrier. The intestinal microbiota are considered to play a vital role in the development of UC[4] and pouchitis. Gionchetti et al[5] demonstrated the efficacy of probiotics such as VSL#3 for the prophylaxis and treatment of pouchitis, and our results are consistent with the results of this study. However, although various studies have shown beneficial effects of probiotics on the prevention and treatment of pouchitis, the specific mechanism remains unclear. Furthermore, the ability of specific bacteria and their combination to improve pouch inflammation is unknown[6].
Interleukin (IL)-1β is a multi-protein complex, which can play an important role in the maintenance of intestinal immune balance through the identification of bacteria and injury-related molecules[7]. IL-6 is an important inflammatory factor secreted by endothelial cells, macrophages, and mast cells, and participates in the activation of lymphocytes[8]. Tumor necrosis factor (TNF)-α plays a key role in intestinal inflammation, involving multiple immune responses, affecting the expression of endothelial cell adhesion molecules and maintaining intestinal permeability[9]. IL-10 is a regulatory cytokine secreted by mononuclear macrophages and plays an anti-inflammatory role[10]. Intestinal inflammation is often accompanied by abnormalities in inflammatory factors such as IL-1β, IL-6, TNF-α and IL-10, which may reflect the severity of inflammation in pouchitis[11]. Furthermore, damage to the intestinal mucosal barrier is often accompanied by destruction of tight junction proteins, especially the loss of zonula occludens protein-1 (ZO-1), leading to increased intestinal permeability and further increasing the intestinal inflammatory response[12]. Rats subjected to IPAA provide an effective model for the study of pouchitis[13]. Shebani et al[14] established a rat model of dextran sulfate sodium (DSS)-induced pouchitis suitable for further studies of the pathogenesis of the disease.
We investigated the therapeutic effect of probiotic LA in a DSS rat model of ileal pouchitis, including determination of the expression of inflammatory markers (IL-1β, IL-6, TNF-α and IL-10) and ZO-1 protein in the intestinal mucosa by immunohistochemistry.
Male Sprague Dawley rats (n = 18) weighing 360-380 g (purchased from the Laboratory Animal Center of the Military Medical Science Academy of the Chinese People’s Liberation Army) were housed individually in a specific pathogen-free animal laboratory at a temperature of 25 °C with a 12 h light/dark cycle, and provided with standard rat chow and running water ad libitum. Animal care and experiments were conducted according to the international guidelines on animal research and ethics.
IPAA was performed by microsurgery and pouchitis was induced by administration of 4% DSS for 4 successive days after postoperative day 31. Rats with pouchitis were divided randomly into three groups: no intervention (NI) group, normal saline (NS) group, and LA group (n = 6 per group). Rats in the NS group received 3 mL/d normal saline by lavage, and rats in the LA group received 3 mL/d LA at a concentration of 1 × 1010 colony-forming units for 7 d by lavage.
Body weight changes, hematochezia, and fecal scores were observed and recorded in all rats before sacrifice under anesthesia on day 7 after LA or normal saline intervention. Fecal scores were evaluated on a 5-point scale according to the method described by Drzymala-Czyż et al[15]: (1) lack of stool; (2) diarrhea; (3) blob of stool; (4) textured stool; and (5) normal stool. The ileal pouch was harvested after sacrifice and washed with normal saline. Half of each sample was fixed in 10% neutral formalin solution for histological examination and immunohistochemistry, and the remaining portion was immediately frozen in liquid nitrogen and stored at -80 °C for RT-PCR analysis.
Tissues were paraffin-embedded, stained with hematoxylin and eosin, and examined under a microscope. Pouch specimens were assessed according to the criteria described by Atila et al[16]. Erosion was evaluated as: 0, negative; 1, focal erosion; 2, erosion in many regions; or 3, extensive erosion. Ulceration was evaluated as: 0, none; 1, focal ulceration of the mucosa in half the superficial regions; 2, total mucosal ulceration at multiple foci; or 3, extensive mucosal ulceration extending to the muscularis mucosa or beyond. Intra-epithelial inflammation was evaluated by counting the number of lymphocytes in 100 epithelial cells at the tips of the villi. Villous atrophy was evaluated as: 0, none; 1, mild; 2, moderate; or 3, severe with villous flattening. Edema at the lamina propria was evaluated as: 0, none or 1, positive. Abscess formation and submucosal inflammation were also evaluated.
IL-1β, IL-6, IL-10 and TNF-α mRNA were detected using a RT-PCR kit (MJ-RESEARCH, United States) according to the manufacturer’s instructions. Total RNA was extracted from pouch tissues using an animal tissue total RNA Extraction Kit (Tiangen, Beijing, China). The primer pairs are shown in Table 1.
| Oligonucleotide sequence (5’-3’) | Product length (bp) | |
| IL-1β | 190 | |
| sense | 5’-AATGCCTCGTGCTGTCTGACC-3’ | |
| antisense | 5’-GTGGGTGTGCCGTCTTTCATCA-3’ | |
| IL-6 | 111 | |
| sense | 5’-GACTTCCAGCCAGTTGCCTTCT-3’ | |
| antisense | 5’-TGGTCTGTTGTGGGTGGTATCC-3’ | |
| IL-10 | ||
| sense | 5’-GGGTTGCCAAGCCTTGTCAGAA-3’ | 196 |
| antisense | 5’-CTTCACCTGCTCCACTGCCTTG-3’ | |
| TNF-α | ||
| sense | 5’-GGGCTCCCTCTCATCAGTTCCA-3’ | 113 |
| antisense | 5’-TGCTCCTCCGCTTGGTGGTT-3’ | |
| R-GAPDH | ||
| sense | 5’-TACCCACGGCAAGTTCAACG -3’ | 122 |
| antisense | 5’-CACCAGCATCACCCCATTTG -3’ |
Samples were subjected to immunohistochemistry to assess the expression of ZO-1 protein, using rabbit anti-ZO-1 (PB0072) (Boster, WuHan, China). Immunohistochemical images were analyzed using Image-Pro Plus6.0 software to assess the optical density.
All statistical analyses were carried out using SPSS 19.0. The data were expressed as the mean ± SD. Data analysis was performed using independent-samples t-tests or one-way ANOVA, and comparisons of two among the three groups were made using Students-Newman-Keuls tests. A difference of P < 0.05 was considered statistically significant.
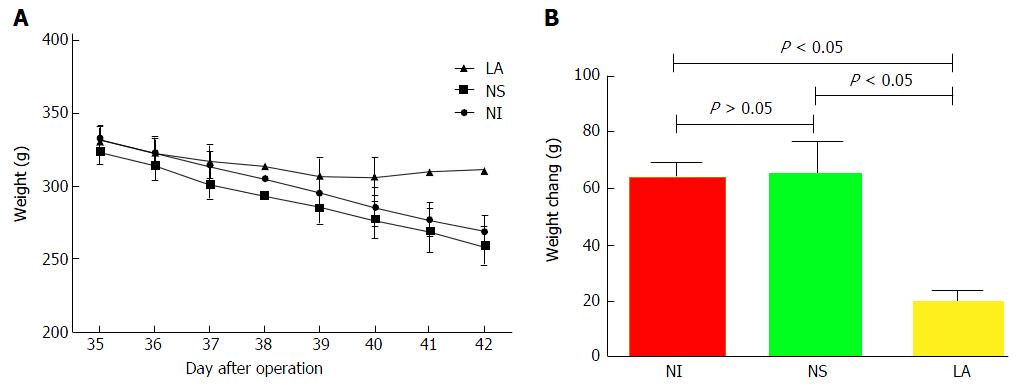
Rat body weight decreased linearly after the generation of pouchitis (72.92 ± 6.60). Following intervention for 7 d, body weight in the NI (63.50 ± 5.99) and NS groups (64.67 ± 11.93) continued to decline (Figure 1A), with no significant difference between the two groups (P > 0.05). However, the weight of rats in the LA group which initially decreased started to increase again on day 4 of lavage with LA (20.17 ± 3.25). The difference in body weights among the three groups was significant (F = 61.34, P < 0.05) (Figure 1B).
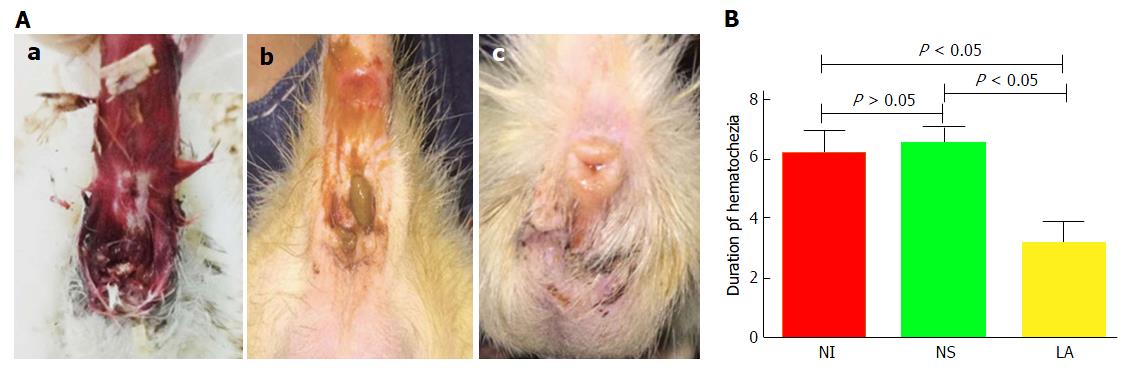
Bloody stools were observed in all groups at the end of the DSS intervention. Hematochezia disappeared in the LA group at 3.17 d ± 0.75 d, and in the NS and NI groups at 6.50 d ± 0.55 d and 6.17 d ± 0.75 d, respectively. The recovery time in the LA group was significantly shorter than in the NS and NI groups (F = 108.70, P < 0.05) (Figure 2).
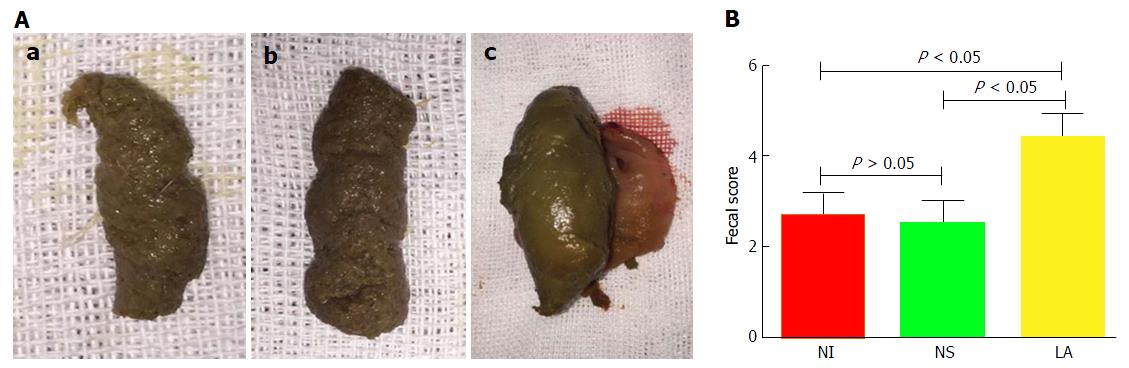
Stools in the NI and NS groups had a similar, loose-paste appearance (Figure 3A) and there was no significant difference in fecal scores between the NI (2.67 ± 0.48) and NS groups (2.50 ± 0.51) (t = 1.16, P > 0.05). However, feces in the LA group (4.42 ± 0.50) were more normal, and the fecal score was significantly higher than in the NI (t = 12.30, P < 0.05) and NS groups (t = 13.09, P < 0.05) (Figure 3B).
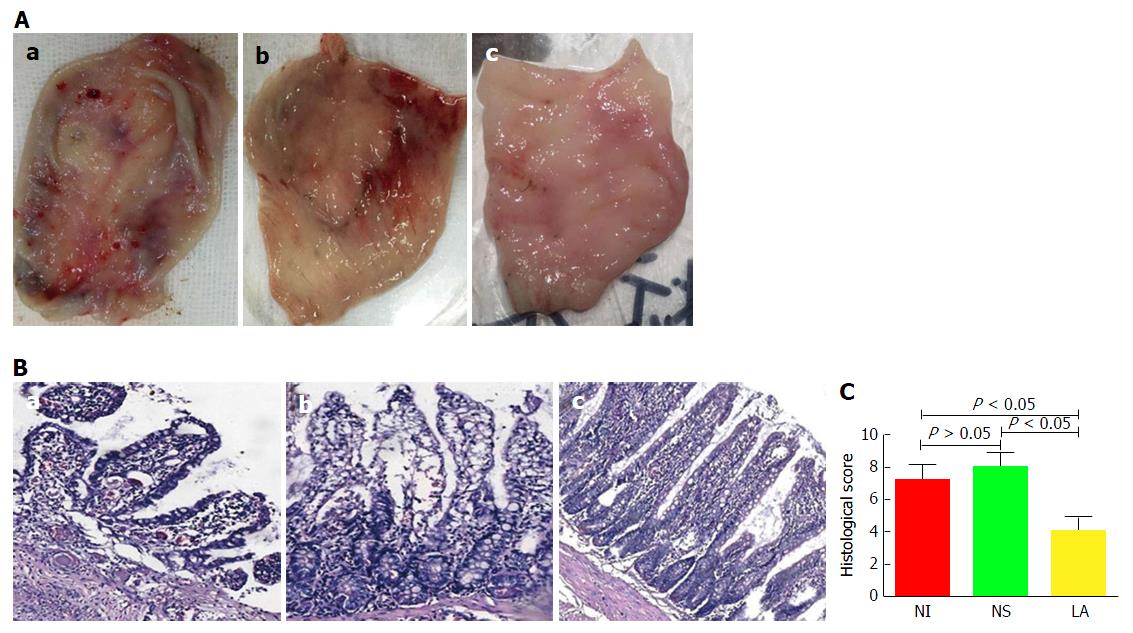
In terms of gross morphology, mucosal surface congestion and edema, toughness, visible multiple ulcers, and scattered bleeding were observed in the NI and NS groups, but mucosal congestion and edema were less evident in the LA group (Figure 4A).
On microscopic examination, the structure of the mucosal villi in the pouch tissue was irregular and disordered, the passivation of the villi, with extensive inflammatory cell infiltration in the central matrix of the villi in the NI and NS groups, while less severe lesions were observed in the LA group (Figure 4B). There was no significant difference in histological scores between the NI (7.17 ± 0.98) and NS groups (8.00 ± 0.89) (t = 1.536, P > 0.05), but the histological score was significantly lower in the LA group (4.00 ± 0.89) compared with the other two groups (F = 5.84, P < 0.05) (Figure 4C).
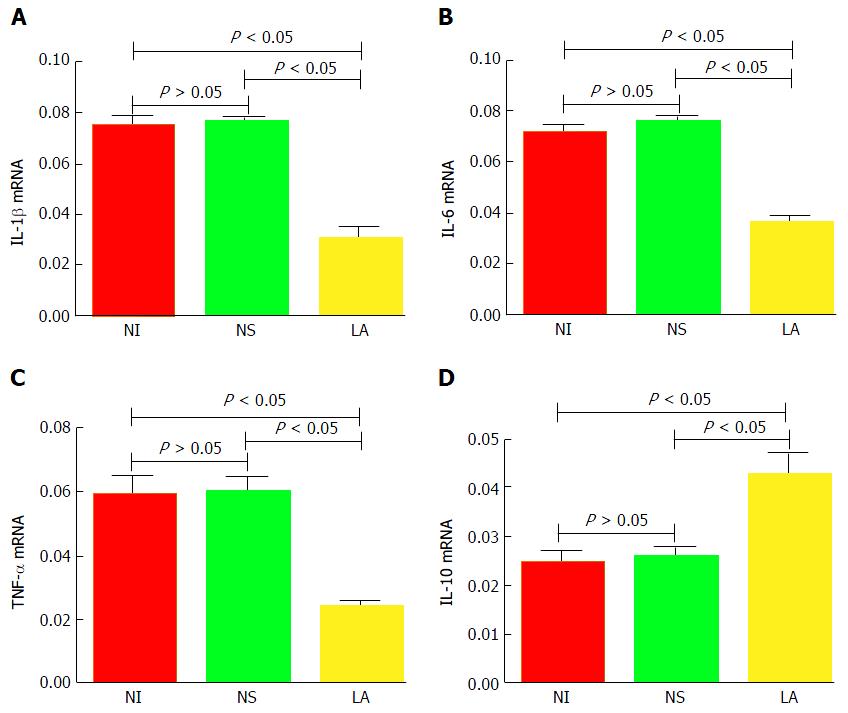
mRNA levels of IL-1β, IL-6, and TNF-α in the LA group were significantly lower than in the NS and NI groups (F = 373.60, P < 0.05; F = 285.50, P < 0.05; F = 132.90, P < 0.05, respectively). In contrast, IL-10 mRNA levels were significantly higher in the LA group compared with the other two groups (F = 61.05, P < 0.05). There was no significant difference in expression levels of inflammatory factors between the NS and NI groups (P > 0.05) (Figure 5).
ZO-1 is a tight junction protein, indicated by yellow staining in the cell membrane (Figure 6A). ZO-1 protein expression levels were significantly lower in the NI (0.27 ± 0.03) and NS groups (0.22 ± 0.08) compared with the LA group (0.35 ± 0.02) (F = 8.23, P < 0.05) (Figure 6B).
The intestinal microflora is generally thought to be involved in the pathogenesis of inflammatory bowel disease (IBD)[17]. The numbers of intestinal Bifidobacterium and Lactobacillus were decreased and Clostridium perfringens was significantly increased in patients with pouchitis[18]. LA is a component of VSL#3, and VSL#3, which is beneficial for maintaining remission in patients with pouchitis[19,20]. Probiotics containing LA can reduce expression of the inflammatory cytokine IL-1β and inhibit inflammatory damage caused by infiltration of polymorphonuclear cells in the tissues. Lammers et al[11] suggested that probiotics could be used to prevent and treat pouchitis, and LA has also shown immunomodulatory effects in in vitro experiments[21-24].
NaCl absorption involves coupling of the Cl-/HCO3- exchanger(s) primarily with the Na+/H+ exchanger 3 at the apical membrane of intestinal epithelia. Disturbances to this process occur in diarrheal diseases[25], and may also be involved in the formation of mucus stools in pouchitis. LA-conditioned medium stimulated intestinal cells to absorb NaCl by different mechanisms in in vitro experiments, and this mechanism may be an important factor in the improvement and treatment of IBD-associated diarrhea symptoms[26,27]. Borthakur et al[26] found that LA increased the exchange effect by increasing cell surface phosphoinositide 3-kinase dependent Cl-/HCO3- channels. Singh et al[27] showed that LA could promote the expression of Na+/H+ exchanger 3 (SLC9A3), which is widespread in epithelial cells of the digestive tract, resulting in improved intestinal absorption of electrolytes and an antidiarrheal effect. Chen et al[28] showed that LA was able to prevent bacterial colitis and activate the immune response with a protective effect on the intestinal mucosa.
Changes in intestinal inflammation are often accompanied by abnormalities in a range of inflammatory cytokines, including IL-1β, IL-6, TNF-α and IL-10, and the level of inflammation can be determined by detecting changes in these inflammatory factors in the pouch mucosa[11]. The results showed that the expression levels of IL-1β, IL-6 and TNF-α were significantly higher in patients with pouchitis than in those with active UC and non-pouchitis. Intestinal tract damage can lead to activation of the inflammatory response resulting in activation of caspase-1 and release of IL-1β[29]. Our study found that the expression of IL-1β in pouch tissues was significantly increased after pouchitis was induced by DSS, and compared with the control group, LA can significantly reduce the expression of IL-1β.
IL-6 expression levels were significantly increased in intestinal tissues of rats with inflammation. The interaction of antigen presenting cells with bacteria in IBD patients was shown to result in abnormal activation of CD4+ T cells, causing continuous release of pro-inflammatory cytokines and increased levels of IL-6 and TNF-α[30]. Our results showed that the expression levels of IL-6 and TNF-α in the mucosa of DSS-induced pouchitis were significantly higher than those in the control group, while the expression was decreased following LA gavage and the pathological score of the pouch was significantly decreased. Sang et al[31] treated rats with colitis using VSL#3 and showed that probiotics could significantly reduce IL-6 expression in intestinal tissues. Chen et al[32] also found that LA could significantly inhibit the expression of TNF-α in the intestinal mucosa in a rat colitis model.
IL-10 has previously been shown to play an anti-inflammatory role in pouchitis[33], and LA significantly increased IL-10 expression in human peripheral blood cells[34]. The results of the current study showed that DSS caused an intestinal inflammatory response in rats, associated with significantly increased levels of IL-1β, IL-6, and TNF-α, and significantly decreased levels of IL-10. This is consistent with the results of Chen et al[35], who used probiotics in a DSS-induced pouchitis model in rats. In addition, IL-6 can increase the amount of myeloperoxidase (MPO) by activating neutrophils, the increase in MPO protein not only reflects the degree of inflammation, but can produce a large number of oxygen free radicals in the intestinal barrier and further aggravate intestinal inflammation.
ZO-1 is a member of the membrane-associated guanylate kinases family[36]. Shen et al[37] showed that abnormal tight junctions resulted in increased permeability of the intestinal barrier, often accompanied by decreased expression of ZO-1 protein. Changes in the intestinal barrier caused by reduced ZO-1 expression in the intestinal tract will further promote the development of intestinal inflammation[12]. ZO-1 decreased following DSS administration in a rat colitis model, and the severity of colitis increased with time[12]. Our results also showed that ZO-1 expression levels were significantly higher in the LA group compared with the NI and NS groups, accompanied by decreased expression of inflammatory factors and improved pathological changes.
In summary, DSS-induced destruction of the intestinal barrier in the pouch manifested as increased levels of pro-inflammatory IL-1β, IL-6 and TNF-α, and decreased levels of anti-inflammatory IL-10, together with decreased expression of the intestinal barrier tight junction protein ZO-1. These factors reflect a complex network with the potential to aggravate mucosal inflammation in the pouch. LA may reduce the expression of pro-inflammatory factors and increase anti-inflammatory factors through a variety of mechanisms, increase expression of the tight junction protein ZO-1 in the intestinal mucosa thus promoting recovery of intestinal mucosal barrier function, and block interactions among various pro-inflammatory factors. Further studies are needed to clarify the potential role of LA in the prevention and treatment of pouchitis.
Ileal pouchitis is a common complication after ileal pouch-anal anastomosis (IPAA) in patients with ulcerative colitis (UC), and occurs in approximately 50% of patients. However, the pathogenesis of pouchitis is unclear and basic studies regarding this complication are lacking. Lactobacillus acidophilus (LA) confers a protective effect on the intestinal barrier, but the therapeutic potential of LA on pouchitis is uncertain.
Gastrointestinal microbiota in the field of inflammatory bowel disease (IBD) and pouchitis is a hot research topic.
Flora imbalance is involved in the pathogenesis of IBD, and has a similar role in the genesis and development of pouchitis. The authors confirmed that LA can be effective in the treatment of pouchitis, which provides a new treatment option for pouchitis.
This study provides a new treatment option for pouchitis. The successful application of LA in the treatment of pouchitis in rat models has indicated that further research on microbial treatment in humans should be carried out.
Pouchitis: An intestinal pouch complication of the IPAA procedure in patients with UC. Symptoms of pouchitis include diarrhea, hematochezia, increased stool frequency and abdominal cramps. LA: LA is a gram-positive bacterium that can form a protein crystal layer on the surface of intestinal cells, thus conferring a protective effect on the intestinal barrier and plays a vital role in the development of UC and pouchitis.
This manuscript is an interesting and well written paper regarding possible facilitatory effects of LA pouchitis in a rat model of IPAA.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Stanciu C S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Li D
| 1. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1543] [Article Influence: 118.7] [Reference Citation Analysis (5)] |
| 2. | Pardi DS, D’Haens G, Shen B, Campbell S, Gionchetti P. Clinical guidelines for the management of pouchitis. Inflamm Bowel Dis. 2009;15:1424-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Hynönen U, Palva A. Lactobacillus surface layer proteins: structure, function and applications. Appl Microbiol Biotechnol. 2013;97:5225-5243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 4. | Martinez C, Antolin M, Santos J, Torrejon A, Casellas F, Borruel N, Guarner F, Malagelada JR. Unstable composition of the fecal microbiota in ulcerative colitis during clinical remission. Am J Gastroenterol. 2008;103:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 5. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. [PubMed] |
| 6. | Batista D, Raffals L. Role of intestinal bacteria in the pathogenesis of pouchitis. Inflamm Bowel Dis. 2014;20:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1783] [Article Influence: 137.2] [Reference Citation Analysis (1)] |
| 8. | Ammori JB, Zhang WZ, Li JY, Chai BX, Mulholland MW. Effects of ghrelin on neuronal survival in cells derived from dorsal motor nucleus of the vagus. Surgery. 2008;144:159-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Apostolaki M, Armaka M, Victoratos P, Kollias G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr Dir Autoimmun. 2010;11:1-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun. 2000;68:3581-3586. [PubMed] |
| 11. | Lammers KM, Vergopoulos A, Babel N, Gionchetti P, Rizzello F, Morselli C, Caramelli E, Fiorentino M, d’Errico A, Volk HD. Probiotic therapy in the prevention of pouchitis onset: decreased interleukin-1beta, interleukin-8, and interferon-gamma gene expression. Inflamm Bowel Dis. 2005;11:447-454. [PubMed] |
| 12. | Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (2)] |
| 13. | Lichtman SN, Wang J, Hummel B, Lacey S, Sartor RB. A rat model of ileal pouch-rectal anastomosis. Inflamm Bowel Dis. 1998;4:187-195. [PubMed] |
| 14. | Shebani KO, Stucchi AF, Fruin B, McClung JP, Gee D, Beer ER, LaMorte WW, Becker JM. Pouchitis in a rat model of ileal J pouch-anal anastomosis. Inflamm Bowel Dis. 2002;8:23-34. [PubMed] |
| 15. | Drzymała-Czyż S, Banasiewicz T, Tubacka M, Tarasiuk-Rusek A, Majewski P, Drews M, Walkowiak J. Discrepancy between clinical and histological effects of DHA supplementation in a rat model of pouchitis. Folia Histochem Cytobiol. 2012;50:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Atila K, Terzi C, Canda AE, Akhisaroglu ST, Avci HS, Sarioglu S, Oktay G, Gulay Z. Partially hydrolyzed guar gum attenuates the severity of pouchitis in a rat model of ileal J pouch-anal anastomosis. Dig Dis Sci. 2009;54:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3430] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 18. | Iwaya A, Iiai T, Okamoto H, Ajioka Y, Yamamoto T, Asahara T, Nomoto K, Hatakeyama K. Change in the bacterial flora of pouchitis. Hepatogastroenterology. 2006;53:55-59. [PubMed] |
| 19. | Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: meta-analysis of randomized controlled trials. Inflamm Bowel Dis. 2014;20:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 20. | Gionchetti P, Calabrese C, Lauri A, Rizzello F. The therapeutic potential of antibiotics and probiotics in the treatment of pouchitis. Expert Rev Gastroenterol Hepatol. 2015;9:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Sahay B, Ge Y, Colliou N, Zadeh M, Weiner C, Mila A, Owen JL, Mohamadzadeh M. Advancing the use of Lactobacillus acidophilus surface layer protein A for the treatment of intestinal disorders in humans. Gut Microbes. 2015;6:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Li L, Jiang YJ, Yang XY, Liu Y, Wang JY, Man CX. Immunoregulatory effects on Caco-2 cells and mice of exopolysaccharides isolated from Lactobacillus acidophilus NCFM. Food Funct. 2014;5:3261-3268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Huang IF, Lin IC, Liu PF, Cheng MF, Liu YC, Hsieh YD, Chen JJ, Chen CL, Chang HW, Shu CW. Lactobacillus acidophilus attenuates Salmonella-induced intestinal inflammation via TGF-β signaling. BMC Microbiol. 2015;15:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Elawadli I, Brisbin JT, Mallard BA, Griffiths MW, Corredig M, Sharif S. Differential effects of lactobacilli on activation and maturation of mouse dendritic cells. Benef Microbes. 2014;5:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl/HCO3 exchanger couples with Na/H exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology. 2008;135:1645-1653.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr. 2008;138:1355-1359. [PubMed] |
| 27. | Singh V, Raheja G, Borthakur A, Kumar A, Gill RK, Alakkam A, Malakooti J, Dudeja PK. Lactobacillus acidophilus upregulates intestinal NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1393-G1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res. 2005;58:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3880] [Cited by in RCA: 4543] [Article Influence: 302.9] [Reference Citation Analysis (0)] |
| 30. | Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 330] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 31. | Sang LX, Chang B, Wang BY, Liu WX, Jiang M. Live and heat-killed probiotic: effects on chronic experimental colitis induced by dextran sulfate sodium (DSS) in rats. Int J Clin Exp Med. 2015;8:20072-20078. [PubMed] |
| 32. | Chen L, Zou Y, Peng J, Lu F, Yin Y, Li F, Yang J. Lactobacillus acidophilus suppresses colitis-associated activation of the IL-23/Th17 axis. J Immunol Res. 2015;2015:909514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Bulois P, Tremaine WJ, Maunoury V, Gambiez L, Hafraoui S, Leteurtre L, Cortot A, Sandborn WJ, Colombel JF, Desreumaux P. Pouchitis is associated with mucosal imbalance between interleukin-8 and interleukin-10. Inflamm Bowel Dis. 2000;6:157-164. [PubMed] |
| 34. | Vissers YM, Snel J, Zuurendonk PF, Smit BA, Wichers HJ, Savelkoul HF. Differential effects of Lactobacillus acidophilus and Lactobacillus plantarum strains on cytokine induction in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 2010;59:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Chen LL, Wang XH, Cui Y, Lian GH, Zhang J, Ouyang CH, Lu FG. Therapeutic effects of four strains of probiotics on experimental colitis in mice. World J Gastroenterol. 2009;15:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (2)] |
| 36. | Pan L, Chen J, Yu J, Yu H, Zhang M. The structure of the PDZ3-SH3-GuK tandem of ZO-1 protein suggests a supramodular organization of the membrane-associated guanylate kinase (MAGUK) family scaffold protein core. J Biol Chem. 2011;286:40069-40074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Shen ZY, Zhang J, Song HL, Zheng WP. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-α-regulated mechanism. World J Gastroenterol. 2013;19:3583-3595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |