Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4689
Peer-review started: December 30, 2016
First decision: March 16, 2017
Revised: March 30, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: July 14, 2017
Processing time: 195 Days and 1.3 Hours
Diarrhea after bariatric procedures, mainly those with malabsorptive elements including Roux-Y Gastric Bypass and Biliopancreatic Diversion, is common and an essential determinant of quality of life and micro- and macronutrient deficiencies. Bariatric surgery is the only sustainably successful method to address morbid obesity and its comorbidities, particularly gaining more and more importance in the specific treatment of diabetic patients. Approximately half a million procedures are annually performed around the world, with numbers expected to rise drastically in the near future. A multitude of factors exert their influence on bowel habits; preoperative comorbidities and procedure-related aspects are intertwined with postoperative nutritional habits. Diagnosis may be challenging owing to the characteristics of post-bariatric surgery anatomy with hindered accessibility of excluded segments of the small bowel and restriction at the gastric level. Conventional testing measures, if available, generally yield low accuracy and are usually not validated in this specific population. Limited trials of empiric treatment are a practical alternative and oftentimes an indispensable part of the diagnostic process. This review provides an overview of causes for chronic post-bariatric surgery diarrhea and details the particularities of its diagnosis and treatment in this specific patient population. Topics of current interest such as the impact of gut microbiota and the influence of bile acids on morbid obesity and especially their role in diarrhea are highlighted in order to provide a better understanding of the specific problems and chances of future treatment in post-bariatric surgery patients.
Core tip: Bariatric surgery is the only sustainable therapy for morbid obesity and its comorbidities. Postoperative diarrhea is common and an essential determinant of quality of life and micro- and macronutrient deficiencies. The distinctive anatomic changes after bariatric procedures with exclusion of various length of small bowel have a severe impact not only on diagnostic but also puts limits on therapeutic means. This review provides an overview of causes for chronic diarrhea in the particular context of post-bariatric patients, and details specific problems in diagnosis and treatment of this challenging patient population.
- Citation: Borbély YM, Osterwalder A, Kröll D, Nett PC, Inglin RA. Diarrhea after bariatric procedures: Diagnosis and therapy. World J Gastroenterol 2017; 23(26): 4689-4700
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4689.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4689
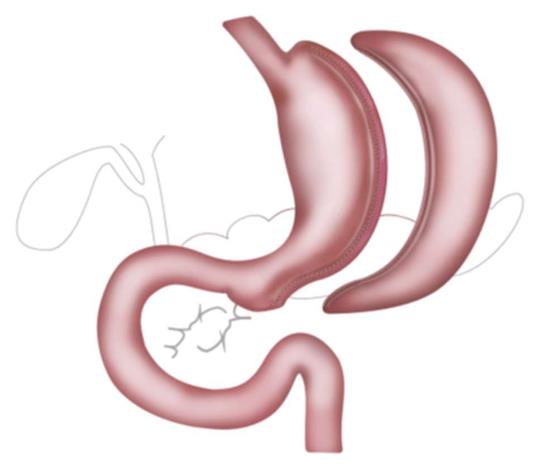
Bariatric surgery was demonstrated to be the most efficacious method to achieve sustainable weight loss and resolution of co-morbidities among the morbidly obese. Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG) and to a lesser extent bilio-pancreatic diversion with duodenal switch (BPD-DS) are the most commonly applied procedures (Figure 1). Around 470000 bariatric procedures have been performed worldwide in 2013, with numbers expected to rise in the future as the threshold, currently at a body mass index of 35 kg/m2, will be lowered to 30 kg/m2 in diabetic patients[1-4]. Weight loss is achieved by different mechanisms, all having various effects on bowel habits. The implied alterations of the anatomy not only affect the sensitivity and specificity of diag–nostic procedures but also the pharmacodynamics and bioavailability of the used medication, potentially resulting in treatment failure[5].
Bariatric surgery, especially BPD-DS and distal RYGB, leads to a significant change of bowel habits with enhanced frequency of malodorous flatus and diarrhea[6]. Diarrhea exposes patients at risk for fecal incontinence - up to 50% of patients after BPD-DS are affected - and has a major impact on Quality of Life as well as on nutrient and vitamin absorption[7-9]. In addition, morbid obesity per se, and in particular its associated comorbidities, broadens the spectrum of possible causes for diarrhea.
The goal of this review is to detail causes for chronic diarrhea in the specific post-bariatric surgery context, especially after RYGB, and to give a focused overview of its diagnosis and treatment, with the intention to depict the differences and difficulties compared to an evaluation for diarrhea in non-operated patients[10-12]. Reasons nonspecific for post-bariatric surgery patients, such as cancer-related diarrhea, are not covered, even though the incidence of cancer is higher in the bariatric than in the non-obese population[13].
Bariatric surgery results in a radical change of life with lifestyle modifications eventually resulting in change of food preference, meal size and frequency. The imposed restriction, especially in the earlier postoperative period, leads to a more liquid diet with decreased fiber intake[14]. While adaptive processes towards a stable metabolism and energy intake take at least 1 year[15], patients rarely benefit from an extensive evaluation prior to 6 mo postoperatively.
There are inconsistent data about postoperative food consumption, mainly because of self-reporting, incongruent assessment time points, changing food selection over time, and influence of nutritional counselling[16]. Overall energy intake is reduced after surgery; however, the proportion of fat, proteins and carbohydrates seems to remain constant[17]. In our practice, we observe an increased consumption of dietary and fat-reduced products in a purpose to eat “healthier”. However, the use of non-absorbable sweeteners such as sorbitol in these products can lead to similar effects as carbohydrate malabsorption[18].
Sleeve gastrectomy has become the most popular bariatric procedure in the last years. The greater curvature of the stomach is resected alongside a bougie preserving around 5 cm of antrum (Figure 1).
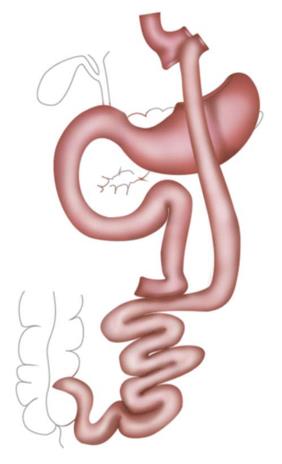
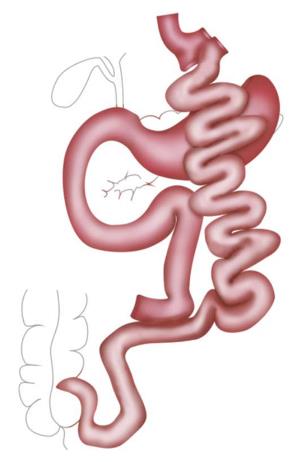
RYGB was the most popular procedure for years. A small gastric pouch is created followed by a pouch-jejunostomy, thereby forming an alimentary Roux-Y limb of up to 150 cm and a biliopancreatic limb of around 50 cm resulting in a common channel of various lengths depending on the length of whole small bowel. In distal RYGB, the length of the common channel, mostly around 100 cm, is the determined factor and the alimentary limb is of variable length (Figures 2 and 3).
The gastric volume is reduced akin to a LSG but with a generally bigger bougie size. The duodenum is transected near the pylorus and a duodeno-jejunostomy is created to form a common channel of around 80 cm.
Adjustable gastric banding (AGB) experienced a massive decline in the past decade due to a failure of long-term weight loss. Currently it is used almost exclusively concomitant to or after RYGB to reduce pouch extensibility. Apart from vagotomy as intraoperative complication, it has little influence on stool consistency, and when, patients rather tend to constipation[14].
There is a consistent relationship between obesity and diarrhea[19]. The incidence of diarrhea in a preoperative bariatric population is around 8%[20], being twice as high as in lean people. A possible reason for this might be a higher intake of poorly absorbed sugars[21]. Indeed, digestive symptoms in general, including diarrhea, are frequent among obese patients, both before and after bariatric surgery[22,23].
There is a change of bowel habits after every bariatric procedure, even though the severity of symptoms differs between the individual techniques. Up to 75% of patients suffer from alterations of bowel habits and faecal transit time after RYGB[20]. Diarrhea is a common symptom after RYGB[6,20,24], and usual after BPD[14,25]. Length of the common channel, i.e., the amount of absorptive surface, seems to play a role, given the higher frequency of diarrhea in long limb/distal RYGB patients than after short limb/proximal RYGB procedures[26], in BPD compared to RYGB[25], and in BPD patients with shorter common channels[27].
Short bowel syndrome, defined by lack of absorptive surface, occurs in around 4% of patients after bariatric surgery[28]. The reported average small bowel length in obese patients ranges from 300 to 900 cm, with considerable variability between 230 cm and 1510 cm[29-31]. However, there is a remarkable intra- and interoperator variability when it comes to determination of bowel length, both in open and laparoscopic procedures[32,33]. In the most commonly performed technique, the proximal RYGB, only the lengths of the alimentary (AL) and the biliopancreatic limbs (BPL) are defined and counted through, whereas the common channel remains of variable, unknown size. While AL of 100-150 cm and BPL of 45-85 cm are commonly used[30,34,35], there is ongoing debate about the delineation of optimal limb lengths. Nonetheless, this debate does not respect absorptive capacity of small bowel and even less its adaptation over time. Even though there is considerable progress in assessing intestinal malabsorption, no direct test with sufficient sensitivity and specificity is currently available, not to mention the diagnostic issues due to the altered anatomy after RYGB[36]. Initial treatment consists of supportive measures; surgical options are lengthening of the common channel, enteral nutrition via gastrostomy into the gastric remnant, and restitution of normal anatomy if still possible[37]. In the United States, 6.3% of home parenteral nutrition patients have a history of bariatric surgery and over two-thirds of them underwent RYGB[38].
Carbohydrates were propagated in a low-fat diet for years; they have a good palatability and are readily available in a high-caloric liquid form[39]. Liquids transit rapidly through the intestine and produce lesser satiety than the solid form. Sugar-sweetened beverages have a considerable impact on body weight, and there is clear association between their consumption and weight gain[40,41].
Even though a diet with a moderate amount of carbohydrates is recommended after bariatric surgery[42], the proportion of ingested macronutrients - lipids, proteins and carbohydrates - remains constant, whereas the overall energy intake that is reduced[18]. Lack of the enzyme lactase in the intestinal mucosa leads to lactose malabsorption and intolerance with diarrhea. While symptoms are dependent on small bowel transit time, there is a poor correlation between lactose malabsorption and intolerance[18,43]. Lactase activity is diminished progressively in adulthood[44], and the influence of bariatric surgery on this process in unclear[6]. Furthermore, there is no data on symptoms after bariatric surgery in a population of non-Western European heritage, i.e., with a higher prevalence of lactase deficiency. On the other hand, a Scandinavian study found a lactose intolerance rate of 30% after jejuno-ileal bypass[45].
Fructose as monosaccharide is widely used as a sweetener in fat-reduced, low-caloric food. In contrast to the disaccharide form, its glucose-independent absorption capacity is limited[46]. Hydrogen breath tests can be used in the diagnosis of carbohydrate malabsorption. However, 18% of patients of European descent are hydrogen non-excretors resulting in a possible false-negative result. Furthermore, the effect of excluded small bowel segments on the test accuracy has not yet been elucidated. Abstaining from or at least reducing lactose and fructose in meals might be most productive for both diagnosis and therapy, even though enzyme replacement therapies, as referred to as lactase and xylose isomerase, are available[18].
Micronutrients are absorbed in the mid to distal jejunum[47]. BPD-DS and to a lesser extent distal RYGB exclude these segments and are associated with macro- and micronutrient deficiencies[9,48]. Hypoalbuminemia occurs in up to 18% of BPD-DS patients[49], further aggravated by a protein intake of half of the recommended amount of 60-120 g protein daily in bariatric patients[47,50]. Hypoalbuminemia is associated with severe diarrhea in every fourth patient ending up with the need for parenteral nutrition[51]. The associated pathogenesis resembles the Kwashiorkor-type malabsorption of severely undernourished children resulting in a reduced production of gastric acid, pancreatic atrophy, small intestinal bowel overgrowth (SIBO) and alterations of the gut microbiota[52]. Treatment is usually initiated by employing supportive measures, rehydration and volume replacement, together with parenteral feeding, akin to World Health Organization Treatment guidelines for children[53]. Alternatively, surgical measures, such as limb length reshaping and reversal of RYGB, may be considered.
Endocrine causes for diarrhea are rather related to morbid obesity and its comorbidities than to bariatric surgery. Ninety percent of patients with type 2 diabetes mellitus (T2DM) are overweight or obese[54]. Metabolic/bariatric surgery, mainly RYGB, is gaining importance in the treatment of T2DM[4,55]. However, minimally symptomatic patients with T2DM may become symptomatic after RYGB. Furthermore, depending on preoperative duration and severity of T2DM, the relapse rate after a disease-free postoperative interval is reported to be up to 11%[56]. Several factors have an influence on diarrhea in T2DM-patients: dietetic, sugar-free food, association of T2DM to celiac disease[57], and T2DM-induced disturbance of the enteric nerve system leading to altered gut motility, again resulting in SIBO and exocrine pancreatic insufficiency (EPI).
Loss of weight and fat volume after bariatric surgery[58] may require adaptation of drug apportioning otherwise leading to postoperative overdosage, e.g., of thyroid hormones. Thus, switch from LSG to LRYGB for gastroesophageal reflux disease requires monitoring despite absent weight change, as L-thyroxine is absorbed in the (partly excluded) small bowel[59].
In human adults, the gut microbiota is a complex and dynamic ecosystem that coevolves with its host[60], and remains remarkably constant slightly fluctuating around an individual core of stable colonisers. Low diversity of an individual’s fecal bacteria is associated with a more pronounced overall obesity and dyslipidemia, impaired glucose homeostasis, and considerable low-grade inflammation[61]. Dietary changes, use of proton pump inhibitors or (recurrent as well as short- and long-term) antibiotic treatments may result in transient alterations of the gut microbiota composition[62,63]. It is still a matter of debate whether dietary intake or host genetics exert the stronger influence on microbial composition[64]. Not only development of obesity but also body weight reduction following bariatric surgery is, at least partly, attributed to alterations in gut microbiota. RYGB, as against SG, was demonstrated to substantially diminish the diversity of gut microbiota[65] paralleled by an increase in the proportion of Gammaproteobacteria and a decrease in Clostridia[66,67]. SG, however, was shown to cause a change in the Bacteroidetes/Firmicutes ratio, indeed, with a distinct increase in Bacteroidetes and a decline in the abundance of Firmicutes[68]. While the exact mechanisms remain unclear, change of the individual’s microbiota composition is considered to be a key factor of postoperative body weight reduction and may be one of the potential contributors to a stable weight loss after bariatric surgery[65,69]. Even though one would assume the importance of the microbiome regarding occurrence and/or resolution of diarrhea after bariatric surgery, this has not yet been elucidated. Bacteroides, normally increased after SG, was found to be substantially decreased in patients with idiopathic chronic diarrhea[70]. These results were confirmed by another group reporting an enrichment of Bacteroides, among other phyla, in controls when compared to diarrhea cases, irrespective of whether they were or not Clostridium difficile-associated[71]. These results cast a possible relationship between the normally observed post-bariatric shift of Bacteroides within the microbiota composition and diarrhea into doubt. Further studies addressing this question are warranted.
SIBO, defined as an excessive amount of bacteria in the small bowel[72], has a prevalence of 2.5% in healthy subjects[73], and up to 41% in obese patients, probably due to an impaired small intestinal motility[74]. In fact, side-side anastomoses with longer blind ends such as in candy cane syndrome are susceptible to SIBO[75,76]. A Brazilian group described a frequent occurrence of bacterial overgrowth in both the gastric pouch and the gastric remnant after RYGB in morbidly obese subjects, when assessed after a mean follow-up of 7.3 years[77]. These patients, however, did not complain of consistent or prolonged symptoms suggestive of SIBO, namely diarrhea, malabsorption, abdominal pain, intestinal obstruction, or extradigestive complaints (polyarthritis, dermatologic abnormalities, progressive liver insufficiency), after a mean follow-up of almost 15 years[78]. Another group reported a more than two-fold rise of SIBO after RYGB. Yet, weight loss itself does not seem to favor SIBO, given the comparable rate of bacterial overgrowth before and after exclusively restrictive surgery, such as AGB[23]. The influence of proton pump inhibitors, caloric intake and dietary composition is another topic of debate[23,79]. Diagnostic insecurities and a high prevalence of SIBO complicate the exact determination of its effects. In fact, it was shown that two thirds of patients after RYGB had digestive symptoms, but none of those were more frequent in patients with SIBO[23]. Consequences of SIBO after bariatric surgery are unclear. The nutrients escaping digestion in the small bowel due to SIBO might yield elevated levels of short- and medium-chain fatty acids through metabolization in the large bowel, implying a higher caloric uptake[80,81]. However, data on expected resulting reduced weight loss is conflicting[23,82]. The altered anatomy with excluded small bowel segments severely inflicts diagnostic measures; aspiration and culture might be impossible despite advanced endoscopic techniques, and breath testing underlies the same restrictions[83].
Alteration of gastrointestinal climate caused by obesity, antibiotic therapy or surgery is a risk factor for clostridium-associated colitis[84], even though it may occur in absence of the aforementioned factors[23]. Furthermore, it may present as a protein-losing enteropathy with hypoalbuminemia without fulminant inflammation[85]. Individual types of stool tests can yield the diagnosis. However, the high rate of asymptomatic carriers demands for a combination of symptoms and positive test results[84]. Treatment aims at reestablishing a diverse microbial flora. Long-standing medical wisdom suggested treatment with only oral antibiotics, metronidazole rather than vancomycin, and avoiding antimotility agents such as loperamid. The latter lacks substantive data[86]; the former must be questioned, at least in post-RYGB and -BPD-patients. In view of the anatomic alterations after bariatric surgery and given the pharmacokinetics of metronidazole - it is almost completely absorbed in the small bowel - intravenous vancomycine might the preferred primary treatment option[87,88]. Fecal microbiota transplantation is a novel method to treat recurrent infections, and has also gained interest in the bariatric community due to its effects on weight loss[89].
An “addiction-transfer” away from food may be an explanation for the increased number of impulse control disorders after bariatric surgery[90]; a quarter of the bariatric population has an eating disorder that impedes weight loss[91]. Amongst other substances, alcohol and nicotine both lead to diarrhea when consumed in excessive amounts[92]. Several cohort studies showed increased risk for alcohol abuse after bariatric surgery[93], further complicated by a faster rise of blood alcohol concentration[94]. Preoperative history of substance misusage is associated with postoperative abuse, as for alcohol ranging up to 12%[95,96]. Furthermore, consumption of excessive amounts of (sugar-free) drops, sometimes with the purpose of covering halitosis, must be kept in mind.
Vagotomy during RYGB can be performed either intentionally to enhance weight loss via earlier satiety and lessened food intake[97,98], as esophageal lengthening procedure concomitant to hiatal hernia repair[99] or inadvertently as intraoperative complication due to the proximity of the vagal nerves to the gastric pouch[100]. Diarrhea occurs in around 10% of patients after truncal and to a lesser extent after more distal vagotomy; severe, debilitating diarrhea occurs in 2%-3%[101]. Controversially, intentional vagotomy to gain esophageal length in hiatal hernia repair did not lead to higher rates of side effects[98,99]. Reasons for postvagotomy diarrhea are not completely elucidated, a change of microbial climate by altered intestinal motility and gastric hypoacidity certainly plays a role[102]. Beneath the usual dietary modifications proposed for RYGB, bulking agents to decrease the water content of the stool should be introduced. A short-term trial with octreoid can be attempted, however, isolated postvagotomy diarrhea is less responsive than dumping syndrome. In severe cases, surgical options such as alteration of limb lengths or even reversal of RYGB should be discussed.
The role of bile acids in morbid obesity, its comorbidity and weight loss in post-bariatric patients experiences increased interest, as they seem to be profoundly involved in the postoperative metabolic improvement. The target of interest are FX- and TGR5-receptors and their influence on bile acid metabolism with subsequently increased hormonal - in particular incretine - answer and change of gut microbiota[103,104].
Bile acid malabsorption results from a disturbed enterohepatic cycle and bile acid production. About 95% of bile acids are reabsorbed in the ileum, an additional small percentage is absorbed in the colon with bowel motility, medication, microbiota and food composition as influencing factors[105-107]. FXR is involved in the regulation of bile acid production, in entero- and hepatocytes[108]. BAM is categorized as either idiopathic, secondary to ileal dysfunction, such as after resection, or unrelated to ileal dysfunction, mainly due to SIBO or cholecystectomy. A disturbed synthesis in idiopathic BAM might also play role in irritable bowel syndrome (IBS)-D patients, same as in post-cholecystectomy patients[109]. Cholecystectomy concomitant to a bariatric procedure was a standard of care in the last decades[110]. BAM might further be a cause for post-vagotomy diarrhea[102]. The short common channel after BPD and distal RYGB predisposes to BAM due to reduced reabsorption rate and diminished time for bile acids to exert its effects on digestion. So far, there is no data about the rate of BAM stratified for subtypes of bariatric procedures. Bile acid malabsorption can be detected by fecal bile acid quantification, radiolabelled Selenium homotaurocholic acid testing or determination of serum-C4-concentration[111]; however, they all are expensive, rather difficult to apply in clinical practice or not standardized. Cholestyramine, a bile-acid binder, is an effective treatment with an efficacy up to 96%[112]. Due to the downsides of the above-mentioned testing procedures, it is used in diagnosis in an empirical trial[12].
Gastrectomy and RYGB both can lead to secondary EPI resulting in steatorrhea[34,113] with subsequent deficiencies of fat-soluble vitamins. Depending on type of RYGB, proximal or distal, the prevalence of EPI is up to 19%-48% respectively[34]. Changes in caloric content, composition and physical properties of meals after RYGB lead to a diminished, uncoordinated pancreatic response to nutrient stimuli[114]. The altered anatomy after RYGB leads to a shorter amount of contact time of enzymes with chyme. In addition, the degradation of pancreatic enzymes is accelerated in absence of food in the biliopancreatic limb[114]. In rare cases, left pancreatic resection for dumping syndrome or nesidioblastosis is performed, leading to primary endocrine and EPI[115]. Testing for EPI proves difficult due to altered anatomy. Direct stimulation tests require the the intubation of the duodenum either in a transgastric way or via a double-balloon technique and measure the exocrine pancreatic response after stimulation with CCK or secretin. Indirect stimulation test, such as measurement of fecal fat or elastase-1 are cheaper, more readily available but pose other problems. The former requires the ingestion of a defined amount of fat, which proves difficult after RYGB-induced, altered perception of food. In the latter, a normal result does not preclude EPI, as only the released amount of enzymes, but not its effect on chyme is measured; the shortened contact time within the common channel is not reflected. The recommended high-fat diet, based on lipids as strongest stimulators of exocrine pancreatic response, is hard to apply in post-RYGB patients[34]. Pancreatic enzyme replacement therapy is the mainstay of EPI treatment. Substitution is adapted to symptoms[114]; if using pancrealipase, removing the acid-resistant coating is imperative as the amount of produced acid in the gastric pouch is only minimal[34].
Diarrhea is one of many abdominal and systemic symptoms of mostly early dumping syndrome (DS), caused by a rapid exposure of the small bowel with undigested nutrients. Prevalence of DS after RYGB is reported to be up to 75%, and after SG up to 45%. Even though bothersome at least, DS is seen not seen as complication but rather as desirable feature by a few surgeons[116], it is thought to be an essential component of postoperative weight loss. Diagnosis relies mainly on symptom-based questionnaires like Sigstad’s scoring system, together with an oral glucose tolerance test or a mixed-meal test. However, those tests have a high sensitivity, but low specificity and are complicated by the small gastric pouch[117]. First-line treatment of DS is directed at a change of diet towards a more fibre- and protein-rich regimen with a low proportion of rapid-absorbable carbohydrates. Dietary supplements such as pectin or glucomannan have a poor tolerability and may interfere with a post-bariatric diet[117]. Acarbose affects mainly late DS, whilst diarrhea is associated rather with early DS. Somatostatin analogues are effective treatment options for both early and late DS, however, the have diarrhea as common side effect. Total parenteral nutrition is less practical, but sometimes inevitable option, other surgical possibilities consist in either remnant gastrostomy, reversal of RYGB or measure to enhance restriction of the pouch, such as AGB or pouch reshapings, only in very rare cases pancreatic resection have to be performed[118,119].
The cardinal symptom of both, Crohn’s disease and ulcerative colitis is diarrhea. It is unclear whether obesity and the subsequent proinflammatory state have a role in the pathogenesis of Crohn’s disease[120]. Even though bariatric surgery leads to a normalization of this state, bacterial overgrowth might lead to a local activation of innate immune factors favoring inflammation[121]. Whether bariatric surgery is of benefit remains to be elucidated, case series show a favorable outcome with LSG as procedure of choice[122-126]. Even though in most instances inflammatory bowel disease will be known prior to surgery, it might be missed in preoperative evaluation as it might occur later or is not captured, as morbid obesity seems to be associated with more colonic disease[127]. Of note, fecal calprotectin as measure for tissue inflammation is elevated after bariatric surgery[128].
Approximately 40% of patients suffering from celiac disease (CD), traditionally associated with malabsorption and insufficient body weight, are overweight or obese at diagnosis[129,130]. They, therefore, could be potential candidates for bariatric surgery. Diarrhea is classically the hallmark of symptomatic coeliac disease. However, a trend toward silent or atypical forms has been observed[131]. Triggers including pregnancy, traveller’s diarrhea, gastroenteritis, and some type of gastrointestinal surgery were reported[132]. Indeed, a recent publication described the rapid onset of CD after bariatric surgery, i.e., a duodenal switch procedure[133]. After all, CD should be considered as a differential diagnosis in patients presenting with persistent diarrhea after bariatric surgery.
A group of Italian surgeons suggested a preoperative work-up of specific CD tests (anti-endomysial and antitransglutaminase antibodies and total Immunoglobulin A) before bariatric procedures. Diagnosis of CD, consisting of harvesting duodenal biopsies is hindered after RGB due to the anatomical changes involved. In these cases, exlusively serologic testing remains an option. Indeed, a negative CD specific serology does not completely exclude the diagnosis of CD though it does make it much less likely[134]. The standard treatment of CD implementing a gluten-free diet (GFD) was shown to be successful in bariatric patients, either. In this line, a complete restoration of the intestinal muscosa within 12 mo after starting GFD was observed in a young adult with an incidentally diagnosed silent form of CD after bariatric surgery (vertical banded gastroplasty) 5 years earlier[135]. However, apart form a few case reports, little is known about onset, course, diagnosis and management of CD following bariatric surgery, particularly sleeve gastrectomy and RGB.
A prevalence of up to 30% of IBS fulfilling the ROME III criteria among morbidly obese patients was reported. These patients are more likely to suffer from profound alterations to quality of life and severe psychological disturbances than those without IBS[136,137]. On the other hand, a recent study of a large cohort showed that obesity is protective of a diagnosis or worsening of IBS[138]. Visceral adiposity, rather than general obesity, has been associated with an increased risk of diarrhea dominant IBS[139]. However, the association between the two entities remains unclear[140]. Increase of intra-abdominal pressure owing to excess of visceral fat, local tissue as well as systemic inflammation mediated by adipokines and cytokines originating from abdominal adipocytes[141-143], and altered gut microbiota[144] have been suggested as possible mechanisms that link obesity and IBS. Bariatric surgery, i.e., RGB, may improve the IBS symptoms[137]. Yet, the impact of bariatric surgery on visceral hypersensitivity and outcome of IBS is still unknown. Nevertheless, it might be advisable to systematically screen IBS and other functional bowel disorders in patients eligible for bariatric surgery.
There are a multitude of reasons for diarrhea in post-bariatric patients. Diagnosis can be challenging, as they are often intertwined and the influence of inconsistent, mood-dependent elements, must not be underestimated. The special anatomy after RYGB and BPD with excluded bowel segments complicates testing and the interpretation of results. Thus, empiric therapy of limited time will help in diagnosis and treatment.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Samanta I, Serban ED S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25:1822-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1163] [Article Influence: 116.3] [Reference Citation Analysis (1)] |
| 2. | Cefalu WT, Rubino F, Cummings DE. Metabolic Surgery for Type 2 Diabetes: Changing the Landscape of Diabetes Care. Diabetes Care. 2016;39:857-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Cummings DE, Cohen RV. Bariatric/Metabolic Surgery to Treat Type 2 Diabetes in Patients With a BMI & lt; 35 kg/m2. Diabetes Care. 2016;39:924-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: a Joint Statement by International Diabetes Organizations. Obes Surg. 2017;27:2-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Stein J, Stier C, Raab H, Weiner R. Review article: The nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 6. | Potoczna N, Harfmann S, Steffen R, Briggs R, Bieri N, Horber FF. Bowel habits after bariatric surgery. Obes Surg. 2008;18:1287-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Roberson EN, Gould JC, Wald A. Urinary and fecal incontinence after bariatric surgery. Dig Dis Sci. 2010;55:2606-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Søvik TT, Karlsson J, Aasheim ET, Fagerland MW, Björkman S, Engström M, Kristinsson J, Olbers T, Mala T. Gastrointestinal function and eating behavior after gastric bypass and duodenal switch. Surg Obes Relat Dis. 2013;9:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Nett P, Borbély Y, Kröll D. Micronutrient Supplementation after Biliopancreatic Diversion with Duodenal Switch in the Long Term. Obes Surg. 2016;26:2469-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | DuPont HL. Persistent Diarrhea: A Clinical Review. JAMA. 2016;315:2712-2723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Schiller LR, Pardi DS, Sellin JH. Chronic Diarrhea: Diagnosis and Management. Clin Gastroenterol Hepatol. 2017;15:182-193.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 12. | Thomas PD, Forbes A, Green J, Howdle P, Long R, Playford R, Sheridan M, Stevens R, Valori R, Walters J. Guidelines for the investigation of chronic diarrhoea, 2nd edition. Gut. 2003;52 Suppl 5:v1-15. [PubMed] |
| 13. | Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Afshar S, Kelly SB, Seymour K, Woodcock S, Werner AD, Mathers JC. The Effects of Bariatric Procedures on Bowel Habit. Obes Surg. 2016;26:2348-2354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Peterli R, Borbély Y, Kern B, Gass M, Peters T, Thurnheer M, Schultes B, Laederach K, Bueter M, Schiesser M. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258:690-694; discussion 695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 16. | Mathes CM, Spector AC. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol Behav. 2012;107:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Odstrcil EA, Martinez JG, Santa Ana CA, Xue B, Schneider RE, Steffer KJ, Porter JL, Asplin J, Kuhn JA, Fordtran JS. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Hammer HF, Hammer J. Diarrhea caused by carbohydrate malabsorption. Gastroenterol Clin North Am. 2012;41:611-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Ho W, Spiegel BM. The relationship between obesity and functional gastrointestinal disorders: causation, association, or neither? Gastroenterol Hepatol (N Y). 2008;4:572-578. [PubMed] |
| 20. | Petereit R, Jonaitis L, Kupčinskas L, Maleckas A. Gastrointestinal symptoms and eating behavior among morbidly obese patients undergoing Roux-en-Y gastric bypass. Medicina (Kaunas). 2014;50:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Aro P, Ronkainen J, Talley NJ, Storskrubb T, Bolling-Sternevald E, Agréus L. Body mass index and chronic unexplained gastrointestinal symptoms: an adult endoscopic population based study. Gut. 2005;54:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Bouchoucha M, Devroede G, Benamouzig R. Are floating stools associated with specific functional bowel disorders? Eur J Gastroenterol Hepatol. 2015;27:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Sabate JM, Coupaye M, Ledoux S, Castel B, Msika S, Coffin B, Jouet P. Consequences of Small Intestinal Bacterial Overgrowth in Obese Patients Before and After Bariatric Surgery. Obes Surg. 2017;27:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Ocón Bretón J, Pérez Naranjo S, Gimeno Laborda S, Benito Ruesca P, García Hernández R. [Effectiveness and complications of bariatric surgery in the treatment of morbid obesity]. Nutr Hosp. 2005;20:409-414. [PubMed] |
| 25. | Sileri P, Franceschilli L, Cadeddu F, De Luca E, D’Ugo S, Tognoni V, Camperchioli I, Benavoli D, Di Lorenzo N, Gaspari AL. Prevalence of defaecatory disorders in morbidly obese patients before and after bariatric surgery. J Gastrointest Surg. 2012;16:62-66; discussion 66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Fysekidis M, Bouchoucha M, Bihan H, Reach G, Benamouzig R, Catheline JM. Prevalence and co-occurrence of upper and lower functional gastrointestinal symptoms in patients eligible for bariatric surgery. Obes Surg. 2012;22:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Wasserberg N, Hamoui N, Petrone P, Crookes PF, Kaufman HS. Bowel habits after gastric bypass versus the duodenal switch operation. Obes Surg. 2008;18:1563-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | McBride CL, Petersen A, Sudan D, Thompson J. Short bowel syndrome following bariatric surgical procedures. Am J Surg. 2006;192:828-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Gondolesi G, Ramisch D, Padin J, Almau H, Sandi M, Schelotto PB, Fernandez A, Rumbo C, Solar H. What is the normal small bowel length in humans? first donor-based cohort analysis. Am J Transplant. 2012;12 Suppl 4:S49-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Mahawar KK, Kumar P, Parmar C, Graham Y, Carr WR, Jennings N, Schroeder N, Balupuri S, Small PK. Small Bowel Limb Lengths and Roux-en-Y Gastric Bypass: a Systematic Review. Obes Surg. 2016;26:660-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Teitelbaum EN, Vaziri K, Zettervall S, Amdur RL, Orkin BA. Intraoperative small bowel length measurements and analysis of demographic predictors of increased length. Clin Anat. 2013;26:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Muise ED, Tackett JJ, Callender KA, Gandotra N, Bamdad MC, Cowles RA. Accurate assessment of bowel length: the method of measurement matters. J Surg Res. 2016;206:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Tacchino RM. Bowel length: measurement, predictors, and impact on bariatric and metabolic surgery. Surg Obes Relat Dis. 2015;11:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Borbély Y, Plebani A, Kröll D, Ghisla S, Nett PC. Exocrine Pancreatic Insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Madan AK, Harper JL, Tichansky DS. Techniques of laparoscopic gastric bypass: on-line survey of American Society for Bariatric Surgery practicing surgeons. Surg Obes Relat Dis. 2008;4:166-172; discussion 172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Nikaki K, Gupte GL. Assessment of intestinal malabsorption. Best Pract Res Clin Gastroenterol. 2016;30:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Chousleb E, Patel S, Szomstein S, Rosenthal R. Reasons and operative outcomes after reversal of gastric bypass and jejunoileal bypass. Obes Surg. 2012;22:1611-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Mundi MS, Vallumsetla N, Davidson JB, McMahon MT, Bonnes SL, Hurt RT. Use of Home Parenteral Nutrition in Post-Bariatric Surgery-Related Malnutrition. JPEN J Parenter Enteral Nutr. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | van Dam RM, Seidell JC. Carbohydrate intake and obesity. Eur J Clin Nutr. 2007;61 Suppl 1:S75-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Bes-Rastrollo M, Martínez-González MA, Sánchez-Villegas A, de la Fuente Arrillaga C, Martínez JA. Association of fiber intake and fruit/vegetable consumption with weight gain in a Mediterranean population. Nutrition. 2006;22:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274-288. [PubMed] |
| 42. | Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4:S73-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 43. | Ladas S, Papanikos J, Arapakis G. Lactose malabsorption in Greek adults: correlation of small bowel transit time with the severity of lactose intolerance. Gut. 1982;23:968-973. [PubMed] |
| 44. | Welsh JD, Poley JR, Bhatia M, Stevenson DE. Intestinal disaccharidase activities in relation to age, race, and mucosal damage. Gastroenterology. 1978;75:847-855. [PubMed] |
| 45. | Gudmand-Höyer E, Asp NG, Skovbjerg H, Andersen B. Lactose malabsorption after bypass operation for obesity. Scand J Gastroenterol. 1978;13:641-647. [PubMed] |
| 46. | Jones HF, Butler RN, Brooks DA. Intestinal fructose transport and malabsorption in humans. Am J Physiol Gastrointest Liver Physiol. 2011;300:G202-G206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 47. | Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8:544-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 48. | Clements RH, Katasani VG, Palepu R, Leeth RR, Leath TD, Roy BP, Vickers SM. Incidence of vitamin deficiency after laparoscopic Roux-en-Y gastric bypass in a university hospital setting. Am Surg. 2006;72:1196-1202; discussion 1203-1204. [PubMed] |
| 49. | Currò G, Centorrino T, Cogliandolo A, Dattola A, Pagano G, Barbera A, Navarra G. A clinical and nutritional comparison of biliopancreatic diversion performed with different common and alimentary channel lengths. Obes Surg. 2015;25:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Hwang TL, Lue MC, Nee YJ, Jan YY, Chen MF. The incidence of diarrhea in patients with hypoalbuminemia due to acute or chronic malnutrition during enteral feeding. Am J Gastroenterol. 1994;89:376-378. [PubMed] |
| 52. | Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 868] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 53. | World Health Organization. Updates on the management of severe acute malnutrition in infants and children. 2013;. |
| 54. | Tentolouris N, Andrianakos A, Karanikolas G, Karamitsos D, Trontzas P, Krachtis P, Christoyannis F, Tavaniotou E, Nikolia Z, Kaskani E. Type 2 diabetes mellitus is associated with obesity, smoking and low socioeconomic status in large and representative samples of rural, urban, and suburban adult Greek populations. Hormones (Athens). 2012;11:458-467. [PubMed] |
| 55. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1179] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 56. | Seki Y, Kasama K, Haruta H, Watanabe A, Yokoyama R, Porciuncula JP, Umezawa A, Kurokawa Y. Five-Year-Results of Laparoscopic Sleeve Gastrectomy with Duodenojejunal Bypass for Weight Loss and Type 2 Diabetes Mellitus. Obes Surg. 2017;27:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Mones RL. Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. J Pediatr Gastroenterol Nutr. 2009;48:645-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | de Aquino LA, Pereira SE, de Souza Silva J, Sobrinho CJ, Ramalho A. Bariatric surgery: impact on body composition after Roux-en-Y gastric bypass. Obes Surg. 2012;22:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Wenzel KW, Kirschsieper HE. Aspects of the absorption of oral L-thyroxine in normal man. Metabolism. 1977;26:1-8. [PubMed] |
| 60. | Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R. Evolution of mammals and their gut microbes. Science. 2008;320:1647-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2974] [Cited by in RCA: 2546] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 61. | Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2727] [Cited by in RCA: 3196] [Article Influence: 266.3] [Reference Citation Analysis (2)] |
| 62. | Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4554-4561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1850] [Cited by in RCA: 1641] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 63. | Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1907] [Cited by in RCA: 1736] [Article Influence: 102.1] [Reference Citation Analysis (1)] |
| 64. | Davis CD. The Gut Microbiome and Its Role in Obesity. Nutr Today. 2016;51:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 255] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 65. | Shao Y, Ding R, Xu B, Hua R, Shen Q, He K, Yao Q. Alterations of Gut Microbiota After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in Sprague-Dawley Rats. Obes Surg. 2017;27:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 66. | Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1399] [Article Influence: 87.4] [Reference Citation Analysis (1)] |
| 67. | Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049-3057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 898] [Cited by in RCA: 890] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 68. | Damms-Machado A, Mitra S, Schollenberger AE, Kramer KM, Meile T, Königsrainer A, Huson DH, Bischoff SC. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int. 2015;2015:806248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 69. | Chakravartty S, Tassinari D, Salerno A, Giorgakis E, Rubino F. What is the Mechanism Behind Weight Loss Maintenance with Gastric Bypass? Curr Obes Rep. 2015;4:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Swidsinski A, Loening-Baucke V, Verstraelen H, Osowska S, Doerffel Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology. 2008;135:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 71. | Schubert AM, Rogers MA, Ring C, Mogle J, Petrosino JP, Young VB, Aronoff DM, Schloss PD. Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. MBio. 2014;5:e01021-e01014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 72. | Rezaie A, Pimentel M, Rao SS. How to Test and Treat Small Intestinal Bacterial Overgrowth: an Evidence-Based Approach. Curr Gastroenterol Rep. 2016;18:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 73. | Sabaté JM, Jouët P, Harnois F, Mechler C, Msika S, Grossin M, Coffin B. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 74. | Madrid AM, Poniachik J, Quera R, Defilippi C. Small intestinal clustered contractions and bacterial overgrowth: a frequent finding in obese patients. Dig Dis Sci. 2011;56:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 75. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 383] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (4)] |
| 76. | Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (NY). 2007;3:112-122. [PubMed] |
| 77. | Ishida RK, Faintuch J, Paula AM, Risttori CA, Silva SN, Gomes ES, Mattar R, Kuga R, Ribeiro AS, Sakai P. Microbial flora of the stomach after gastric bypass for morbid obesity. Obes Surg. 2007;17:752-758. [PubMed] |
| 78. | Ishida RK, Faintuch J, Ribeiro AS, Ribeiro U, Cecconello I. Asymptomatic gastric bacterial overgrowth after bariatric surgery: are long-term metabolic consequences possible? Obes Surg. 2014;24:1856-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Ierardi E, Losurdo G, Sorrentino C, Giorgio F, Rossi G, Marinaro A, Romagno KR, Di Leo A, Principi M. Macronutrient intakes in obese subjects with or without small intestinal bacterial overgrowth: an alimentary survey. Scand J Gastroenterol. 2016;51:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (3)] |
| 80. | Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 1783] [Article Influence: 118.9] [Reference Citation Analysis (0)] |
| 81. | Jeppesen PB, Mortensen PB. Colonic digestion and absorption of energy from carbohydrates and medium-chain fat in small bowel failure. JPEN J Parenter Enteral Nutr. 1999;23:S101-S105. [PubMed] |
| 82. | Andalib I, Shah H, Bal BS, Shope TR, Finelli FC, Koch TR. Breath Hydrogen as a Biomarker for Glucose Malabsorption after Roux-en-Y Gastric Bypass Surgery. Dis Markers. 2015;2015:102760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Choung RS, Ruff KC, Malhotra A, Herrick L, Locke GR, Harmsen WS, Zinsmeister AR, Talley NJ, Saito YA. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther. 2011;33:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 84. | Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 85. | Rybolt AH, Bennett RG, Laughon BE, Thomas DR, Greenough WB, Bartlett JG. Protein-losing enteropathy associated with Clostridium difficile infection. Lancet. 1989;1:1353-1355. [PubMed] |
| 86. | Koo HL, Koo DC, Musher DM, DuPont HL. Antimotility agents for the treatment of Clostridium difficile diarrhea and colitis. Clin Infect Dis. 2009;48:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27:1169-1172. [PubMed] |
| 88. | Al-Jashaami LS, DuPont HL. Management of Clostridium difficile Infection. Gastroenterol Hepatol (NY). 2016;12:609-616. [PubMed] |
| 89. | Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73:147-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 342] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 90. | Mitchell JE, Steffen K, Engel S, King WC, Chen JY, Winters K, Sogg S, Sondag C, Kalarchian M, Elder K. Addictive disorders after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2015;11:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Israel A, Sebbag G, Fraser D, Levy I. Nutritional behavior as a predictor of early success after vertical gastroplasty. Obes Surg. 2005;15:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 92. | Smith EW, Smith KA, Maibach HI, Andersson PO, Cleary G, Wilson D. The local side effects of transdermally absorbed nicotine. Skin Pharmacol. 1992;5:69-76. [PubMed] |
| 93. | King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, Courcoulas AP, Pories WJ, Yanovski SZ. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307:2516-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 306] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 94. | Pepino MY, Okunade AL, Eagon JC, Bartholow BD, Bucholz K, Klein S. Effect of Roux-en-Y Gastric Bypass Surgery: Converting 2 Alcoholic Drinks to 4. JAMA Surg. 2015;150:1096-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | Suzuki J, Haimovici F, Chang G. Alcohol use disorders after bariatric surgery. Obes Surg. 2012;22:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 96. | Li L, Wu LT. Substance use after bariatric surgery: A review. J Psychiatr Res. 2016;76:16-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 97. | Ikramuddin S, Blackstone RP, Brancatisano A, Toouli J, Shah SN, Wolfe BM, Fujioka K, Maher JW, Swain J, Que FG. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 98. | van Wezenbeek MR, van Oudheusden TR, Smulders JF, Nienhuijs SW, Luyer MD. Transection versus preservation of the neurovascular bundle of the lesser omentum in primary Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016;12:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Oelschlager BK, Yamamoto K, Woltman T, Pellegrini C. Vagotomy during hiatal hernia repair: a benign esophageal lengthening procedure. J Gastrointest Surg. 2008;12:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Sapala JA, Wood MH, Schuhknecht MP. Vagotomy at the time of gastric bypass: can it be harmful? Obes Surg. 2004;14:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Johnston D, Humphrey CS, Walker BE, Pulvertaft CN, Goligher JC. Vagotomy without diarrhoea. Br Med J. 1972;3:788-790. [PubMed] |
| 102. | al-Hadrani A, Lavelle-Jones M, Kennedy N, Neill G, Sutton D, Cuschieri A. Bile acid malabsorption in patients with post-vagotomy diarrhoea. Ann Chir Gynaecol. 1992;81:351-353. [PubMed] |
| 103. | Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 694] [Cited by in RCA: 747] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 104. | Kohli R, Seeley RJ. Diabetes: The search for mechanisms underlying bariatric surgery. Nat Rev Endocrinol. 2013;9:572-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 105. | Swell L, Gustafsson J, Schwartz CC, Halloran LG, Danielsson H, Vlahcevic ZR. An in vivo evaluation of the quantitative significance of several potential pathways to cholic and chenodeoxycholic acids from cholesterol in man. J Lipid Res. 1980;21:455-466. [PubMed] |
| 106. | Barkun AN, Love J, Gould M, Pluta H, Steinhart H. Bile acid malabsorption in chronic diarrhea: pathophysiology and treatment. Can J Gastroenterol. 2013;27:653-659. [PubMed] |
| 107. | Thomas LA, Veysey MJ, Bathgate T, King A, French G, Smeeton NC, Murphy GM, Dowling RH. Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology. 2000;119:806-815. [PubMed] |
| 108. | Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1242] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 109. | Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 110. | Worni M, Guller U, Shah A, Gandhi M, Shah J, Rajgor D, Pietrobon R, Jacobs DO, Ostbye T. Cholecystectomy concomitant with laparoscopic gastric bypass: a trend analysis of the nationwide inpatient sample from 2001 to 2008. Obes Surg. 2012;22:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 111. | Camilleri M, Nadeau A, Tremaine WJ, Lamsam J, Burton D, Odunsi S, Sweetser S, Singh R. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734-e43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 112. | Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 113. | Friess H, Böhm J, Müller MW, Glasbrenner B, Riepl RL, Malfertheiner P, Büchler MW. Maldigestion after total gastrectomy is associated with pancreatic insufficiency. Am J Gastroenterol. 1996;91:341-347. [PubMed] |
| 114. | Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54 Suppl 6:vi1-v28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 115. | Vanderveen KA, Grant CS, Thompson GB, Farley DR, Richards ML, Vella A, Vollrath B, Service FJ. Outcomes and quality of life after partial pancreatectomy for noninsulinoma pancreatogenous hypoglycemia from diffuse islet cell disease. Surgery. 2010;148:1237-1245; discussion 1245-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 116. | Laurenius A, Engström M. Early dumping syndrome is not a complication but a desirable feature of Roux-en-Y gastric bypass surgery. Clin Obes. 2016;6:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 117. | van Beek AP, Emous M, Laville M, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. 2017;18:68-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 118. | Borbély Y, Winkler C, Kröll D, Nett P. Pouch Reshaping for Significant Weight Regain after Roux-en-Y Gastric Bypass. Obes Surg. 2017;27:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Z’graggen K, Guweidhi A, Steffen R, Potoczna N, Biral R, Walther F, Komminoth P, Horber F. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg. 2008;18:981-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 120. | Desreumaux P, Ernst O, Geboes K, Gambiez L, Berrebi D, Müller-Alouf H, Hafraoui S, Emilie D, Ectors N, Peuchmaur M. Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology. 1999;117:73-81. [PubMed] |
| 121. | Ahn DH, Crawley SC, Hokari R, Kato S, Yang SC, Li JD, Kim YS. TNF-alpha activates MUC2 transcription via NF-kappaB but inhibits via JNK activation. Cell Physiol Biochem. 2005;15:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Keidar A, Hazan D, Sadot E, Kashtan H, Wasserberg N. The role of bariatric surgery in morbidly obese patients with inflammatory bowel disease. Surg Obes Relat Dis. 2015;11:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 123. | Janczewska I, Nekzada Q, Kapraali M. Crohn’s disease after gastric bypass surgery. BMJ Case Rep. 2011;2011:pii: bcr0720103168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | Shoar S, Shahabuddin Hoseini S, Naderan M, Mahmoodzadeh H, Ying Man F, Shoar N, Hosseini M, Bagheri-Hariri S. Bariatric surgery in morbidly obese patients with inflammatory bowel disease: A systematic review. Surg Obes Relat Dis. 2017;13:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 125. | Gianos M, Abdemur A, Szomstein S, Rosenthal R. Laparoscopic sleeve gastrectomy as a step approach for morbidly obese patients with early stage malignancies requiring rapid weight loss for a final curative procedure. Obes Surg. 2013;23:1370-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Kotze PG, Bremer-Nones R, Kotze LM. Is there any relation between gastric bypass for morbid obesity and the development of Crohn’s disease? J Crohns Colitis. 2014;8:712-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 127. | Ungar B, Kopylov U, Goitein D, Lahat A, Bardan E, Avidan B, Lang A, Maor Y, Eliakim R, Ben-Horin S. Severe and morbid obesity in Crohn’s disease patients: prevalence and disease associations. Digestion. 2013;88:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Carswell KA, Vincent RP, Belgaumkar AP, Sherwood RA, Amiel SA, Patel AG, le Roux CW. The effect of bariatric surgery on intestinal absorption and transit time. Obes Surg. 2014;24:796-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 129. | Ukkola A, Mäki M, Kurppa K, Collin P, Huhtala H, Kekkonen L, Kaukinen K. Changes in body mass index on a gluten-free diet in coeliac disease: a nationwide study. Eur J Intern Med. 2012;23:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 130. | Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten-free diet. Am J Gastroenterol. 2006;101:2356-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 131. | Rampertab SD, Pooran N, Brar P, Singh P, Green PH. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 132. | Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 593] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 133. | Pané A, Orois A, Careaga M, Saco A, Ortega E, Vidal J, Leyes P, Amor AJ. Clinical onset of celiac disease after duodenal switch: a case report. Eur J Clin Nutr. 2016;70:1078-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 134. | Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1156] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 135. | de’Angelis N, Carra MC, Vincenzi F. Gluten-free diet in obese patients with celiac disease: an enemy of the bariatric surgeon? Obes Surg. 2012;22:995-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 136. | Schneck AS, Anty R, Tran A, Hastier A, Amor IB, Gugenheim J, Iannelli A, Piche T. Increased Prevalence of Irritable Bowel Syndrome in a Cohort of French Morbidly Obese Patients Candidate for Bariatric Surgery. Obes Surg. 2016;26:1525-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 137. | Clements RH, Gonzalez QH, Foster A, Richards WO, McDowell J, Bondora A, Laws HL. Gastrointestinal symptoms are more intense in morbidly obese patients and are improved with laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 138. | Carter D, Beer-Gabel M, Tzur D, Levy G, Derazne E, Novis B, Afek A. Predictive factors for the diagnosis of irritable bowel syndrome in a large cohort of 440,822 young adults. J Clin Gastroenterol. 2015;49:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 139. | Lee CG, Lee JK, Kang YS, Shin S, Kim JH, Lim YJ, Koh MS, Lee JH, Kang HW. Visceral abdominal obesity is associated with an increased risk of irritable bowel syndrome. Am J Gastroenterol. 2015;110:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 140. | Pickett-Blakely O. Obesity and irritable bowel syndrome: a comprehensive review. Gastroenterol Hepatol (NY). 2014;10:411-416. [PubMed] |
| 141. | Revelo XS, Luck H, Winer S, Winer DA. Morphological and inflammatory changes in visceral adipose tissue during obesity. Endocr Pathol. 2014;25:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 142. | Clément K, Vignes S. [Inflammation, adipokines and obesity]. Rev Med Interne. 2009;30:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Akiho H, Ihara E, Nakamura K. Low-grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol. 2010;1:97-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 144. | Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 621] [Article Influence: 62.1] [Reference Citation Analysis (0)] |