Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4654
Peer-review started: December 30, 2016
First decision: March 16, 2017
Revised: March 31, 2017
Accepted: June 19, 2017
Article in press: June 19, 2017
Published online: July 7, 2017
Processing time: 190 Days and 4.6 Hours
To systematically review literature upon aetiology of nosocomial spontaneous bacterial peritonitis (N-SBP) given the rising importance of multidrug-resistant (MDR) bacteria.
A literature search was performed on MEDLINE and Google Scholar databases from 2000 to 15th of November 2016, using the following search strategy: “spontaneous” AND “peritonitis”.
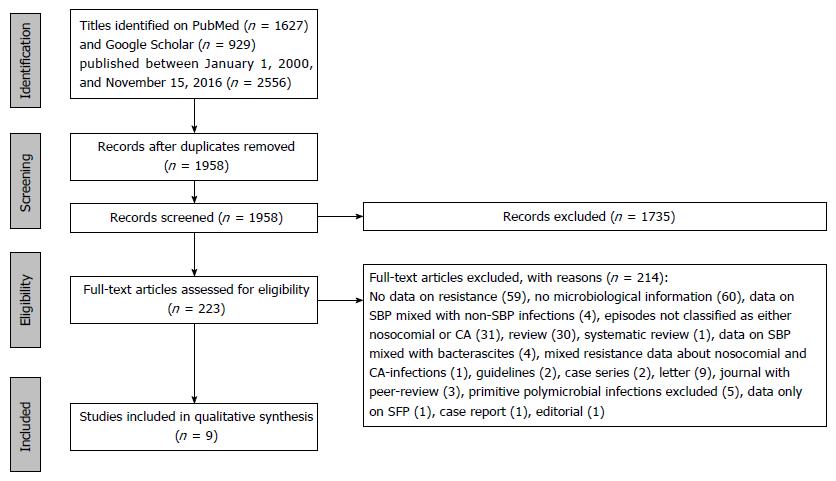
The initial search through electronic databases retrieved 2556 records. After removing duplicates, 1958 records remained. One thousand seven hundred and thirty-five of them were excluded on the basis of the screening of titles and abstract, and the ensuing number of remaining articles was 223. Of these records, after careful evaluation, only 9 were included in the qualitative analysis. The overall proportion of MDR bacteria turned out to be from 22% to 73% of cases across the studies.
N-SBP is caused, in a remarkable proportion, by MDR pathogens. This should prompt a careful re-assessment of guidelines addressing the treatment of this clinical entity.
Core tip: Nosocomial spontaneous bacterial peritonitis (N-SBP) develops in up to one-third of cirrhotic patients. The overall 30-d survival for N-SBP is only 20%, also due to an inadequate empirical antibiotic therapy (EAT). The aim of our Sistematic Review is to describe N-SBP bacterial aetiology and the prevalence of multiple drug resistance (MDR) pathogens to suggest which EAT may be adequate in these entities. After careful evaluation 9 studies were identified. The overall proportion of MDR bacteria was up to 22%-73% of cases. EAT with a carbapenem plus daptomycin and a Beta-lactam active against methicillin-resistant cocci should be considered in centers with a high prevalence of MDR bacteria.
- Citation: Fiore M, Maraolo AE, Gentile I, Borgia G, Leone S, Sansone P, Passavanti MB, Aurilio C, Pace MC. Nosocomial spontaneous bacterial peritonitis antibiotic treatment in the era of multi-drug resistance pathogens: A systematic review. World J Gastroenterol 2017; 23(25): 4654-4660
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4654.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4654
Bacterial infections are a well-known cause of morbidity and mortality in cirrhotic patients, being a leading aetiology of progression in liver failure[1]. Subjects suffering from liver cirrhosis can be considered as immunocompromised[2] and, as such, are more prone to infections, whose incidence and severity is greater than in non-cirrhotic individuals. Spontaneous bacterial peritonitis (SBP) and urinary tract infections are the most frequent infections in this setting[3].
SBP is defined as a bacterial infection, usually monomicrobial, of ascitic fluid, without an evident source of sepsis in the peritoneum or adjacent tissues, occurring in subjects with decompensated liver diseases[4]. Diagnosis relies on ascitic fluid cell analysis (polymorphonuclear cell count ≥ 250/mm3) and ascitic fluid culture, but positivity of microbiological findings is inconstant, generally lower than 50%; thereby, many cases are classified as culture-negative[5].
The fundamental pathogenic mechanism underlying SPB is the bacterial translocation from the gut. It is well-known how in cirrhotic patients the composition of gut microbiota is altered both from a quantitative and qualitative perspective[6] Moreover, in this context gut’s barrier function and immune response to translocating microbes are hindered[6]. In light of these considerations, it does not come as surprise the historical prevalence of Gram-negative enteric bacteria among the etiological agents of SBP, influencing the empirical therapeutic choice[7]. However, over the last years physicians have been facing an important change in the epidemiology features of SBP, in particular, and of bacterial infections, in broader sense in cirrhotic patients. Indeed, since early 2000s, factors such as the prophylactic oral antibiotic therapy with quinolones used to prevent SBP have led up to the remarkable increase of Gram-positive bacteria as causative agents, as well as the occurrence of infections by multi-drug resistant (MDR) organisms[8,9].
The most frequently isolated MDR bacteria are extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, non-fermentable Gram-negative bacilli (such as Pseudomonas aeruginosa), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant enterococci (VRE)[10]. This trend has been applying to all the major types of bacterial infection in cirrhotic patients, including SBP[4].
Recently, SBP caused by MDR bacteria has been exclusively associated with nosocomial infections[11]. Resistant pathogens are one the main reason why SBP is still today a potentially life-threating infection in hospitalized patients, being the mortality rate up to 20%[12]. This rate is dramatic in view of the fact that more than a third of decompensated ESLD patients develop nosocomial SBP (N-SBP) during hospitalization[13]. Nosocomial SBP seems to have a higher risk to be provoked by MDR bacteria. The selection of the appropriate empiric regimen, pending the culture results, is a crucial decision because any delay implies an increase of the mortality rates[14].
The increasing failure of traditional treatment options for nosocomial SBP, namely third-generation cephalosporins as recommended by previous guidelines[5], has prompted a re-evaluation of suggested empirical therapeutic regimens, which should be based on broader-spectrum agents, such as piperacillin/tazobactam, meropenem ± glycopeptide[14] or meropenem plus daptomycin[12] . For instance, the latter proved to be more effective than ceftazidime alone in a recent randomized controlled trial involving patients with nosocomial SBP[12]. According to the most recent recommendations, third-generation cephalosporins should be restricted only to selected patients, particularly in case of community-acquired infections[3].
A “grey area” is represented by the so-called healthcare-associated infections, whose difference with nosocomial infections appears to be extremely tenuous in terms of epidemiology and outcome[15].
The aim of this systematic review is to provide a comprehensive overview of the microbiological features, both from an etiological and resistance standpoint, of N-SBP in order to have an insight into the MDR forms and inform physicians’ empirical choice depending on the context wherein infection occurs.
This systematic review was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[16].
Spontaneous peritonitis (SP) is an infection of ascitic fluid of cirrhotic patients without a definitive alternative intra-abdominal source of infection. If a microbiological culture is performed on ascitic fluid, the growth of bacteria makes diagnosis of SBP, instead the growth of fungi makes diagnosis of Spontaneous fungal peritonitis (SFP)[17]. Cases were defined nosocomial if the diagnosis was made after 48[11] or 72[12] h of hospitalization. Multidrug-resistance was defined as to at least one agent in three or more anti-infective agents categories[18].
The MEDLINE and Google Scholar databases[19] were screened from 2000 to 15th of November 2016, using the following search strategy “spontaneous” AND “peritonitis”. References were managed using both Endnote X5 and Zotero. Handsearching of additional records was performed screening the reference list of the retrieved full-text articles.
Two independent reviewers (Fiore M and Maraolo AE) screened titles and abstracts to establish eligibility for full-text review. No geographical restriction was placed. Studies were included if (1) published in full and written in English; (2) published in peer-reviewed journal; (3) clearly defined the onset of SBP (nosocomial alone or as opposed to non-nosocomial setting) in cirrhotic patients; and (4) specified the aetiology and the resistance profile of SBP causative agents, among the culture-positive cases. Studies were excluded if (1) primitive polymicrobial infections were not taken into account; (2) aetiology and resistance profile of SBP causative agents could not be extricated from the presented data (e.g., aetiology and bacterial susceptibility referring to both SBP and bacterascites, or to both infections of ascitic fluid and bacterial infections other than SBP); (3) they were case series or case reports; (4) they were not empirical studies (e.g., reviews, editorials, commentaries, book chapters); and (5) they were only published as abstracts, congress posters, letters to the editors.
Full text articles resulting from the end of the data selection process were reviewed by Fiore M and Maraolo AE to identify (1) study characteristics as authors, journal, clinical setting and country, observation time span and design; (2) temporal definition of nosocomial cases; (3) ratio between nosocomial and non-nosocomial episodes; (4) number of culture-positive cases among the total episodes; (5) ratio between bacterial and fungal cases of spontaneous peritonitis; (6) ratio between cases due to Gram-positive bacteria and to Gram-negative bacteria; (7) proportion of MDR strains in the overall sample; (8) proportion of MDR strains among Gram-positive bacteria; and (9) proportion of MDR strains among Gram-negative bacteria. Results were crosschecked and any disagreement was resolved through a third reviewer’s opinion (Leone S).
Figure 1 depicts the whole selection process involving the studies selected for this systematic review. The initial search through electronic databases retrieved 2556 records. After removing duplicates, 1958 records remained. One thousand seven hundred and thirty-five of them were excluded on the basis of the screening of titles and abstract, and the ensuing number of remaining articles was 223. Of these records, after careful evaluation, only 9 were included in the qualitative analysis, meeting both the aforementioned inclusion and exclusion criteria.
Tables 1 and 2 describe the main features of included studies[12,20-27]. Eight of the 9 studies were conducted in University Hospitals. Eight observational studies (5 retrospective and 3 prospective) and one randomized controlled trial (RCT); the majority of the studies was conducted on a European population (2 Germany, 2 Italy, 1 Spain), followed by the Asia (2 South Korea, 1 China) and North America (1 Canada). A total of 1.201 positive culture of ascitic fluid of cirrhotic patients were included. Five hundred and twenty positive culture were nosocomial SP, of these 19 were fungal peritonitis and 501 were bacterial peritonitis, respectively. Identification of pathogens in ascitic fluids from patients with N-SBP is approximately 50%[12,27] as that reported historically in peritonitis not nosocomial[5]. The review shows that recently the bacterial spectrum seems to have changed with high prevalence of Gram-positive bacteria (29.3%-62.5%). The percentage of MDR varies from 36.8 to 50% for the Gram-positive and from 30% to 66.6% for the Gram-negative bacteria.
| Ref. | Country, clinical setting | Study years | Study design | N-SP definition (hours after admission) | N-SP/non N-SP |
| Song et al[20] | South Korea, University Hospital | 1998-2003 | RC | > 72 | 32/74 |
| Cheong et al[21] | South Korea, University Hospital | 2000-2007 | RC | > 72 | 126/110 |
| Fernández et al[22] (first cohort) | Spain, University Hospital | 2005-2007 | PC | > 48 | 32/94 |
| Fernández et al[22] (second cohort) | Spain, University Hospital | 2010-2011 | PC | > 48 | 7/26 |
| Chaulk et al[23] | Canada, University Hospital | 2003-2011 | RC | > 48 | 17/60 |
| Li YT et al[24] | China, University Hospital | 2011-2013 | RC | > 48 | 99/207 |
| Friedrich et al[25] | Germany, University Hospital | 2007-2013 | RC | > 48 | 89/49 |
| Piano et al[12] | Italy, University Hospital plus Private Care Center | 2011-2014 | RCT | > 72 | Only NSP, 31 cases |
| Salerno et al[26] | Italy, multicenter | 2007-2009 | PC | > 48 | 24/32 |
| Lutz et al[27] | Germany, University Hospital | 2012-2016 | PC | > 48 | 63/29 |
| Ref. | N-SP culture-positive/N-SP total | N-SBP/N-SFP | N-SBP by GPB/culture-positive N-SPB | N-SPB by MDR bacteria/culture-positive N-SPB | N-SBP by MDR-GPB/N-SBP by GPB | N-SBP by MDR-GNB/N-SBP by GNB |
| Song et al[20] | Only culture positive cases | 32/0 | NA | NA | NA | 12/18 (66.6)1 |
| Cheong et al[21] | Only culture positive cases | 123/3 | 37/119 (31.0) | NA | NA | 23/82 (28.0) |
| Fernández et al[22] (first cohort) | NA | 32/0 | NA | 7/32 (21.9) | NA | NA2 |
| Fernández et al[22] (second cohort) | NA | 7/0 | NA | 2/7 (28.5) | NA | NA3 |
| Chaulk et al[23] | NA | 17/0 | NA | 7/17 (41.1) | NA | NA |
| Li et al[24] | Only culture positive cases | 92/7 | 27/92 (29.3) | 29/62 (46.8)4 | 7/19 (36.8)5 | 22/43 (51.2)6 |
| Friedrich et al[25] | NA | 81/8 | 37/118 (31.3) | 59/81 (72.8) | NA | NA |
| Piano et al[12] | 16/31 (51.6) | 16/0 | 10/16 (62.5) | 6/16 (37.5) | 3/6 (50.0) | 3/10 (30.0) |
| Salerno et al[26] | NA | 24/0 | NA | 6/24 (25.0) | NA | NA |
| Lutz et al[27] | 30/63 (47.6) | 29/1 | 14/29 (48.2) | 9/30 (30.0) | NA | NA |
The included studies clearly shows the emerging patterns of third-generation cephalosporins resistance in causative bacteria of N-SBP. In the study of Piano et al[12] only 60% of Gram-negative bacteria are susceptible to third-generation cephalosporins; enterococci (24%) and staphylococci (19%) are the most commonly Gram-positive bacteria isolated. In the study of Friedrich et al[25] the overall percentage of enterococci (26.1%) is similar and they are intrinsically cephalosporin-resistant. The rate of MRSA, among Staphylococcus aureus strains, is as high as 85.7% in the study of Li et al[24]. Resistance of Enterococcus spp. to ampicillin varies from 37.5%[12] to 75%, with a 8.3% of VRE[25]. The rate of ESBL among Enterobacteriaceae ranged from 28%[21] to 51.2%[24]. The proportion of ESBL among specific strains such as E. coli can be as high as 66.7%[20].
Bacterial infections represent a relevant complication of cirrhosis, especially in hospitalized patients[28]. Moreover, infections are the key driver of the so-called acute-on-chronic liver failure[29], defined by a recent consensus as a syndrome in patients with advanced liver disease characterized by acute decompensation provoking liver failure and at least one extrahepatic organ failure, resulting in an increased short-term mortality[30] A prompt recognition, allocating the patient in the proper care setting[31], and an adequate treatment of the precipitating factor are essential to improve patients’ outcomes[32]. SBP-associated septic shock has a poor prognosis (mortality 80%). Appropriate antimicrobial therapy should be given as soon as possible: non-administration corresponds to an increase of 1.86 times hospital mortality per hour[33].
Over the last years, a new threat has emerged with respect to bacterial infections in cirrhosis in the form of the changing epidemiological pattern (increase of Gram-positive bacteria) and the rise of antimicrobial resistance[34]. Possible reasons for this phenomenon were the large preventive use of quinolones and the growing degree of instrumentalization of the cirrhotic patient, especially in the nosocomial context[35].
Unfortunately, even the most authoritative guidelines[5,36] relying on outdated microbiological data, do not provide a useful guidance. They still support the use of third-generation cephalosporins for SBP, taking into account the risk of infections by MDR bacteria or the difference between nosocomial and community-acquired forms[37]. On the contrary, a position statement in 2013 by the European Association for the Study of the Liver recommended different therapeutic options according to the onset of the infection, namely third-generation cephalosporins for community-acquired infection and piperacillin/tazobactam or meropenem plus daptomycin for nosocomial episodes[3]. Experts recommend cephalosporins[4] or piperacillin/tazobactam, meropenem ± glycopeptide[14].
Piperacillin/tazobactam is inadequate therapy for patients with life-theatering infections due to ESBL-producing Enterobacteriaceae[38,39].
Meropenem is carbapenem antibiotic which has excellent bactericidal activity in vitro against ESBL-producing Enterobacteriaceae. Breadth of spectrum is due, in part, to stability to all serine-based beta-lactamases, including those which hydrolyse third-generation cephalosporins. Meropenem is poorly active against staphylococci and enterococci[40].
Glycopeptides active against MDR Gram-positive cocci give concerns owing to the potential risk of nephrotoxicity. The rate of acute kidney injury is higher in patients with concomitant acute liver failure, which may be related to hemodynamic instability or development of hepatorenal syndrome[41]. Furthermore, when teicoplanin is administered intravenously at 10 mg/kg of body weight, low concentrations are achieved in peritoneal fluid[42].
Daptomycin is a lipopeptide active against MDR Gram-positive pathogens, including drug-resistant and drug-susceptible Staphylococcus aureus and VRE[43]. Decreased susceptibility to daptomycin (DAPR) reported in MRSA is frequently accompanied with a paradoxical decrease in beta-lactam resistance, a process known as the “see-saw” effect. Despite the observed discordance in resistance phenotypes, the combination of daptomycin/beta-lactams has been proven clinically effective for the prevention and treatment of infections due to DAPR-MRSA strains[44]. Therefore Daptomycin in monotherapy does not seem to be the treatment of choice for SBP due to MRSA[45].
In conclusion, third-generation cephalosporins have poor microbial coverage for treatment of N-SBP. Cirrhotic patients with N-SBP in clinical setting with high prevalence of VRE, MRSA, ESBL should receive as empirical antibiotic therapy: High dose of Daptomicyn (i.e., 8-12 mg/kg per 24 h) plus Meropenem (i.e., 1 g/8 h) plus a β-lactam active against MRSA[46].
When the culture is positive and susceptibility data are available an antibiotic with a narrower spectrum should be promptly initiated (early de-escalation strategy); this in the case of the individuation of non-MDR bacteria limits the selection of antibiotic resistances[47].
In areas with high prevalence of MDR bacteria, the late start of Broad-Spectrum EAT for N-SBP should be discouraged[48].
The authors deeply thank Dr. Muhammed Niyas (MBBS, MD, DNB), Senior Resident in Infectious Diseases at All India Institute of Medical Sciences of New Delhi (AIIMS, India), for his valuable pro bono help in revising the manuscript in order to improve and polish language.
Spontaneous bacterial peritonitis (SBP) is one the most frequent type of infection in cirrhotic patients. Bacterial infections remarkably worsen the prognosis of these subjects, in case of not adequate treatment. The therapeutic choice has become increasingly difficult in the light of the growing importance of multidrug-resistant (MDR) bacteria as causative agents of bacterial infections in patients with advanced liver disease.
This is the first systematic review aimed at summarizing the evidence from literature concerning the epidemiology of nosocomial cases of SBP, in order to highlight the importance of MDR bacteria.
This systematic review, focused on SBP, confirms the epidemiological change involving bacterial infections in cirrhotic patients as whole, namely the rise of Gram-positive isolates, and, more alarming, the notable increase of MDR bacteria among nosocomial cases.
The results of this systematic review suggest that the burden of MDR bacteria as causative agents of nosocomial SBP is worrisome. Further studies, especially randomized clinical trials, are warranted so as to understand the best treatment of these cases. In the meanwhile, the use of broad-spectrum agents as empiric therapy is a prudent and reasonable choice, especially in settings where resistance rate is high.
The authors did clearly represent the current problematics in the field of nosocomial spontaneous peritonitis and its possible solutions with the proposition of new guidelines. It provides very useful information for clinicians.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Knehtl M S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol. 2016;8:307-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (2)] |
| 2. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 847] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 3. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 644] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 4. | Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis--bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41:1116-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1131] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 6. | Lutz P, Nischalke HD, Strassburg CP, Spengler U. Spontaneous bacterial peritonitis: The clinical challenge of a leaky gut and a cirrhotic liver. World J Hepatol. 2015;7:304-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Fagiuoli S, Colli A, Bruno R, Burra P, Craxì A, Gaeta GB, Grossi P, Mondelli MU, Puoti M, Sagnelli E. Management of infections in cirrhotic patients: report of a consensus conference. Dig Liver Dis. 2014;46:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Bartoletti M, Giannella M, Lewis RE, Viale P. Bloodstream infections in patients with liver cirrhosis. Virulence. 2016;7:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Alexopoulou A, Papadopoulos N, Eliopoulos DG, Alexaki A, Tsiriga A, Toutouza M, Pectasides D. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int. 2013;33:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Fernandez J, Arroyo V. Bacterial Infections in Cirrhosis: A Growing Problem with Significant Implications. Clin Liver Dis. 2013;2:102-105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Alexopoulou A, Vasilieva L, Agiasotelli D, Siranidi K, Pouriki S, Tsiriga A, Toutouza M, Dourakis SP. Extensively drug-resistant bacteria are an independent predictive factor of mortality in 130 patients with spontaneous bacterial peritonitis or spontaneous bacteremia. World J Gastroenterol. 2016;22:4049-4056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, Cavallin M, Gola E, Sticca A, Loregian A. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: Results of a randomized, controlled clinical trial. Hepatology. 2016;63:1299-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 14. | Solà E, Solé C, Ginès P. Management of uninfected and infected ascites in cirrhosis. Liver Int. 2016;36 Suppl 1:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 16. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47158] [Article Influence: 2947.4] [Reference Citation Analysis (0)] |
| 17. | Lahmer T, Brandl A, Rasch S, Schmid RM, Huber W. Fungal Peritonitis: Underestimated Disease in Critically Ill Patients with Liver Cirrhosis and Spontaneous Peritonitis. PLoS One. 2016;11:e0158389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6072] [Cited by in RCA: 8760] [Article Influence: 625.7] [Reference Citation Analysis (0)] |
| 19. | Bramer WM, Giustini D, Kramer BM. Comparing the coverage, recall, and precision of searches for 120 systematic reviews in Embase, MEDLINE, and Google Scholar: a prospective study. Syst Rev. 2016;5:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Song JY, Jung SJ, Park CW, Sohn JW, Kim WJ, Kim MJ, Cheong HJ. Prognostic significance of infection acquisition sites in spontaneous bacterial peritonitis: nosocomial versus community acquired. J Korean Med Sci. 2006;21:666-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, Koh KC, Lee NY, Song JH, Peck KR. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 434] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 23. | Chaulk J, Carbonneau M, Qamar H, Keough A, Chang HJ, Ma M, Kumar D, Tandon P. Third-generation cephalosporin-resistant spontaneous bacterial peritonitis: a single-centre experience and summary of existing studies. Can J Gastroenterol Hepatol. 2014;28:83-88. [PubMed] |
| 24. | Li YT, Yu CB, Huang JR, Qin ZJ, Li LJ. Pathogen profile and drug resistance analysis of spontaneous peritonitis in cirrhotic patients. World J Gastroenterol. 2015;21:10409-10417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 25. | Friedrich K, Nüssle S, Rehlen T, Stremmel W, Mischnik A, Eisenbach C. Microbiology and resistance in first episodes of spontaneous bacterial peritonitis: implications for management and prognosis. J Gastroenterol Hepatol. 2016;31:1191-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Salerno F, Borzio M, Pedicino C, Simonetti R, Rossini A, Boccia S, Cacciola I, Burroughs AK, Manini MA, La Mura V. The impact of infection by multidrug-resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int. 2017;37:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 27. | Lutz P, Nischalke HD, Krämer B, Goeser F, Kaczmarek DJ, Schlabe S, Parcina M, Nattermann J, Hoerauf A, Strassburg CP. Antibiotic resistance in healthcare-related and nosocomial spontaneous bacterial peritonitis. Eur J Clin Invest. 2017;47:44-52. [PubMed] |
| 28. | Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 29. | Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet. 2015;386:1576-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 30. | Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin HC, Gines P, Kim WR, Kamath PS; World Gastroenterology Organization Working Party. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 31. | Lindvig KP, Teisner AS, Kjeldsen J, Strøm T, Toft P, Furhmann V, Krag A. Allocation of patients with liver cirrhosis and organ failure to intensive care: Systematic review and a proposal for clinical practice. World J Gastroenterol. 2015;21:8964-8973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Arabi YM, Dara SI, Memish Z, Al Abdulkareem A, Tamim HM, Al-Shirawi N, Parrillo JE, Dodek P, Lapinsky S, Feinstein D, Wood G, Dial S, Zanotti S, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Antimicrobial therapeutic determinants of outcomes from septic shock among patients with cirrhosis. Hepatology. 2012;56:2305-2315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Karvellas CJ, Abraldes JG, Arabi YM, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study. Aliment Pharmacol Ther. 2015;41:747-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Merli M, Lucidi C. Bacterial resistance in cirrhotic patients: an emerging reality. J Hepatol. 2012;56:756-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Acevedo J. Multiresistant bacterial infections in liver cirrhosis: Clinical impact and new empirical antibiotic treatment policies. World J Hepatol. 2015;7:916-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 519] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 37. | Fiore M. Letter: the emergence of multi-drug resistant spontaneous bacterial peritonitis: a new challenge for the hepatologist? Aliment Pharmacol Ther. 2016;43:944-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | In the literature. Piperacillin-tazobactam and extended-spectrum β-lactamase--producing Escherichia coli. Clin Infect Dis. 2013;57:iii-iiv. [PubMed] |
| 39. | Retamar P, López-Cerero L, Muniain MA, Pascual Á, Rodríguez-Baño J; ESBL-REIPI/GEIH Group. Impact of the MIC of piperacillin-tazobactam on the outcome of patients with bacteremia due to extended-spectrum-β-lactamase-producing Escherichia coli. Antimicrob Agents Chemother. 2013;57:3402-3404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Edwards JR. Meropenem: a microbiological overview. J Antimicrob Chemother. 1995;36 Suppl A:1-17. [PubMed] |
| 41. | Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Stamatiadis D, Papaioannou MG, Giamarellos-Bourboulis EJ, Marinaki S, Giamarellou H, Stathakis CP. Pharmacokinetics of teicoplanin in patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int. 2003;23:127-131. [PubMed] |
| 43. | Eisenstein BI, Oleson FB Jr, Baltz RH. Daptomycin: from the mountain to the clinic, with essential help from Francis Tally, MD. Clin Infect Dis. 2010;50 Suppl 1:S10-S15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Renzoni A, Kelley WL, Rosato RR, Martinez MP, Roch M, Fatouraei M, Haeusser DP, Margolin W, Fenn S, Turner RD. Molecular Bases Determining Daptomycin Resistance-Mediated Resensitization to β-Lactams (Seesaw Effect) in Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2016;61:pii: e01634-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Fiore M, Andreana L, Leone S. Treatment of spontaneous bacterial peritonitis: beyond the current international guidelines. Liver Int. 2016;36:918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Fiore M, Andreana L. The Possible Role of Anti-Methicillin-Resistant Staphylococcus Aureus Antimicrobial Agents in Spontaneous Bacterial Peritonitis. Infect Dis Rep. 2015;7:6286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Fiore M. Spontaneous bacterial peritonitis due to multidrug resistant bacteria: are the current guidelines outdated? Eur J Gastroenterol Hepatol. 2016;28:731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Fiore M. Nosocomial spontaneous bacterial peritonitis: discussing a specific infection treatment algorithm. Liver Int. 2016;36:1074-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |