Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4529
Peer-review started: February 9, 2017
First decision: February 23, 2017
Revised: March 3, 2017
Accepted: June 12, 2017
Article in press: June 12, 2017
Published online: July 7, 2017
Processing time: 154 Days and 13.1 Hours
To evaluate the protective effects of glutamine in a model of portal hypertension (PH) induced by partial portal vein ligation (PPVL).
Male Wistar rats were housed in a controlled environment and were allowed access to food and water ad libitum. Twenty-four male Wistar rats were divided into four experimental groups: (1) control group (SO) - rats underwent exploratory laparotomy; (2) control + glutamine group (SO + G) - rats were subjected to laparotomy and were treated intraperitoneally with glutamine; (3) portal hypertension group (PPVL) - rats were subjected to PPVL; and (4) PPVL + glutamine group (PPVL + G) - rats were treated intraperitoneally with glutamine for seven days. Local injuries were determined by evaluating intestinal segments for oxidative stress using lipid peroxidation and the activities of glutathione peroxidase (GPx), endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) after PPVL.
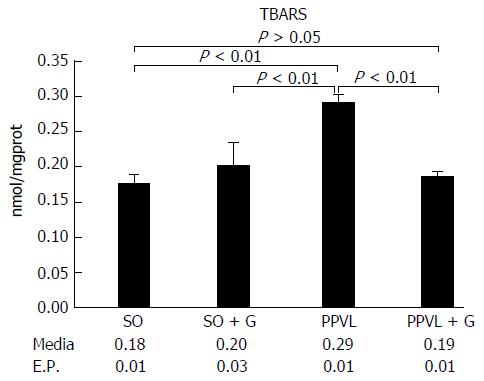
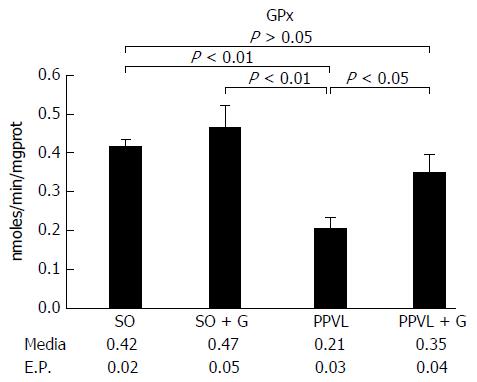
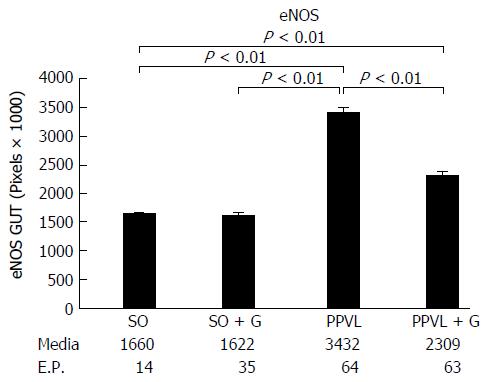
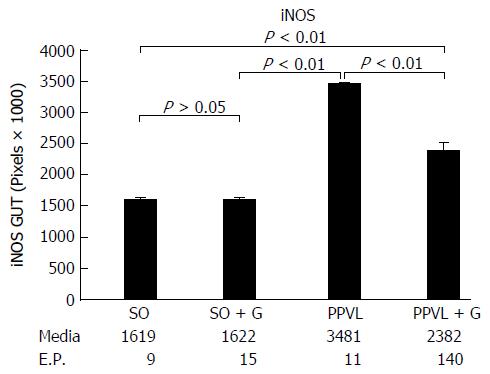
Lipid peroxidation of the membrane was increased in the animals subjected to PH (P < 0.01). However, the group that received glutamine for seven days after the PPVL procedure showed levels of lipid peroxidation similar to those of the control groups (P > 0.05). The activity of the antioxidant enzyme GTx was decreased in the gut of animals subjected to PH compared with that in the control group of animals not subjected to PH (P < 0.01). However, the group that received glutamine for seven days after the PPVL showed similar GTx activity to both the control groups not subjected to PH (P > 0.05). At least 10 random, non-overlapping images of each histological slide with 200 × magnification (44 pixel = 1 μm) were captured. The sum means of all areas, of each group were calculated. The mean areas of eNOS staining for both of the control groups were similar. The PPVL group showed the largest area of staining for eNOS. The PPVL + G group had the second highest amount of staining, but the mean value was much lower than that of the PPVL group (P < 0.01). For iNOS, the control (SO) and control + G (SO + G) groups showed similar areas of staining. The PPVL group contained the largest area of iNOS staining, followed by the PPVL + G group; however, this area was significantly smaller than that of the group that underwent PH without glutamine (P < 0.01).
Treatment with glutamine prevents gut mucosal injury after PH in rats.
Core tip: Portal hypertension (PH) is characterized by an increased portal pressure gradient. The progressive increase in portal pressure leads to the formation of portosystemic shunts and intestinal hypoxia. Many enzymes have been implicated in this process, among them endothelial nitric oxide synthase and inducible nitric oxide synthase. Some substances, such as glutamine, have been studied as protective agents against oxidative stress. In an experimental model of PH induced by partial portal vein ligation, we have found that glutamine reduced lipid peroxidation and preserved intestinal glutathione peroxidase activity, suggesting a protective role of this amino acid in the setting of PH.
- Citation: Zabot GP, Carvalhal GF, Marroni NP, Licks F, Hartmann RM, da Silva VD, Fillmann HS. Glutamine prevents oxidative stress in a model of portal hypertension. World J Gastroenterol 2017; 23(25): 4529-4537
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4529.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4529
Portal hypertension (PH) is a clinical entity defined by a haemodynamic increased portal pressure gradient, directly related to the portal blood flow and vascular resistance[1,2]. Anatomically, the causes of PH can be classified as pre-hepatic (portal vein thrombosis or splenic), intra-hepatic (cirrhosis) and post-hepatic (Budd-Chiari syndrome)[3].
PH triggers disorders of the gastrointestinal tract, compromising the function of the small intestine. Gastrointestinal bleeding is present in 80%-90% of patients with PH due to the progressive dilatation of the vessels, which rupture and lead to haemorrhage[4]. Such a high bleeding rate points to the need to study the pathophysiology of PH to minimize its consequences. The partial ligation model of the portal vein is the model most widely used to study pre-hepatic PH; it was developed by Sikuler in 1985, and several experimental studies have shown that, in animals, partial portal vein ligation (PPVL) produces abnormalities that are equivalent to those of PH in humans[5].
The development of hyper dynamic circulation can cause structural changes in the intestinal wall as well as spontaneous bacterial infections and sepsis[6]. The increase in splanchnic vascular resistance and venous flow leads to congestion in the portal system, causing an intermittent intestinal hypo perfusion[7]. Oxidative stress damages the integrity of the intestinal mucosa and leads to lipid peroxidation[8,9]. Likewise, there is an increased production of nitric oxide (NO) in this hyper dynamic state, producing nitrosative stress[10]. NO is the main mediator of cytotoxic immune cells and plays a messenger/modulatory role in many important biological processes[11]. However, it is highly toxic, especially in cases of oxidative stress and in situations of antioxidant system deficiency[12]. The physiological and pathophysiological effects of the nitric oxide isoforms [endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS)] are related to their concentration levels[13]. When operating at low concentrations, these isoforms behave as messengers and cell protection factors (antioxidants), interacting with transition metals and other free radicals. Under high concentrations and upon forming dinitrogen trioxide (N2O3) or peroxynitrite (ONOO), NO behaves as an active species of nitrogen, responsible for numerous cytotoxic actions in a framework known as nitrosative stress[14]. A high NO concentration was observed in PH, this increase being intimately related to the development of hyper dynamic circulation and oxidative stress[15].
To protect against the damage posed by oxidative stress, we need a system to prevent the formation of free radicals and neutralize oxidative damage, a function performed by antioxidants. The enzymatic system is one of the most important defence systems because it catalytically removes free radicals and other reactive species. Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) are some of the most prominent antioxidant enzymes. The peroxidase GPx enzyme is characteristically located in the cytosol and mitochondrial matrix[16-18].
Similar to the enzymes involved in removing free radicals, there are other substances with antioxidant properties capable of preventing oxidative chain reactions, such as those involved in lipid peroxidation (LPO). These substances are known as non-enzymatic antioxidant defences[19,20].
Glutamine is the most commonly found free amino acid in plasma, acting on oxygen-free radicals and playing an important role in attenuating inflammatory bowel disease[21]. It is used for hepatic synthesis of urea, for renal ammoniagenesis and for gluconeogenesis and also serves as the main respiratory fuel for many cell types[8]. Thus, it is vital to the regulation of the intracellular oxidative balance[22].
The progression of different diseases is triggered by oxidative stress. In PH, it promotes the development of collateral circulation. Thus, antioxidant therapy appears to be a promising strategy to minimize the complications of PH[15].
The objectives of this study were to evaluate the effects of glutamine in rats with pre-hepatic PH submitted to an experimental model of PPVL, to assess LPO and determine the activity of the enzyme GTx in the intestine. Additionally, we aimed to quantify the expression of the enzymes eNOS and iNOS in the intestine through immunohistochemistry.
Animal care was performed in compliance with the normative resolution 04/97 of the Research and Ethics Committee of the Health Research Group and Graduate Teaching Hospital of Porto Alegre (Hospital de Clínicas de Porto Alegre-HCPA)[23].
Twenty-four male Wistar rats weighing between 250 and 350 grams, were used from the State Foundation for Research and Production in Health (FEPPS-RS). The animals were divided into 4 groups of 6. During the experiment, the animals were kept in plastic boxes of 47 cm × 34 cm × 18 cm lined with wood, in a cycle of 12 h light/dark and temperature between 20 and 25 °C. Water and feed are given ad libitum.
After trichotomy, rats were anaesthetized with ketamine and xylazine solution [45 mg/kg intraperitoneally (ip)]. After midline laparotomy, the bowel loops were removed gently and were covered with gauze moistened with saline, and then, the portal vein was isolated. A 20G needle was placed on the portal vein and tied with 3.0 silk sutures. Subsequently, the needle was gently removed, and the occurrence of portal vein thrombosis was noted[5]. Sham-operated (SO) control animals underwent the same procedures but without partial ligature of the portal vein. After surgery, the animals were treated with glutamine or saline solution, depending on the group. Glutamine was administered beginning on day 8 after surgery, intraperitoneally, at a dosage of 0.75 g/kg for 7 d[12]. Control animals received vehicle (saline, 0.9% NaCl) in a volume of 0.6 mL intraperitoneally for the same period. At the end of the experiments, intestinal segments (10 cm) were removed for histological examination and biochemical studies.
Rats were randomly allocated into the following groups: (1) control group (SO): rats were subjected to the simulation of surgery and vehicle administration (NaCl) (n = 6); (2) control + glutamine group (SO + G): rats were subjected to the simulation of surgery and glutamine administration (n = 6); (3) PH group (PPVL): rats were subjected to PPVL and vehicle administration (NaCl) (n = 6); (4) PPVL + glutamine group (PPVL + G): rats were subjected to PPVL and glutamine administration (n = 6).
Thiobarbituric acid reactive substances: Tissue samples were placed in test tubes, and solutions were added in the following order: 0.75 mL of 10% trichloroacetic acid (TCA), 0.25 mL of homogenate, 0.5 mL of 0.67% thiobarbituric acid (TBA), and 0.25 mL of distilled water. Thiobarbituric acid reactive substances (TBARS) were measured by heating the homogenate with thiobarbituric acid and then measuring the consequent formation of a coloured product in a spectrophotometer at 535 nm. The coloration is due to the presence of malondialdehyde and other substances from biological lipid peroxidation[24].
The determination of selenium glutathione peroxidase was based on the method of Guntzler Flohé and consisted of measuring the nicotinamide adenine dinucleotide phosphate dehydrogenase (NADPH) consumption rate in a system containing total glutathione (GSH), wherein the oxidation is recorded spectrophotometrically at a wavelength of 340 nm. The GPx activity can be studied by measuring the NADPH consumption rate in a system containing GSH[25].
This technique consists of determining the activity of the enzyme spectrophotometrically by measuring the rate of oxidation of NADPH in a reaction.
To this end, 2.7 mL of phosphate regulating solution of Na+ and K+ (100 mmol/L, pH 7.0) was placed in a quartz cuvette with 50 μL of NADPH (10 mmol/L), 150 μL of butylhydroperoxide (BOOH) (10 mmol/L) and 50 μL of glutathione reductase (12 U/mL). The mixture was read for 1 min and was identified as the baseline, followed by the addition of 50 μL of GSH (100 mmol/L) and 50 mL of homogenate. The samples were incubated at 25 °C for 5 min and then absorbance was read at 340 nm. The activity was expressed in nmol/min/mg protein[25].
For the preparation of the slides and subsequent immunohistochemical analysis, 3-μm-thick sections were prepared using a microtome (Leica SM 2000R, Germany). The sections were placed on slides pre-treated with HistoGrip (Zymed, United States) and were left in the oven at 60 °C for 24 h.
The sections were then deparaffinized by incubation with xylene for 10 min three times, followed by rehydration with a sequence of decreasing concentrations of ethanol (absolute, 90%, 80% and 70%) for 3 min per dilution. Next, the sections were washed three times in distilled water.
Antigen retrieval was performed by heating in a pTLINK platform (DAKO) and then treating the slides for 40 min at 98 °C with the Envision Flex high pH antigen retrieval solution (DAKO). Immediately thereafter, the slides were washed with PBS buffer at a pH of 7.2. Endogenous peroxidase was blocked by incubation in a solution of 3% H2O2 in methanol for two 15-min intervals, followed by washing three times with PBS buffer at a pH of 7.2.
The sections were then incubated using a Sequenza (Thermo Shandon, United States) immunostaining station, were left for 2 h at room temperature and then were diluted with the dilution solution (Antibody Diluent with background reducing components, Dako, United States) and the following antibodies: Anti-eNOS (Thermo Scientific, PA3-031A, United States) 1:800, anti-iNOS (PA5-16855 Thermo Scientific, United States) 1:800. After incubation with the primary antibody, the sections were washed three times with PBS buffer at a pH of 7.2. For the amplification of the antigen-antibody reaction for anti-eNOS, we used the HRP Envision Flex System (Dako, United States) in accordance with the manufacturer's recommendations. Next, the slides were washed with PBS buffer at a pH of 7.2 and were incubated with diaminobenzidine solution (Dako Liquid DAB Substrate Chromogen System, United States) for 5 min. After washing in distilled water, the slides were counterstained with Harris haematoxylin for 1 min and were washed with water until complete removal of the dye, followed by incubation in a 37-mmol/L ammonia solution for 15 s. Finally, the slides were dehydrated in absolute ethanol (four 2-min incubations) and were treated twice with xylene for 5 min. The slides were then mounted with Entellan synthetic medium (Merck, Germany)[20,26,27].
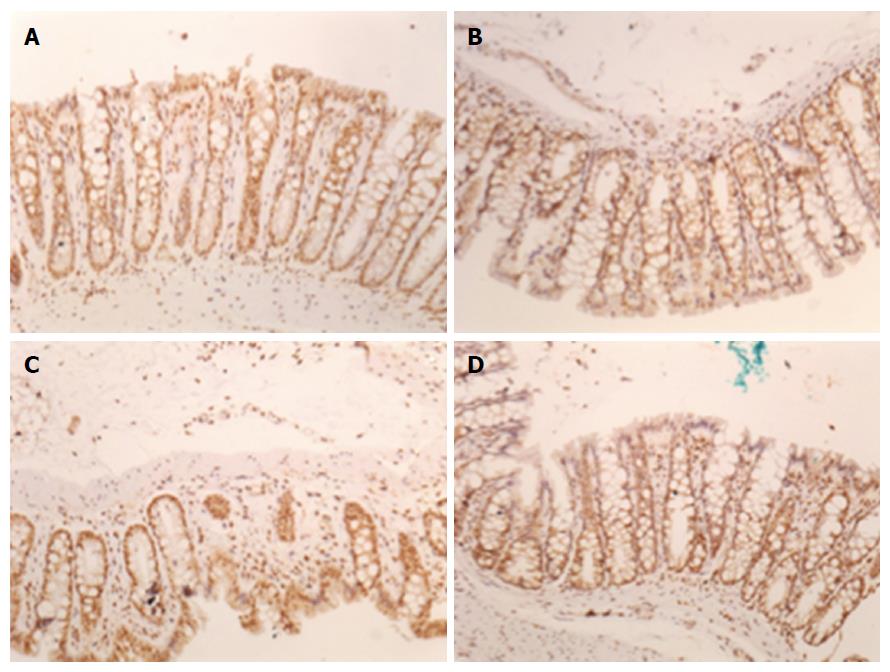
We used a digital analysis system composed of a Zeiss Axioskop 40 microscope (Oberkochen, Germany) with Neofluar lenses connected by a Roper Scientific video camera (Media Cybernetics, Rockville, United States) to a computer with an Image Capture Pro kit (Media Cybernetics, Rockville, MD, United States) capture card. Image-Pro Plus version 7.0 (Media Cybernetics, Rockville, United States) was used to analyse the digital images. Images were captured in the TIFF (True Image File Format) format without compression by the same examiner with the same light intensity pattern for all photos. Images were captured of at least fifteen random, non-overlapping fields for each histological slide at 200 × magnification (44 pixel = 1 μm). The hot spot method was used to select fields on slides with focal positivity for the markers. Colour selection was performed interactively by three trained observers and was then applied to all samples by the automated digital image analysis system. The initial area considered was 0.01 cm.
Quantitative data were initially described as the means and SE. For comparison of the groups, one-way analysis of variance (ANOVA) was used, followed by the SNK post hoc test (Student-Newman-Keuls). The level of significance for the experiment was P = 0.05. Data were analysed using SPSS software version 22.0. A biomedical statistician performed the statistical review.
Oxidative stress, quantified by gut membrane lipid peroxidation was increased in the group of animals subjected to PPVL (P < 0.01). On the other hand, animals that received glutamine for seven days after the procedure exhibited levels of lipid peroxidation similar to those of the control groups (animals not subjected to PPVL and animals receiving glutamine without PPVL). These levels were also significantly different from those of the group of animals that was submitted solely to PPVL (P < 0.01; Figure 1).
The activity of GTx was decreased in the gut of animals subjected to PPVL compared with that of animals not subjected to PPVL (P < 0.01; Figure 2). Importantly, the group that received glutamine for seven days after PPVL showed similar GTx activity to that of groups without PPVL. The difference in GTx activity in animals subjected to PPVL and in those that received glutamine after PPVL (P < 0.05), suggests that glutamine is a protective factor for PH.
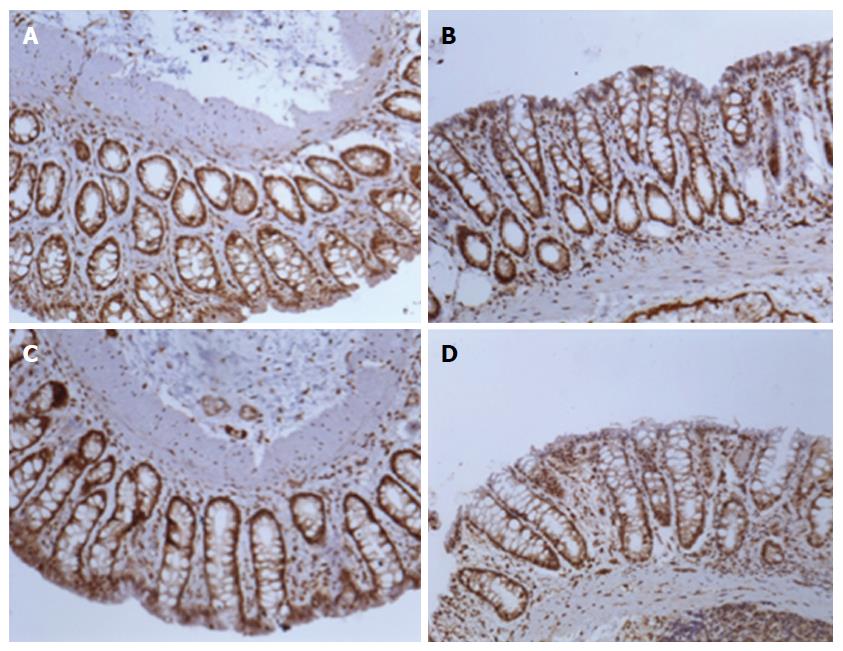
For digital analysis of the images, the program Image-Pro Plus version 7.0 (Media Cybernetics, Rockville, United States) was used. They were captured in the True Image File Format (TIFF) format, with at least ten fields, without random overlap, for each histological slide at 200 × (44 pixel = 1 μm). Slides with focal positivity for the markers were selected by the hot spot method. The initial area considered was 0.01 cm. The sum of the stained areas was calculated for each group. The control (SO) and control + G (SO + G) groups presented with similar areas of staining. The PPVL group presented the largest area. The second group with larger staining areas was the PPVL + G, but with values much lower than those of the PPVL group, resembling the area found in the control groups with and without glutamine. These results were statistically significant (P < 0.01). These results are shown in Figures 3 and 4.
Figures 5 and 6 show the immunohistochemical expression of iNOS. The control (SO) and control + G (SO + G) groups presented with similar areas of staining (P > 0.05). Regarding eNOS, the PPVL group also presented the largest stained area. The second group with a larger staining area was the PPVL + G, again with values much lower than those of the PPVL group. These results were also statistically significant (P < 0.01).
Glutamine promotes intracellular oxidative balance[28]. It is a precursor of glutathione, one of the most important non-enzymatic cellular antioxidants, and is the most abundant free amino acid in plasma, acting on oxygen-free radicals[29].
Other researchers have used the PPVL model to study pre-hepatic PH[5]. Our work demonstrates that glutamine treatment exerts important protective effects in pre-hepatic PH in an animal model. We have shown that after PPVL glutamine: (1) reduced oxidative stress determined by the lipid peroxidation of the intestinal mucosa; (2) maintained levels of GTx activity; and (3) reduced the expression of eNOS and iNOS.
We have observed greater levels of lipid peroxidation in the group of animals that underwent PPVL. Glutamine significantly decreased lipid peroxidation in the PPVL model. Schimpl et al[8] evaluated pre-hepatic PH with a model of common bile duct ligation (CBDL). They evaluated the effects of glutamine and allopurinol on bacterial translocation in PH and obstructive jaundice. These authors concluded that, in PH and common bile duct ligation (CBDL) in rats, there was significant bacterial translocation, ileal mucosal lipid peroxidation, and neutrophil derived myeloperoxidase (MPO) activity. Bacterial translocation, ileal mucosal malondialdehyde (MDA) concentrations and MPO activities were significantly reduced by the combined use of allopurinol and glutamine.
Huang et al[30] studied the oxidative stress related to intrahepatic PH after ligature of the bile duct in rats. They identified an increase in the MDA levels in plasma as well as reduced levels of plasma GTx. We have found reduced levels of GTx activity in animals submitted to PPVL compared with controls (SO and SO + G). When glutamine was administered intraperitoneally to animals submitted to PPVL, there was a less pronounced reduction if GTx activity levels. Rodríguez-Vilarrupla et al[17] stressed the importance of antioxidants as enzymatic systems in PH syndrome. The effects of glutamine on eNOS activity were already investigated Marques et al[22]; however, these authors have used a model of PH gastropathy. They have also evaluated the activity of the nitric oxide (eNOS) by immunohistochemistry. In their study, lipid peroxidation and NO were significantly increased in PPVL, but the addition of glutamine has attenuated eNOS expression[22]. Oxidative stress was also evidenced by Gonzales et al[31] in a pre-hepatic PPVL rat model, through an increased concentration of TBARS. Additionally, these authors have shown a decrease in the antioxidant enzymes SOD, CAT and glutathione peroxidase (GTx). Moreover, they studied the antioxidant role of haem oxygenase-1 (HO-1) and suggested a beneficial role of HO-1 overexpression.
eNOS favours the decrease of blood pressure and assists in the inhibition of platelet aggregation. iNOS forms NO induced by certain cytotoxins, being closely related to defensive body processes[14]. In our study, the PH group (PPVL) showed the largest area of staining for eNOS. The PPVL + G group had the second highest amount of stained areas, but with a mean value much lower than that of the PH group (P < 0.05). For iNOS, the control (SO) and control + G (SO + G) groups showed similar areas of staining. The PH group (PPVL) contained the largest area of iNOS staining, followed by the PPVL + G group; however, this area was significantly lower than that of the group that underwent PH without glutamine (PPVL).
Jalan et al[32] tested the hypothesis that ammonia modulates human hepatic stellate cell activation in vitro and in vivo, in a BDL model, using l-ornithine phenylacetate. This substance significantly reduced the plasmatic levels of ammonia and portal pressure, which was associated with increased eNOS activity[32].
Iwakiri et al[14] stated that eNOS and iNOS have different roles; most commonly, eNOS prevents the occurrence of disease whereas iNOS favours its progress.
Kajita et al[33] observed that iNOS expression in vascular resident macrophages contributed to the circulatory dysfunction of splanchnic vascular smooth muscle contractions in PH rats[33]. Xu et al[34], in a study with male Sprague-Dawley rats submitted to intra-hepatic PH induced by the injection of CCl4, noted that iNOS contributes to the haemodynamic alterations of PH secondary to increased levels of NO[34].
Oxidative stress is also responsible for hepatic encephalopathy. Pilar Carbonero-Aguilar et al[35], suggest that it may be resultant from increased ammonia concentration in the brain.
Arias et al[36], using an experimental model through the triple portal vein ligation method, demonstrated, for the first time, a relationship between inflammation, astrocyte damage and neurons and cerebral metabolic compromise.
The use of antioxidants in the prevention or treatment of various diseases related to oxidative stress seems to be a viable alternative in the attempt to minimize or reverse damage. Glutamine was initially used prophylactically in patients undergoing radiotherapy, resulting in a decreased incidence and decreased severity of actinic enteritis[37]. This substance was most extensively investigated in relation to the oxidative stress caused by exercise. Cruzat et al[38] showed that, in exhaustive exercise, there is a significant reduction in serum glutamine. This decrease is accompanied by a significant increase in the inflammatory activity and oxidative stress levels measured by the lipid peroxidation rate. Glutamine has also been beneficial as a nutritional supplement in severely debilitated patients, such as those with multiple trauma or in those undergoing major surgery[39,40]. Antioxidant therapy improves the prognosis and reduces the overall complication rates in debilitated patients[41]. Tang et al[42] utilized TPN containing glutamine and recombinant human growth hormone in the postoperative care of patients after PH surgery. They found that this supplementation enhanced immune function, modulated the inflammatory response, and prevented the intestinal membrane atrophy.
This is the first publication describing the role of glutamine in the intestinal oxidative stress in an animal model of PPVL.
In conclusion, the present study demonstrates that intraperitoneally administration of glutamine at a dose of 0.75 g/kg during 7 d, starting on the 8th postoperative day after PPVL, prevented lipid peroxidation and maintained GTx levels. The expression of eNOS and iNOS were reduced upon intraperitoneal glutamine during the 7 d after PPVL in rats. Our work confirms the findings of previously published experimental research on glutamine as a protective factor in murine models of PH. Additional research is necessary to ascertain the benefits of glutamine as protective element against intestinal disorders secondary to PH in humans.
To the Pontifical Catholic University of Rio Grande do Sul, in particular to the Graduate Program in Medicine and Health Sciences, for the opportunity to carry out this study and have made available a scholarship.
Portal hypertension (PH) is often secondary to the obstruction of the intra- or extra-hepatic portal flow. The progressive increase in pressure in the portal system leads to dilation of the blood vessels, with consequent formation of porto-systemic shunts. The development of hyperdynamic circulation can cause structural changes in the intestinal wall and lipid peroxidation as well as oxidative stress. The physiological and pathophysiological effects of nitric oxide isoforms are related to their concentration levels. Glutamine has been investigated as a potential preventive agent against damage caused by inflammatory processes caused by oxidative agents.
Being the most abundant amino acid in the plasma, glutamine has many roles including the regulation of macrophage activity, the modulation of reactive oxygen species, the hepatic synthesis of urea, renal ammoniagenesis, and gluconeogenesis, serving also as fuel for many cell types.
The first studies with glutamine were in patients undergoing radiotherapy, when there was a decreased incidence and severity of actinic enteritis associated with its use. It was also observed that glutamine plays a major role in the immune defence barrier of the intestinal mucosa through its participation in the formation of immunoglobulins, especially IgA. Furthermore, it was demonstrated that glutamine decreases the inflammatory enterocolitis induced by methotrexate and reduces bacterial translocation in animals with abdominal sepsis. These data demonstrate that glutamine prevents lipid peroxidation after partial portal vein ligation (PPVL) in a murine model.
This study suggests that glutamine reduces oxidative stress by decreasing lipid peroxidation and by preserving glutathione peroxidase (GTx) levels. The immunohistochemical expression of endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) were reduced upon intraperitoneal administration of glutamine for seven days starting on the eighth postoperative day after PPVL in rats.
PH: a clinical entity defined by a haemodynamically increased portal pressure gradient that is directly related to the portal blood flow and vascular resistance. Lipid peroxidation: oxidative stress that causes an alteration in cell membrane permeability that favours bacterial translocation and the activation of the inflammatory response. GTx: an antioxidant enzyme that catalytically removes free radicals and other active oxidative species. eNOS and iNOS: isoforms of nitric oxide, which is a mediator of cytotoxic immune cells that have messenger/modulator roles in many important biological processes.
It is a well-writen paper. The current work evaluates the protective effects of glutamine in a model of PH induced by PPVL.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arias JL, Hashimoto N S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Kim MY, Baik SK, Lee SS. Hemodynamic alterations in cirrhosis and portal hypertension. Korean J Hepatol. 2010;16:347-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Miñano C, Garcia-Tsao G. Clinical pharmacology of portal hypertension. Gastroenterol Clin North Am. 2010;39:681-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Majid S, Azam Z, Shah HA, Salih M, Hamid S, Abid S, Jafri W. Factors determining the clinical outcome of acute variceal bleed in cirrhotic patients. Indian J Gastroenterol. 2009;28:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Sikuler E, Kravetz D, Groszmann RJ. Evolution of portal hypertension and mechanisms involved in its maintenance in a rat model. Am J Physiol. 1985;248:G618-G625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Hernández-Guerra M, García-Pagán JC, Bosch J. Increased hepatic resistance: a new target in the pharmacologic therapy of portal hypertension. J Clin Gastroenterol. 2005;39:S131-S137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Bosch J, Pizcueta P, Feu F, Fernández M, García-Pagán JC. Pathophysiology of portal hypertension. Gastroenterol Clin North Am. 1992;21:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Schimpl G, Pesendorfer P, Steinwender G, Feierl G, Ratschek M, Höllwarth ME. Allopurinol and glutamine attenuate bacterial translocation in chronic portal hypertensive and common bile duct ligated growing rats. Gut. 1996;39:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int. 2013;33:1457-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Kanwar S, Kubes P, Tepperman BL, Lee SS. Nitric oxide synthase activity in portal-hypertensive and cirrhotic rats. J Hepatol. 1996;25:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Röth E, Hejjel L, Jaberansari M, Jancso G. The role of free radicals in endogenous adaptation and intracellular signals. Exp Clin Cardiol. 2004;9:13-16. [PubMed] |
| 12. | Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1222] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 13. | Cerqueira NF, Hussni CA, Yoshida WB, Sequeira JL, Padovani CR. Effects of pentoxifylline and n-acetylcysteine on injuries caused by ischemia and reperfusion of splanchnic organs in rats. Int Angiol. 2008;27:512-521. [PubMed] |
| 14. | Iwakiri Y, Kim MY. Nitric oxide in liver diseases. Trends Pharmacol Sci. 2015;36:524-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 15. | Fernando B, Marley R, Holt S, Anand R, Harry D, Sanderson P, Smith R, Hamilton G, Moore K. N-acetylcysteine prevents development of the hyperdynamic circulation in the portal hypertensive rat. Hepatology. 1998;28:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Batinić-Haberle I, Rebouças JS, Spasojević I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 411] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 17. | Rodríguez-Vilarrupla A, Bosch J, García-Pagán JC. Potential role of antioxidants in the treatment of portal hypertension. J Hepatol. 2007;46:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Closa D, Folch-Puy E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life. 2004;56:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, Marroni N, González-Gallego J. Quercetin prevents oxidative stress and NF-kappaB activation in gastric mucosa of portal hypertensive rats. Biochem Pharmacol. 2004;68:1939-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Licks F, Marques C, Zetler C, Martins MI, Marroni CA, Marroni NP. Antioxidant effect of N-acetylcysteine on prehepatic portal hypertensive gastropathy in rats. Ann Hepatol. 2014;13:370-377. [PubMed] |
| 21. | Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P. Molecular mechanisms of glutamine action. J Cell Physiol. 2005;204:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 320] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Marques C, Mauriz JL, Simonetto D, Marroni CA, Tuñon MJ, González-Gallego J, Marrón NP. Glutamine prevents gastric oxidative stress in an animal model of portal hypertension gastropathy. Ann Hepatol. 2011;10:531-539. [PubMed] |
| 23. | Goldim JR, Raymundo MM. Pesquisa em saúde e direitos dos animais. 2nd ed. Porto Alegre: HCPA, 1997. . |
| 24. | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7899] [Cited by in RCA: 7984] [Article Influence: 169.9] [Reference Citation Analysis (0)] |
| 25. | Flohe L, Günzler WA, Schock HH. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973;32:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 896] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Gaffey MJ, Mills SE, Swanson PE, Zarbo RJ, Shah AR, Wick MR. Immunoreactivity for BER-EP4 in adenocarcinomas, adenomatoid tumors, and malignant mesotheliomas. Am J Surg Pathol. 1992;16:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Zhao X, Deng B, Xu XY, Yang SJ, Zhang T, Song YJ, Liu XT, Wang YQ, Cai DY. Glycyrrhizinate reduces portal hypertension in isolated perfused rat livers with chronic hepatitis. World J Gastroenterol. 2013;19:6069-6076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Calder PC, Yaqoob P. Glutamine and the immune system. Amino Acids. 1999;17:227-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Gianotti L, Alexander JW, Gennari R, Pyles T, Babcock GF. Oral glutamine decreases bacterial translocation and improves survival in experimental gut-origin sepsis. JPEN J Parenter Enteral Nutr. 1995;19:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 141] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Huang YT, Hsu YC, Chen CJ, Liu CT, Wei YH. Oxidative-stress-related changes in the livers of bile-duct-ligated rats. J Biomed Sci. 2003;10:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Gonzales S, Perez MJ, Perazzo JC, Tomaro ML. Antioxidant role of heme oxygenase-1 in prehepatic portal hypertensive rats. World J Gastroenterol. 2006;12:4149-4155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Jalan R, De Chiara F, Balasubramaniyan V, Andreola F, Khetan V, Malago M, Pinzani M, Mookerjee RP, Rombouts K. Ammonia produces pathological changes in human hepatic stellate cells and is a target for therapy of portal hypertension. J Hepatol. 2016;64:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Kajita M, Murata T, Horiguchi K, Iizuka M, Hori M, Ozaki H. iNOS expression in vascular resident macrophages contributes to circulatory dysfunction of splanchnic vascular smooth muscle contractions in portal hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;300:H1021-H1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Xu J, Cao H, Liu H, Wu ZY. Role of nitric oxide synthase and cyclooxygenase in hyperdynamic splanchnic circulation of portal hypertension. Hepatobiliary Pancreat Dis Int. 2008;7:503-508. [PubMed] |
| 35. | Carbonero-Aguilar P, Diaz-Herrero M, Campo J, Cremades O, Romero-Gómez M, Bautista J. Hyperammonemia induced oxidative stress in cirrhotic rats without promoting differential protein expression in the brain cortex: A 2D-DIGE analysis. Adv Biosci Biotechnol. 2012;3:1116-1123. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Arias N, Méndez M, Gómez-Lázaro E, Azpiroz A, Arias JL. Main target of minimal hepatic encephalopathy: Morphophysiological, inflammatory and metabolic view. Physiol Behav. 2015;149:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Klimberg VS, Souba WW, Dolson DJ, Salloum RM, Hautamaki RD, Plumley DA, Mendenhall WM, Bova FJ, Khan SR, Hackett RL. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990;66:62-68. [PubMed] |
| 38. | Cruzat VF, Rogero MM, Tirapegui J. Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem Funct. 2010;28:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Heyland DK, Dhaliwal R, Day AG, Muscedere J, Drover J, Suchner U, Cook D; Canadian Critical Care Trials Group. REducing Deaths due to OXidative Stress (The REDOXS Study): Rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proc Nutr Soc. 2006;65:250-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Demirkan A, Savaş B, Melli M. Endotoxin level in ischemia-reperfusion injury in rats: effect of glutamine pretreatment on endotoxin levels and gut morphology. Nutrition. 2010;26:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, Radella F, Garcia I, Maier RV. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 336] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 42. | Tang ZF, Ling YB, Lin N, Hao Z, Xu RY. Glutamine and recombinant human growth hormone protect intestinal barrier function following portal hypertension surgery. World J Gastroenterol. 2007;13:2223-2228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |