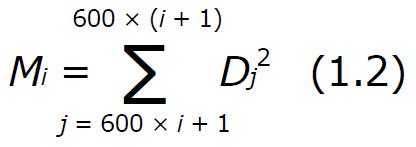Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4517
Peer-review started: January 9, 2017
First decision: April 14, 2017
Revised: April 29, 2017
Accepted: June 12, 2017
Article in press: June 12, 2017
Published online: July 7, 2017
Processing time: 181 Days and 22.1 Hours
To enhance the clinical utility of electrogastrography (EGG), which has been recorded since 1922, but is clinically unutilized.
An innovative method to salvage the promise of EGG is proposed by introducing a preliminary procedure, while maintaining the electrodes, standardized equipment, and signal processing utilized in the well-established EGG testing of today. The proposed enhanced EGG (EEGG) protocol involves swallowing an ingestible capsule containing miniature electronic oscillator embedded in an expandable, self-disintegratable, biocompatible pseudobesoar residing in the stomach for the duration of the test. The benefits of the proposed approach are outlined, tested and discussed in details.
Experiments were performed on eight mongrel dogs (6F, 4M, 23.8 ± 3.3 kg). Four were administered an active EEGG capsule, while the rest were given a deactivated (battery removed) capsule. Pharmacologically facilitated gastric motility revealed a significant (P < 0.01) Pearson correlation between gastric motility indices obtained by force transducers implanted directly on the stomach, and the motility indices obtained by EEGG. A particular emphasis was made on preserving standard EGG-related hardware and software in order to facilitate the introduction of the proposed EEGG in environments which already utilize standard EGG testing. The expanded intragastric pseudobezoar containing the miniature electronic oscillator was retained during the tests, and could be disintegrated on demand.
Enhancing standard EGG by an ingestible, self-expanding and self-disintegrating pseudobesoar containing a miniature electronic oscillator can be an important avenue for clinical applicability of this test.
Core tip: Intrinsic gastric electrical activity is of specific nature that restricts the clinical applicability of its non-invasive measurements known as electrogastrography (EGG). This study proposes the utilization of an ingestible, self-expanding and self-disintegrating pseudobesoar containing a miniature electrical oscillator to enhance the clinical utility of EGG in diagnosing functional dyspepsia and gastroparesis.
- Citation: Poscente MD, Mintchev MP. Enhanced electrogastrography: A realistic way to salvage a promise that was never kept? World J Gastroenterol 2017; 23(25): 4517-4528
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4517.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4517
Cutaneous recordings of gastric electrical activity (GEA), known as electrogastrography (EGG), have been recorded since 1922[1] and has been groomed to become a single, non-invasive test for the early diagnosis of functional gastric dyspepsia and gastroparesis. However, to this day remain clinically unutilized, although two distinct Current Procedural Terminology (CPT) codes associated with EGG have been introduced in the United States for diagnostic purposes[2]. Despite numerous unsubstantiated claims regarding the clinical utility of EGG, the prevailing attitude addressing the limitations of EGG for clinical decision-making is that they have overwhelmingly cast their shadow over this tempting non-invasive test which was promoted as a primary clinical tool for diagnosing functional dyspepsia[2-4]. In this study we discuss an innovative method to salvage the promise of EGG, by introducing a preliminary procedure to the patient preparation, while maintaining the electrodes, standardized equipment, and signal processing utilized in the standard EGG testing of today, with the hope that this technique can revive the promise of EGG as a clinical diagnostic test for functional dyspepsia and gastroparesis.
Functional dyspepsia is a multifaceted disorder of the upper gastrointestinal tract that affects between 5% and 11% of the world population[5], and is defined by the so called Rome IV criteria, which include: (1) sensation of pain and/or burning in the epigastrium; (2) early satiety (inability to finish a normal-sized meal); (3) distinct feeling of fullness during and immediately after meal; and (4) combination of these symptoms in a repetitive manner[6]. Gastric motility abnormalities such as delayed emptying, impaired initial distribution of a meal within the stomach, impaired accommodation to a meal, antral hypomotility, gastric electrical dysrhythmias (tachygastrias, bradygastrias, and mixed dysrhythmias), and delayed gastric emptying have all been identified in patients with functional dyspepsia[7]. The portfolio of dyspeptic symptoms strongly suggests an impaired gastric motility association with the disorder. However, although antral hypomotility and delayed gastric emptying are frequent in patients with functional dyspepsia[6], the clinical importance of these findings remains uncertain, mainly for two reasons: (1) they do not always correlate with symptoms; and (2) the link to impaired gastric motility has not been demonstrated[8] due to the lack of a single, reliable, 24-h (or longer) ambulatory test for assessing gastric motility in settings not different than the normal daily routine of the patient, as is presently investigated in the evaluation of esophageal reflux[9].
In this situation, the availability of a reliable long-term, ambulatory gastric motility test could possibly correlate the presence of dyspeptic symptoms to gastric motor function abnormalities, or, alternatively, to dispel such relationship altogether. This testing deficiency prompted researchers to search for alternative causes and reasons that could possibly be associated with functional dyspepsia[10]. Thus, presently, the diagnosis of functional dyspepsia is predominantly symptom-based, with the support of several tests, including most typically antral manometry and gastric emptying, but in what could be classified as a scientific curiosity associated with mild desperation, researchers are actively seeking other valid reasons for the dyspeptic symptoms to manifest themselves[10,11].
EGG, the cutaneous, non-invasive recordings of GEA, seemed to be perfectly suited to offer the missing link between gastric motility abnormalities and dyspeptic symptoms, as it was supposed to be a true representation of the intrinsic, omnipresent GEA, which in turn controls gastric motility[12]. Not quite, unfortunately. Although arguments pro[13] and con[14] have been circulating in the research community for the last 30 years, the prevalent objective scientific opinion outlines the following 4 major limitations associated with plain EGG:
(1) EGG cannot reliably represent gastric contractions or their shape, pattern, frequency, coupling and strength[15]; (2) Although EGG seems to represent a heavily integrated picture of the intrinsic gastric electrical rhythm, it fails where its reliability is needed most - in truthfully representing objectively existing internal gastric electrical abnormalities - dysrhythmias, tachygastrias and bradygastrias[16]; (3) Although postprandial EGG somewhat differs from fasting EGG, these differences have not led to any reliable clinical association with abnormal gastric motility, even if a standardized meal is administered[17]; and (4) The intrinsic electrical activity of the stomach is a complex, multi-component phenomenon, and the omnipresent rhythmic electrical component (also known as electrical control activity, or ECA) is a necessary, but not sufficient condition for gastric contractions to occur. Because of the different physiological nature of the gastric pump compared to the cardiac pump, the electrical fields of the stomach and of the heart differ substantially, with the stomach resembling functionally an “asynchronous pump”, irregularly exhibiting intermittent contractions during the presence of which the underlying electrical activity controlling it grows in complexity by the superposition over ECA of the so-called electrical response activity (ERA), which in turn is of two types, plateau and spikes[18]. Only the presence of the latter has been associated with meaningfully strong contractions. However, unfortunately, although often of high amplitude, spikes are of very short duration, which results in relatively low electrical power that dissipates before it can influence the cutaneous EGG recordings in any reliable and meaningful manner. In contrast, the heart can be regarded as a “synchronous” pump, with its electrical and mechanical activity corresponding quite intimately and with mutually reflected changes[19].
Unfortunately, the tempting promise of EGG as a non-invasive test for assessing gastric motor function similarly to the way its overwhelmingly successful sister, electrocardiography (ECG) can assess the motor function of the heart, continue to overshadow the above limitations, which in our opinion are objectively prohibitive. Although the arguments pro- and con-EGG can continue forever, the facts are clear: one after another, the assessment panels of state insurance companies in the United States label EGG as an investigative, but not as a diagnostic test, and refuse to offer any reimbursement for it, despite that two distinct current procedural terminology (CPT) codes associated with EGG have been introduced long time ago[20]. It is our opinion, therefore, that unless a major change in the EGG paradigm is proposed, the test will slowly decay and die its natural death. Thus, in our view, the standard EGG testing of today can be regarded not as a promise, only partially kept[21], but as a promise not kept at all, at least in the context of objectively assessing gastric motility.
Recently, and with much fanfare, the so-called “SmartPill” has been introduces as a possible gastric motility test, at least as a minimally invasive tool to measure gastric emptying[22]. Although this could represent a welcome replacement of traditional scintigraphy for measuring gastric emptying, it has been already discussed that gastric emptying test alone could not be the long searched single diagnostic test for dyspepsia, and by itself is not a direct gastric motility test[23,24].
Our present opinion is that in order to reliably assess gastric motility, particularly in ambulatory settings closely resembling the daily routine of the patient, three necessary conditions need to be met: (1) A “smart” capsule needs to be ingested by the patient; (2) The capsule should be retained in the stomach for the duration of the test, but should has the capability to disintegrate and safely exit the gut naturally after the testing is completed; and (3) Existing testing equipment and established procedures need to be used in order to optimise the path to clinical utilization of the technique, and respectively, to its reimbursement by insurance companies as a legitimate diagnostic test.
Recently, we have been exploring the utilization of passive, temporary, controllable pseudobezoars as ingestible implements for the treatment of obesity[25]. The safety of these implements has been established in various tests on animals and humans[25]. It has been already discussed that the intrinsic gastric electrical oscillator is a complex, multicomponent and dynamic phenomenon, whose relationship with actual gastric motility is so multifaceted that plain EGG cannot reliably assess it. Thus, we recently proposed to enhance an ingestible pill containing a temporary, controllable pseudobezoar with a miniature electronic oscillator of known and strong enough emitting frequency, optimized for good transluminal transmission[26-28]. This is a way to essentially enhance the intrinsic gastric electrical oscillator with a strong, simple, single-frequency source, which could be easily detected cutaneously by traditional EGG equipment and can be consequently processed by the same signal processing tools that plain EGG testing of today employs. Thus, not only long-term gastric retention could be achieved, but also the internal manipulation of the oscillator-containing pseudobezoar by gastric motility would be able to manifest itself cutaneously in a far more reliable fashion. Thus, our proposition is: (1) Convert an ingestible, passive, temporary, controllable pseudobezoar capsule into an active oscillator source, while preserving its gastric retentive capability; and (2) Utilize routine EGG testing equipment, electrode arrangement and signal processing tools to record the electric power dynamics of the ingested gastric-retentive oscillator, with the hope that it would reliably reflect gastric motility.
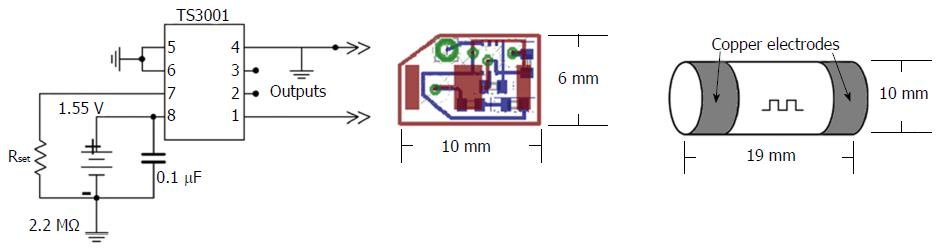
Oscillator: Previous literature demonstrated that 50 kHz is an optimal frequency for luminal electrical impedance measurements to minimize interference with regular muscle, nerve, and organ functions, while enabling effective transmission through the body[26,27]. Body conduction of small low frequency signals has been clinically proven to be safe, and has found application in other GI related technologies[29]. Size and power consumption were taken into account when it was decided to utilize the TS3001 (Silicon Labs, Austin, TX, United States) programmable oscillator integrated circuit that offers a frequency range from 9 to 300 kHz and uses a maximum of 5.4 μA of supply current to maintain operation. The complete design including a battery, the selected oscillator chip, resistor and capacitor fitted onto a 10-mm by 6-mm two sided printed circuit board that was custom manufactured.
Enhanced EGG capsule body: In order to protect the circuitry, the custom-designed electronics were held in a custom capsule that was made for the prototype models. The capsule consisted of a cylindrical body with two cap electrodes on either end, with an outer dimension of 10 mm in diameter and 19 mm in length, (Figure 1). The hollow cylindrical body was made of a machined biocompatible chemically resistant medical grade polyetherimide (PEI) resin (Ultem 2300; Ritter GmbH, Schwabmunchen, Germany). This material was selected for its corrosion resistance, dielectric insulation, and high strength to weight ratio[30]. The inner diameter of the cylindrical body was 8.36 mm, allowing the custom electronics to fit. On either end of the body the last 2.5 mm had recessed threading to allow custom-made caps to be tightly screwed into place. The main constraint on the size of the capsule was the presence of embedded electronics, as well as the need for gastric retentive polymers surrounding the capsule but within a dissolvable pill body. The custom caps were made of machined copper that was threaded to closely fit the body in order to be able to seal the contents of the pill. The inside surface of the copper caps was soldered to thin wires connected to output vias on the circuit board before being screwed together. Particular care was taken to ensure that body fluids did not enter the capsule; the two caps were screwed onto the shell with a layer of ultra-thin polytetrafluoroethylene (PTFE) film in order to provide high quality waterproof seal between threads. Figure 1 shows the full design of the internal circuitry and body of the enhanced EGG (EEGG) capsule.
Gastric retention: In order to provide long term gastric motility measurements and prevent the expulsion of the EEGG pill into the duodenum, a gastric retentive enclosure was incorporated into the prototype. The oscillating EEGG capsule was embedded in 0.15 g of dry, non-toxic, hydrophilic, crosslinked polyacrylate polymer granules contained inside a nonwoven, high permeability 20-gsm polyvinyl alcohol (PVA) mesh. The polymer granules are able to immediately absorb and retain hundreds of times their weight in water, and swell to 30-50 times their dry size in gastric liquids if accompanied by the concurrent intake of strong antacid. Once the polymers have swelled they are unable to dissolve due to the three dimensional crosslinked structure[30], making them an ideal choice for gastric retention when held in place by a permeable mesh structure. The PVA mesh was designed to ensure that upon expansion the whole structure would exceed 1.5 cm in any direction, but not be larger than 4 cm in any direction, with a fully expanded volume between 20 to 30 mL, which is well below the threshold of perception[31]. When pressure is applied the polymer granules linearly retain less water, allowing a certain degree of compliance of the structure to the mechanical activity of the stomach. The mesh was also chosen due to its permeability to fluids, which enable the polymer granules to make adequate contact with the gastric juices. The 40-degree PVA mesh is biodegradable, and will disintegrate within 2-3 d in order to avoid obstruction, and similar gastric retentive technologies have been demonstrated to withstand digestive antral pressures, and produce no adverse mucosal impact or evacuation obstruction issues[25]. The PVA mesh can be immediately disintegrated on demand via the consumption of a glass of hot (> 40 °C) water. Obviously, in human testing, the patients should be advised not to drink hot (> 36 °C) beverages for the duration of the test, unless they need to terminate it.
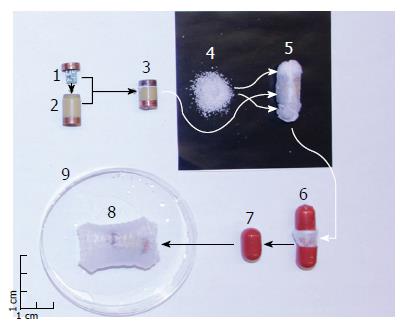
Encapsulation: In order to orally deliver the EEGG capsule and gastric retentive enclosure to the stomach the prototype models were encased in a split hard gelatin shell capsule (AAA DB capsule, Capsugel, Greenwood, SC, United States). The size of the gelatin capsule directly affected how much expanding polymer could be integrated into the design, as the size of the inner EEGG capsule was determined from the internal electronics. For the present feasibility study the emphasis was on demonstrating the effectiveness of the electronics. Figure 2 presents an exploded image of the pill, as well as the order of assembly.
Experiments were performed on eight mongrel dogs (6F, 4M, 23.8 ± 3.3 kg). Four were administered an active EEGG capsule, while the rest were given a deactivated (battery removed) capsule. The dogs were vaccinated and dewormed as per the Canadian Veterinary Medical Association’s recommended yearly protocol regime. Vaccines included Canine distemper/adenovirus, Type 2 Parvovirus/Bordetella/Rabies. Drontal Plus (Bayer HealthCare LLC, Shawnee Mission, KS, United States) was used as an oral dewormer. Each animal underwent a physical examination by a board-certified veterinarian and was found to be in good condition. After 24 h of fasting and 12 h of water deprivation, each animal transorally ingested a single capsule as described above with 500 cc of room temperature water (21.0 °C). The pill swelled to its maximum size in the stomach within 15 min after ingestion to dimensions exceeding 1.5 cm in any direction, and subsequently was unable to pass the pyloric sphincter even when subjected to pharmacologically induced propulsive peristalsis.
Each animal underwent an induction with an intravenous injection of thiopental (Thiotal 15 mg/kg IV, Vetoquinol Canada, Lavaltrie, QC, Canada) and was subsequently maintained on inhalant isoflurane and oxygen (Halocarbon Laboratories, River Edge, NJ, United States) with a vaporizer setting of 1%-3%. The anesthesia was chosen because it did not influence gastric neurotransmitters, and as such would not affect gastric contractions[32]. Individually, the animals were then positioned supine, their abdomens shaved, cleaned, and sterilized with alcohol before performing laparotomy via a median incision vertically along the linea alba to gain access to the stomach.
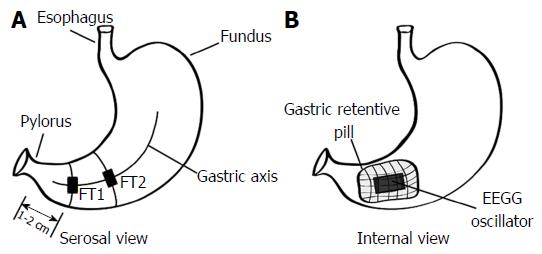
After the incision the location of the ingested pill in the stomach was verified endoscopically using an EPK-700 veterinary endoscope (Pentax, Tokyo, Japan), and the voltage developed on the serosa of the stomach was measured using an oscilloscope (Tektronix, Beaverton, OR, United States) to confirm the presence of an activated or deactivated pill. After this verification, two 90W24 force transducers (RB Products, Stillwater, MN, United States) were surgically sutured to the serosal side of the antral stomach along the gastric axis[33]. The first force transducer was positioned 1-2 cm from the pylorus, and the second was affixed proximally 5-6 cm from the pylorus, along the gastric axis. The mesenteric innervation and the blood supply of the stomach were carefully preserved. The internal position of the EEGG oscillator and the serosal position of the force transducers is shown in Figure 3.
The signals from the force transducers were amplified using a custom-designed multichannel bridge amplifier, and digitized using a PCMCIA DAQ Card-AL-16XE-50 (National Instruments, Austin, TX, United States). The force transducer signals were monitored and analyzed with custom-designed signal processing and visualization software (GAS-6.2, Biomedical Instrumentation Laboratory, University of Calgary, Calgary, Alberta, Canada). Once the force transducers were in place, their functionality was verified mechanically by manual palpation of variable strength, and the offsets and gains were calibrated accordingly for maximal sensitivity.
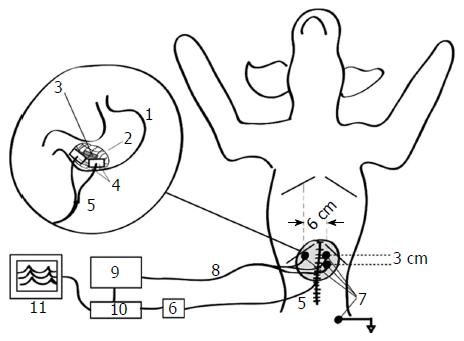
The intragastric position of the pill was then verified mechanically by palpating it to ensure that it had not been compromised during surgery. Following the FT implantations, the abdomen was closed, and after appropriate skin cleaning and preparation, three pediatric ECG electrodes (Conmed, Utica, NY, United States) were placed cutaneously over the stomach along the abdominal projection of the gastric axis, with a ground electrode positioned close to the left hip of the animal[34]. The position of the electrodes was similar to the one associated with impedance epigastrography, since previous studies have suggested optimal electrode placement[35].
The EEGG signals were measured with a custom-designed 16-channel electrogastrograph. The amplification of the electrogastrograph was set to 1000 ×, in order to utilize the range of the analog to digital converter. The cut-off frequencies of the bandpass filter of the custom electrogastrograph were set to the commonly used 0.03-0.1 Hz following the hypothesis that gastric motility signals in the animals will not exceed 6 cycles-per-minute (cpm)[36] and will amplitude-modulate the intraluminal oscillator frequency of 50 kHz, the latter acting only as their carrier. The 0.1-Hz low pass filter would thus act as a demodulator for this transcutaneous signal transmission, and would prevent higher frequency electrophysiological and mechanical processes (e.g., electrocardiographic activity and respiration) from interfering with the signal originating from within the stomach. The signals were then digitized using the same PCMCIA card DAQ Card-AL-16XE-50 (National Instruments, Austin, TX, United States) simultaneously with the force transducer signals, and were subsequently monitored and stored for further analysis using the same custom software. The overall setup of this experiment is shown in Figure 4.
Immediately after the experimental setup was completed, a baseline recording was performed with no pharmacological stimulant for 30 min. Following this recording, bolus neostigmine (0.04 mg/kg, APP Pharmaceuticals, Schaumburg, IL, United States) was administered intravenously as a smooth muscle stimulant to invoke contractions[34]. Thirty minutes of post-neostigmine recordings were subsequently obtained. The total recorded time from each animal was 60 min; 30 min in the basal state, and 30 min in the post-neostigmine state, with a one-minute time interval between them for the intravenous (IV) administration of the bolus neostigmine.
At the end of the experiments the animals were sacrificed by an IV injection of Euthanyl, 480 mg/4.5 kg (Bimeda-MTC Animal Health Inc., Cambridge, ON, Canada). Subsequent retrieval of the expanded pill was performed in order to verify its retention within the stomach, and to confirm the presence of the signal in the active EEGG pills or the lack thereof in the inactive sham pills using an oscilloscope. The post-administration volume of each gastric-retentive pill was measured to quantify expansion dimensions. The study was approved by the Veterinary Sciences Animal Care Committee, University of Calgary, Calgary, Alberta, Canada.
Signal acquisition: The cutaneous biovoltage evoked from the induced EEGG signal was measured via disposable Ag/AgCl ECG electrodes (Cleartrode, Conmed, Utica, NY, United States). Two EEGG channels were bipolarly recorded as the potential differences between two active electrodes measured relative to a common ground electrode placed away from the stomach on the shaved inner thigh of the animal. These bipolar signals were processed to reflect the dynamics of their electrical power, which was hypothesized to correlate highly with contractile activity. Electrodes were placed in a similar configuration to that used in standard EGG recordings (Figure 4)[37].
Signal conditioning: The raw analog signals from the ECG electrodes were amplified and filtered using a custom-designed multichannel electrogastrograph (EGG, James Long Company, Caroga Lake, NY, United States), which measured the voltages relative to the ground electrode using analog gain of 10000. The high pass filtering cut-off frequency was set at 0.03 Hz, while the low pass filtering cut-off frequency was 0.1 Hz.
Signal digitization: After being amplified and filtered, the EEGG signal was digitized using a PCMCIA DAQ Card-AL-16XE-50 (National Instruments, Austin, TX, United States). This analog to digital converter can record 16 bit voltages between 0 and 5 volts at a maximum rate of 200 kS/s. Since the low-pass cut-off frequency of the amplifier was set at 0.1 Hz, a sampling frequency of 10 Hz was chosen for simplicity and to avoid aliasing effects. The resolution of this analog-to-digital converter was found to be 0.076 mV ± 0.038 mV.
Signal processing: The signals were viewed using custom signal processing and visualization software (GAS-6.2, Biomedical Instrumentation Laboratory, University of Calgary, Calgary, Alberta, Canada). This software was designed with EGG in mind, and can visualize up to 16 channels simultaneously in real time. Signals are monitored and stored for further analysis.
After the signals were acquired they were further processed using a custom program developed using Matlab (MathWorks, Natick, MA, United States) that normalized each signal before calculating one-minute motility indices, and computing Pearson correlation coefficients between the input signals. Normalization is done because of the inhomogeneity of the tissues between the EEGG transducer capsule and whichever electrode recorded the signal. As a result, EEGG signals are presented in terms of relative units, where the highest value is represented by a 1 and the lowest by a 0. Normalization was performed according to equation (1.1).
DNorm = (DO - DMin)/(DMax - DMin) (1.1)
where, DNorm is the new normalized data point, [dimensionless]; DO is the original point to be normalized, [dimensionless]; DMin is the minimum data point in the entire set, [dimensionless]; and DMax is the maximum data point in the entire set, [dimensionless].
One-minute motility indices[38] effectively low-pass filter the signals, reducing the effect of erroneous outlying data and presenting the general trend of gastric motility. Motility indices are a convenient way to interpret the impedance dynamics of the tissue because they represent the power of the signal[39], which is independent of pill polarity. This is important because the pill is effectively floating within the stomach, and its orientation cannot be predicted so by measuring the power of the signal we can negate this effect. One-minute motility indices are further advantageous because signals from force transducers and EEGG may be out of phase due to the spatial difference in ECG electrodes. In this case if could appear that little to no correlation in activity was present. Motility indices are calculated by taking the sum of squared data points over the course of a minute. In one minute there were 600 samples, as the sampling frequency was 10 Hz. After motility indices were calculated using equation (1.2) they were again normalized according to equation (3.1). This normalization is indicative of the average power, and can be compared across samples even with varying sample rates.
Math 1
where Mi is the motility index M calculated for minute i, [dimensionless]; and Dj is the normalized data at point j, [dimensionless];
Once the general trends of gastric motility are established by the motility indices, Pearson correlation coefficients can be computed in order to compare the different signals, and provide a metric for validation. Pearson correlation coefficients were chosen for statistical validation because they indicate a measure of linearity between two signals. Pearson correlation coefficients with P < 0.05 were considered to be significant[40].
Further processing of the signals was done to assess the contractions per minute visible in each respective channel. Dominant peaks of the frequency for each measuring modality (basal and post-neostigmine) were subjected to a comprehensive statistical analysis using the paired Student’s t-test[40] to evaluate the relationship between the frequency dynamics of EEGG, sham and force transducer measurements. Frequency analysis was done using the Fast Fourier Transform, implemented in the custom visualization and analysis GAS-6.2 software used previously.
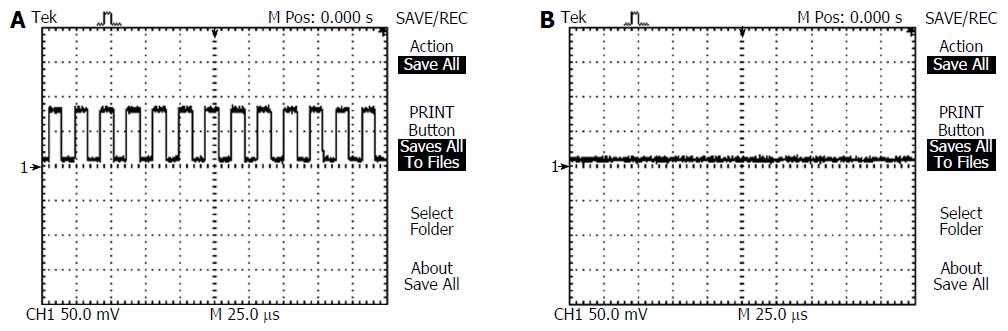
Before the implantation of the force transducers an oscilloscope was used to detect the 50 kHz signal, or lack thereof being emitted by the ingested EEGG capsule. Oscilloscope leads were placed in electrical contact with the serosa of the stomach over the pill, the position of which was approximately determined by manual palpation. A sample oscilloscope recording for an active pill is shown in Figure 5A, while Figure 5B shows a recording of an inactive pill.
The expanded pill was retrieved from each animal after experimentation, in order to verify the pill’s activity using the same oscilloscope used previously, as well as to assess the expansion dimensions and structural integrity of the gastric retentive enclosure during testing. Each experiment revealed that all the EEGG pills remained either active during testing, and did not fail as a result of liquid exposure or battery failure. Conversely, the sham pills remained inactive, as expected. The average post-retrieval volume of the pills was 12.1 ± 0.4 mL, and had dimensions exceeding 1.5 cm in all directions. The presence of the intact pill within the stomach at the conclusion of the experiments indicated that the pharmacologically induced contractions were unable to propel the expanded gastric retentive enclosure into the small intestine, nor were the pressures within the stomach high enough to rupture the gastric retentive enclosure itself.
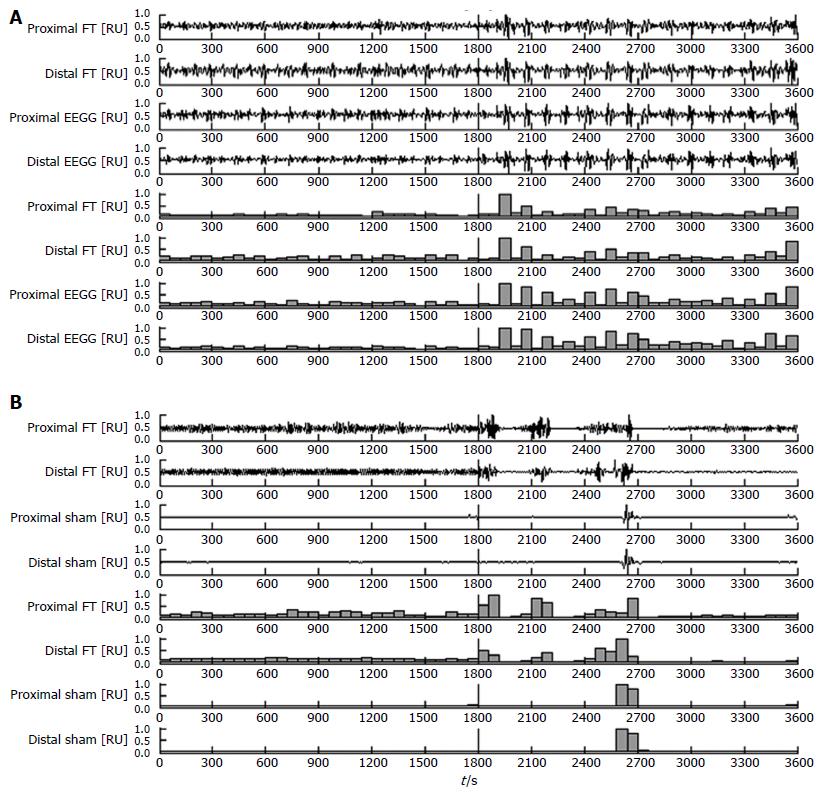
The testing was divided into two sections, baseline and post-neostigmine, and results were recorded and labelled accordingly. During the baseline tests there was sporadic presence of spontaneous contractile activity as measured by the force transducers, and evidenced in the visual comparisons of Figure 6A and B. Following the introduction of neostigmine several gastric motility factors dramatically increased, notably the frequency, regularity, and amplitudes of contractile activity. A typical simultaneous force transducer and EEGG recording for an active capsule is shown in Figure 6A, along with the post processed one-minute motility indices in parallel. Thirty minutes of baseline activity and thirty minutes of post-neostigmine activity was recorded and normalized together, in order to put each recording into perspective with respect to the maximum and minimum of both states combined. In both states there were statistically significant (P < 0.01) correlations demonstrated between the one-minute motility indices of EEGG and force transducer signals. The more distal or proximal cutaneous electrode combinations were compared to their corresponding force transducer. In the case of the battery removed sham pill the results showed no significant correlations between respective motility indices. Another typical simultaneous recording is shown in Figure 6B, for the case of the sham pill. Table 1 summarizes the Pearson correlation coefficients of the one-minute gastric motility indices, divided into baseline or post-neostigmine, and active or sham capsule.
| Modality | State | PCCs proximal FT-proximal | P value | PCCs distal FT-distal | P value |
| Cutaneous GMIs | Cutaneous GMIs | ||||
| EEGG capsule | Baseline | 0.763 ± 0.20 | < 0.01 | 0.674 ± 0.47 | < 0.01 |
| After neostigmine | 0.731 ± 0.12 | < 0.01 | 0.734 ± 0.14 | < 0.01 | |
| Sham capsule | Baseline | 0.160 ± 0.03 | > 0.10 | 0.071 ± 0.02 | > 0.10 |
| After neostigmine | 0.113 ± 0.09 | > 0.10 | 0.051 ± 0.03 | > 0.10 |
Frequency spectral analysis was performed in order to assess the contractions per minute of each signal. As in the case of Pearson correlation coefficients distal and proximal EEGG recordings were compared to the corresponding force transducer. The dominant frequency peaks revealed statistically significant relationships between EEGG and force transducer recordings. The sham study revealed substantial dissociation between the dominant frequency peaks, particularly during the baseline period in which the limited spontaneous gastric activity was sporadic and irregular. Accordingly, there was no significant relationship between the sham study (which can be effectively be considered a standard EGG study) and the force transducers. The averaged values of the dominant peaks in the frequency spectra (0.03-0.1 Hz) are presented in Table 2.
| Modality | State | Channel | cpm |
| EEGG capsule | Baseline | FT | 2.38 ± 1.20 |
| EEGG | 2.42 ± 1.27 | ||
| After neostigmine | FT | 3.55 ± 0.94 | |
| EEGG | 3.58 ± 0.95 | ||
| Sham capsule | Baseline | FT | 2.65 ± 1.15 |
| Sham | 3.94 ± 1.67 | ||
| After neostigmine | FT | 3.84 ± 0.91 | |
| Sham | 4.12 ± 1.56 |
Comparative statistical evaluation of the frequency dynamics of the dominant spectral peaks is presented in Table 3.
The history of cutaneous recordings of human GEA spans almost one full century[1]. However, in contrast to its extremely successful twin sister, electrocardiography (ECG)[19], which became a pivotal clinical tool that immensely enhanced the frontiers of cardiology, EGG, by any objective account, is a failure. The explanation of this failure can easily be sought in various different directions, ranging from the lack of critical, life-saving diagnostic importance, to the lack of scientific consensus in identifying dyspepsia and gastroparesis as clear-cut gastric motility disorders, to the lack of attention of brilliant enough academics and scholars, to a bad luck due to numerous misrepresenting and over-exaggerated EGG-related claims, etc. In our opinion, all these reasons cannot be regarded as serious. In our view, the deep reason for the lack of any clinical utility in the present-day EGG testing is rooted in the nature of the gastric electromagnetic field[17,41,42]. In contrast to the electromagnetic field of the heart[43], its gastric counterpart can be modeled with a dynamically moving annular cylindrical band of electrical dipoles pointing to the center, towards the gastric axis[17,42]. This is a special type of electromagnetic field that presents a highly integrated signature when its electrical potentials are recorded in its outer vicinity. Add to that the humble amplitude range of these biopotentials, their infralow frequency nature, and their secondary integration over the surface area of the recording cutaneous electrodes, and what emerges is the hypothesis that it is the very nature of the gastric electromagnetic field that objectively limits traditional present-day EGG. So, what can we do to revive EGG testing and breathe a new life in its promise as a single diagnostic test for dyspepsia and/or gastroparesis?
Our proposal is simple - if the nature of the gastric electromagnetic field is prohibitive to any meaningful cutaneous, non-invasive EGG testing of gastric motility, we create our own electromagnetic field inside the stomach, which can do the job in a far more meaningful and reliable way. In the present article we present one such possibility - an ingestible, temporary, controllable pseudobezoar containing a point source of a 50-kHz electromagnetic field. Upon swallowing, the structure would swell in the stomach to a size prohibiting its expulsion through the pylorus for at least 48 h. Supplied by a powerful enough battery, this pseudobezoar-based, gastric-retentive electromagnetic oscillator overshadows the spontaneously existing GEA in a powerful, yet meaningful fashion. In fact, canine testing revealed that the cutaneous recordings of this new electromagnetic field reflect gastric contractions in a very reliable fashion. Subsequently, when the battery supplying the electronic oscillator in the pseudobezoar exhausts itself, the structure disintegrates into its constituent fibers (with the encapsulated electronic oscillator and its supplying battery being only several millimeters in diameter), which leave the gastrointestinal system in a natural way. An additional safety feature can be also integrated within the pseudobezoar, allowing its disintegration at any time prior to its self-disintegration, based on ingesting strong antacids, or drinking hot (> 45 °C) liquids.
As with any innovative idea, a lot more needs to be done before this radically new approach in the non-invasive ambulatory assessment of gastric motility becomes a reliable clinical tool for diagnosing gastric dyspepsia and/or gastroparesis. First and foremost, the clinical community should clearly defend and loudly support the need for such a single, non-invasive and inexpensive test. Second, controlled clinical trials on humans should take place in order to explicitly show the real diagnostic value of such testing, including its sensitivity and specificity. Third, the existing EGG insurance codes should be revisited so that the routine EGG is replaced by EEGG and it enters the clinical mainstream, rather than remain forever “a research tool” of little consequence, administered free of charge only now and again and here and there by curious investigators.
In conclusion, EEGG is a new modality to record reliably and non-invasively gastric motility utilizing the same recording setup used in present-day plain EGG. Its clinical utilization promises to revive a non-invasive gastric testing that is fading in oblivion.
Cutaneous recordings of gastric electrical activity, known as electrogastrography (EGG) could not find any reliable clinical applicability for almost a century.
Although a promising non-invasive test to possibly diagnose gastric dyspepsia and/or gastroparesis, EGG is still a research tool with no clinical utilization.
In this study, it is proposed to preserve the overall EGG test protocol and standard equipment but provide enhanced patient preparation by swallowing a self-expandable, self-disintegratable pseudobesoar capsule containing a miniature electronic oscillator.
The proposed enhanced electrogastrography method can be utilized for the early diagnosis of functional dyspepsia and/or gastroparesis.
ECG is so limited in diagnosing for its lack of clinical utility. The innovative method for EGG recording introduced in this paper, which leave the gastrointestinal system in a natural way, is non-invasive, reliable and long-hour ambulatory for gastric motility monitoring. It would become a promising clinical tool for diagnosing gastric motility disorder.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arya V, Liu MJ, Nicodeme F S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Alvarez W. The electrogastrogram and what it shows. JAMA. 1922;78:1116-1119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 220] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Parkman HP, Orr WC. The gastrointestinal motility laboratory. Gastroenterol Clin North Am. 2007;36:515-529, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Lin Z, Eaker EY, Sarosiek I, McCallum RW. Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. Am J Gastroenterol. 1999;94:2384-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | van der Voort IR, Osmanoglou E, Seybold M, Heymann-Mönnikes I, Tebbe J, Wiedenmann B, Klapp BF, Mönnikes H. Electrogastrography as a diagnostic tool for delayed gastric emptying in functional dyspepsia and irritable bowel syndrome. Neurogastroenterol Motil. 2003;15:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Lacy BE, Talley NJ, Locke GR 3rd, Bouras EP, DiBaise JK, El-Serag HB, Abraham BP, Howden CW, Moayyedi P, Prather C. Review article: current treatment options and management of functional dyspepsia. Aliment Pharmacol Ther. 2012;36:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, Crowell MD, Keefer L, Lembo AJ, Parkman HP, Rao SS. Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. Gastroenterology. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 441] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 7. | Kelly KA. Gastric emptying of liquids and solids: roles of proximal and distal stomach. Am J Physiol. 1980;239:G71-G76. [PubMed] |
| 8. | Sarnelli G, Caenepeel P, Geypens B, Janssens J, Tack J. Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol. 2003;98:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Bredenoord AJ, Tutuian R, Smout AJ, Castell DO. Technology review: Esophageal impedance monitoring. Am J Gastroenterol. 2007;102:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Talley NJ, Ford AC. Functional Dyspepsia. N Engl J Med. 2015;373:1853-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 315] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 11. | Yarandi SS, Christie J. Functional Dyspepsia in Review: Pathophysiology and Challenges in the Diagnosis and Management due to Coexisting Gastroesophageal Reflux Disease and Irritable Bowel Syndrome. Gastroenterol Res Pract. 2013;2013:351086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Smout AJ, van der Schee EJ, Grashuis JL. What is measured in electrogastrography? Dig Dis Sci. 1980;25:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 248] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 13. | Randall CW, Zaga-Galante J, Vergara-Suarez A, and Taboada CM. Utilizing Electrogastrography in the Evaluation of Idiopathic Dyspepsia. J Med Diagn Methods. 2014;2014. |
| 14. | Yin J, Chen JD. Electrogastrography: methodology, validation and applications. J Neurogastroenterol Motil. 2013;19:5-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Sha W, Pasricha PJ, Chen JD. Correlations among electrogastrogram, gastric dysmotility, and duodenal dysmotility in patients with functional dyspepsia. J Clin Gastroenterol. 2009;43:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Verhagen MA, Van Schelven LJ, Samsom M, Smout AJ. Pitfalls in the analysis of electrogastrographic recordings. Gastroenterology. 1999;117:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Mirizzi N, Stella R, Scafoglieri U. Model to simulate the gastric electrical control and response activity on the stomach wall and on the abdominal surface. Med Biol Eng Comput. 1986;24:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Couturier D, Rozé C, Paolaggi J, Debray C. Electrical activity of the normal human stomach. A comparative study of recordings obtained from the serosal and mucosal sides. Am J Dig Dis. 1972;17:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Coblentz B, Harvey RM. The relationship between electrical and mechanical events in the cardiac cycle of man. Br Heart J. 1949;11:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Botoman VA, Rao S, Dunlap P, Abell T, Falk GW; Bill Codying and RVS Committee, American Motility Society. Motility and GI function studies billing and coding guidelines: a position paper of the American Motility Society. Am J Gastroenterol. 2003;98:1228-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Bortolotti M. Electrogastrography: a seductive promise, only partially kept. Am J Gastroenterol. 1998;93:1791-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Kloetzer L, Chey WD, McCallum RW, Koch KL, Wo JM, Sitrin M, Katz LA, Lackner JM, Parkman HP, Wilding GE. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil. 2010;22:527-533, e117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Szarka LA, Camilleri M. Methods for measurement of gastric motility. Am J Physiol Gastrointest Liver Physiol. 2009;296:G461-G475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 237] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Deneva MG, Yadid-Pecht O, Fattouche M, Mintchev MP. Utilization of Temporary Controllable Intragastric Pseudobezoars for the Treatment of Obesity. Curr Obesity Rep. 2012;1:68-74. [DOI] [Full Text] |
| 26. | Arriagada AJ, Jurkov AS, Neshev E, Muench G, Andrews CN, Mintchev MP. Design, implementation and testing of an implantable impedance-based feedback-controlled neural gastric stimulator. Physiol Meas. 2011;32:1103-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Zarowitz BJ, Peterson EL, Robert S. Characterization of drug disposition and dosing using bioelectrical impedance. Med Prog Technol. 1993;19:193-198. [PubMed] |
| 28. | Poscente MD, Yadid-Pecht O, Andrews CN, Mintchev MP. Device and method for monitoring internal organs,” US20160206200 A1, 21-Jul-2016. . |
| 29. | Shim HB, Hwang JJ, Kim KS, Seo YD, Kim BH, Lee YW, Cha C, Baek BK. Endoscope and a method for finding its location. US20100168517 A1, 01-Jul-2010. . |
| 30. | Liu M, Guo T. Preparation and swelling properties of crosslinked sodium polyacrylate. J Appl Polym Sci. 2001;82:1515-1520. [RCA] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 762] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 32. | Hall JA, Dunlop CI, Solie TN, Hodgson DS, Twedt DC. Gastric myoelectric and motor activity in dogs after isoflurane anesthesia. Vet Surg. 1995;24:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Reinke DA, Rosenbaum AH, Bennett DR. Patterns of dog gastrointestinal contractile activity monitored in vivo with extraluminal force transducers. Am J Dig Dis. 1967;12:113-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 64] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Poscente MD, Wang G, Filip D, Ninova P, Muench G, Yadid-Pecht O, Mintchev MP, Andrews CN. Transcutaneous intraluminal impedance measurement for minimally invasive monitoring of gastric motility: validation in acute canine models. Gastroenterol Res Pract. 2014;2014:691532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Kee WC, Kingma YJ, Mintchev MP, Bowes KL. Optimal placement of impedance epigastrography electrodes. Ann Biomed Eng. 1996;24:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Kelly KA, Code CF, Elveback LR. Patterns of canine gastric electrical activity. Am J Physiol. 1969;217:461-470. [PubMed] |
| 37. | Pfaffenbach B, Adamek RJ, Kuhn K, Wegener M. Electrogastrography in healthy subjects. Evaluation of normal values, influence of age and gender. Dig Dis Sci. 1995;40:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Stemper TJ. Gastric emptying and its relationship to antral contractile activity. Gastroenterology. 1975;69:649-653. [PubMed] |
| 39. | Dive A, Miesse C, Galanti L, Jamart J, Evrard P, Gonzalez M, Installé E. Effect of erythromycin on gastric motility in mechanically ventilated critically ill patients: a double-blind, randomized, placebo-controlled study. Crit Care Med. 1995;23:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Snedecor GW, Cochran WG. In: Statistical Methods. The Iowa State University Press, Ames, IA, United States, 1967: 258-380. . |
| 41. | Buist ML, Cheng LK, Sanders KM, Pullan AJ. Multiscale modelling of human gastric electric activity: can the electrogastrogram detect functional electrical uncoupling? Exp Physiol. 2006;91:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Mintchev MP, Bowes KL. Conoidal dipole model of electrical field produced by the human stomach. Med Biol Eng Comput. 1995;33:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Barbuti A, Baruscotti M, DiFrancesco D. The pacemaker current: from basics to the clinics. J Cardiovasc Electrophysiol. 2007;18:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |