Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4480
Peer-review started: August 3, 2016
First decision: September 5, 2016
Revised: September 30, 2016
Accepted: October 31, 2016
Article in press: October 31, 2016
Published online: July 7, 2017
Processing time: 338 Days and 11.9 Hours
Colorectal cancer (CRC) is one of the most common human cancers and the cause of about 700000 deaths per year worldwide. Deregulation of the WNT/β-catenin pathway is a key event in CRC initiation. This pathway interacts with other nuclear signaling pathways, including members of the nuclear receptor superfamily and their transcription coregulators. In this review, we provide an overview of the literature dealing with the main coactivators (NCoA-1 to 3, NCoA-6, PGC1-α, p300, CREBBP and MED1) and corepressors (N-CoR1 and 2, NRIP1 and MTA1) of nuclear receptors and summarize their links with the WNT/β-catenin signaling cascade, their expression in CRC and their role in intestinal physiopathology.
Core tip: Colorectal cancer (CRC) is one of the most common human cancers worldwide. Deregulation of the WNT/β-catenin pathway is a key event in CRC initiation. This pathway interacts with other nuclear signaling pathways, including members of the nuclear receptor superfamily and their transcription coregulators. In this review, we provide an overview of the literature dealing with the main coactivators and corepressors of nuclear receptors, and summarize their links with the WNT/β-catenin signaling cascade, their expression in CRC and their role in intestinal physiopathology.
- Citation: Triki M, Lapierre M, Cavailles V, Mokdad-Gargouri R. Expression and role of nuclear receptor coregulators in colorectal cancer. World J Gastroenterol 2017; 23(25): 4480-4490
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4480.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4480
Colorectal cancer (CRC) is the third most common human cancer with more than 1.3 million recorded cases in 2012. Patients are generally treated with surgery, chemotherapy and radiotherapy that are associated with several side effects. Despite important advances in CRC prognosis, diagnosis and treatment during the last decade, it remains the cause of about 700000 deaths per year worldwide[1]. Although CRC can be sporadic or hereditary, in both cases, environmental factors contribute to its development. The main risk factor is age; however, diet, sedentary lifestyle and tobacco smoking also increase the risk of developing CRC[2].
CRCs are complex and heterogeneous epithelial tumors that involve various genetic and epigenetic alterations. The progressive accumulation of these molecular changes contributes to the transformation of normal mucosa into neoplasia[3]. At least three molecular pathways have been identified as involved in CRC pathogenesis[4]. The most common is the chromosomal instability (CIN) pathway, characterized by inactivation of tumor suppressor genes (APC, TP53, SMAD4) and activation of oncogenes (KRAS)[5]. The hallmark of the second molecular pathway is microsatellite instability (MSI) that results from the deregulation of DNA mismatch repair (MMR) genes, leading to genetic hypermutability[6]. Finally, the third molecular mechanism involves gene silencing via aberrant hypermethylation of promoter CpG islands (CpG island methylator phenotype, or CIMP)[7]. A recent study has proposed a unique molecular classification of CRC based on gene expression with four consensus molecular subtypes (CMS): MSI immune (CMS1), canonical (CMS2), metabolic (CMS3) and mesenchymal (CMS4)[8].
Large-scale investigations have identified several critical genes and multiple signaling pathways that are important for CRC initiation and progression, including WNT, Notch, p53, RAS-MAPK, PI3K and TGF-β. The WNT/β-catenin signaling pathway is the most studied in CRC[9]. In normal intestinal cells, WNT expression is detected in cells at the crypt base and is essential for the maintenance of stem cell compartmentalization and, ultimately, for the intestinal tract organization and patterning. The WNT canonical pathway is activated upon binding of the WNT ligands to the Frizzled/LRP receptor. This interaction induces a signaling cascade, leading to the stabilization of β-catenin in the cytoplasm and to its translocation into the nucleus. There, β-catenin interacts with the TCF/LEF transcription factors to modulate specific target genes that are involved in cell proliferation and differentiation, such as MYC, which encodes the Myc proto-oncogene, and CCND1, which encodes the cyclin D1 protein. In the absence of WNT ligands, β-catenin is targeted for proteasomal degradation upon phosphorylation through its association with a destruction complex composed of various scaffold proteins, such as adenomatous polyposis coli (APC), Axin 2, glycogen synthase kinase 3 (GSK3) and casein kinase 1 (CK1). Approximately 70%-80% of sporadic CRCs involve somatic mutations that inactivate APC, leading to β-catenin accumulation and activation of its target genes.
Nuclear receptors (NRs) are ligand-activated transcription factors that directly regulate genes and are involved in several physiological processes, such as growth, homeostasis, differentiation, development and metabolism[10]. NR signaling dysregulation contributes to various human diseases, including cancer[11]. Currently, NRs represent one of the largest families of transcription factors (48 members in humans) that can be classified in three groups (hormone, metabolic and orphan NRs), based on their ligand properties[10,12]. Members of the hormone receptor subfamily generally bind to DNA as homodimers and include estrogen (ERα and ERβ), androgen (AR), progesterone (PR), glucocorticoid (GR) and mineralocorticoid receptors (MR). Metabolic receptors, such as farnesoid X (FXR), liver X (LXR) and peroxisome proliferator-activated receptors (PPARs), bind to DNA as heterodimers with their obligate partner retinoid X receptor (RXR). Orphan receptors include all NRs for which ligands were (or are still) unknown[10]. NRs share a modular structure composed of an N-terminal activation domain, which is important for interactions with coregulators (activators and repressors), a DNA-binding domain, a small hinge region and a C-terminal ligand binding domain that interacts with the ligands and transcriptional coregulators[13].
NRs promote gene transcription mainly through classical gene transactivation and transrepression. These activities are modulated by transcriptional coregulators that allow NRs to target gene promoters and to coordinate transcription. Additionally, NRs can modulate also other nuclear signaling pathways that rely on various transcription factors, such as SP1, AP-1, p53 or NF-κB[14-17]. Moreover, NR activities can be influenced by post-translational modifications whereby they are targeted by other cellular pathways, leading, for instance, to activation of kinases in response to growth factors. Recent investigations have highlighted the role of some NRs, such as ER, PPAR, nuclear vitamin D receptor (VDR), RXR and retinoic acid receptor (RAR), in the regulation of intestinal cell growth and differentiation and also in CRC.
Various NRs regulate the cell cycle, proliferation and apoptosis of intestinal epithelial cells and are now considered as factors that might also modulate CRC development and progression[18]. The characterization of NR localization and expression in normal and tumoral gut epithelium led to a better understanding of their potential role in CRC. On the basis of their expression profile in the normal intestinal epithelium, NRs can be divided in three subgroups. Some NRs, such as NR5A2 (LRH1), PPARβ/δ and thyroid hormone receptor alpha (TRα), are detected mostly in the proliferative compartment of the crypt, suggesting that they might be involved in cell proliferation regulation. Other NRs (including VDR, ERβ, GR, MR and FXRα) are mostly expressed in the differentiated compartment of the intestinal epithelium. The last group includes the NRs LXRβ, PPARα, RARα and RXRβ that are ubiquitously expressed in the intestinal mucosal epithelium[19].
Differently from what observed in the normal intestine, the expression of most NRs is downregulated in patients with familial adenomatous polyposis (FAP) and in ApcMin/+ mice (an animal model of CRC), while only few are upregulated[20]. For instance, loss of ERβ and VDR in mice results in colon cell hyper-proliferation[21,22]. On the other hand, the expression of other NRs, such as EAR-2, is induced in CRC models and is downregulated in HT29 colon cancer cells upon restoration of the activity of wild type APC[19]. In both cases, variations in NR expression/activity seem to be directly related to the WNT signaling pathway and indeed, many NRs cross-talk with the WNT signaling pathway[18].
The canonical WNT/β-catenin pathway is one of the major signaling pathways involved in the establishment of intestinal homeostasis. WNT signaling is fundamental for the maintenance of the intestine proliferative compartment[23]. Components of the WNT pathway can modulate NR function through transcriptional activation or repression[18]. Similarly, NRs can exert dynamic changes in the WNT signaling pathway. For instance, it has been reported that after association with β-catenin, NR5A2 (LRH1) is activated to promote CCND1 transcriptional activation and governs the self-renewal of intestinal crypt cells. Consequently, proliferation of epithelial cells is enhanced, contributing to CRC development[24]. By contrast, β-catenin activity is repressed when associated with VDR, RAR and AR[25-27]. Moreover, components of the WNT pathway can interact with PPARβ/σ. Specifically, PPARβ/σ levels increase in CRC in response to inactivation of the APC gene or after treatment with the potent carcinogen azoxymethane[28]. This suggests that loss of APC expression leads to increased PPARβ/σ expression through the β-catenin/TCF-4 transcriptional pathway[29].
Besides the WNT pathway, NR cross-talk with the Notch pathway that plays a role in both intestinal development and cancer[30,31]. For instance, TRα1 controls several components of the Notch pathway[32,33]. Deciphering the mechanisms underlying NR cross-talk with different signaling pathways could eventually lead to the identification of targets for clinical interventions in CRC and for diagnostic/prognostic purposes.
The expression of NR target genes is modulated by a large set of transcription coregulators that can act as NR activators and repressors. This review focuses on several of these transcription factors (Table 1) that may play a role in intestinal homeostasis and tumorigenesis.
| Name | Description and aliases | Gene ID | MIM | Location |
| NCOA1 | Nuclear receptor coactivator 1 | 8648 | 602691 | 2p23.3 |
| F-SRC-1, KAT13A, RIP160, SRC1, bHLHe42, bHLHe74 | ||||
| NCOA2 | Nuclear receptor coactivator 2 | 10499 | 601993 | 8q13.3 |
| GRIP1, KAT13C, NCoA-2, SRC2, TIF2, bHLHe75 | ||||
| NCOA3 | Nuclear receptor coactivator 3 | 8202 | 601937 | 20q13.12 |
| ACTR, AIB-1, AIB1, CAGH16, CTG26, KAT13B, RAC3, SRC-3, SRC3, TNRC14, TNRC16, TRAM-1, bHLHe42, pCIP | ||||
| NCOA6 | Nuclear receptor coactivator 6 | 23054 | 605299 | 20q11.22 |
| AIB3, ASC2, NRC, PRIP, RAP250, TRBP | ||||
| PPARGC1A | PPAR gamma, coactivator 1 alpha | 10891 | 604517 | 4p15.2 |
| LEM6, PGC-1 (alpha), PGC-1v, PGC1, PGC1A, PPARGC1 | ||||
| EP300 | E1A binding protein p300 | 2033 | 602700 | 22q13.2 |
| histone acetyltransferase p300", KAT3B, p300 | ||||
| CREBBP | CREB-binding protein | 1387 | 600140 | 16p13.3 |
| CREBBP, KAT3A, RTS | ||||
| MED1 | Mediator of RNA polymerase II transcription subunit 1 | 5469 | 604311 | 17q12 |
| TRIP2, MED1, PBP, PPARBP, PPARGBP, CRSP1, CRSP200, DRIP230, RB18A | ||||
| NCOR1 | Nuclear receptor corepressor 1 | 9611 | 600849 | 17p12-p11 |
| N-CoR, N-CoR1, PPP1R109, TRAC1, hN-CoR | ||||
| NCOR2 | Nuclear receptor corepressor 2 | 9612 | 600848 | 12q24.31 |
| CTG26, N-CoR2, SMAP270, SMRT, SMRTE, SMRTE-tau, TNRC14, TRAC, TRAC-1, TRAC1 | ||||
| NRIP1 | Nuclear receptor interacting protein 1 | 8204 | 602490 | 21q11.2-q21.1 |
| RIP140 | ||||
| MTA1 | Metastasis associated 1 | 9112 | 603526 | 14q32.33 |
Nuclear receptor coactivator 1 (NCoA-1), also known as SRC-1/RIP160, was the first identified member of the SRC family. It belongs to the structurally homologous p160 family of coactivators[34]. This family includes three members (SRC-1, -2 and -3) characterized by the presence of three distinct structural domains: (1) the bHLH-PAS domain that facilitates the interaction with several transcription factors; (2) the central NR-interacting domain; and (3) two C-terminal activation domains[35]. Several NRs are regulated by NCoA-1, including PR, GR, ERα, TR, RXR and PPARγ[34,36,37]. Interestingly, GST pull-down and coimmunoprecipitation assays showed that NCoA-1 binds directly to β-catenin, the key mediator of the canonical WNT signaling pathway[38,39]. The bHLH-S/T domain (amino acids 1 to 580) and the domain required for NCoA-1 histone acetyltransferase (HAT) activity (amino acids 1080 to 1442) appear to be involved in this interaction, together with the Arm3-10 domains of β-catenin (amino acids 234 to 585). Upon binding to β-catenin, NCoA-1 specifically enhances β-catenin transactivation activity, as indicated by transient transfection experiments using the TOP-Flash reporter plasmid[39]. It should be mentioned that coactivator-associated arginine methyltransferase 1 (CARM1), which is associated with the different p160 family members and participates in their coactivator function, also can bind to β-catenin and increase TCF-4 mediated transactivation[40].
In the colon mucosa, NCOA1 expression is confined mostly to the crypts[41]. Although, the NCOA1 gene (with NCOA2) was identified by a ChIP-seq based genome-wide analysis as a possible TCF-4 target in SW620 CRC cells[42], to our knowledge, no other study reported data on NCOA1 expression and role in CRC.
NCoA-2, also referred to as TIF2/GRIP-1/SRC-2, was identified soon after the discovery of NCoA-1[43]. NCoA-2 is involved in mammary gland morphogenesis, energy balance and lipid metabolism[44,45]. Indeed, the NCOA2 gene is expressed in many tissues[43,46], including colon[47]. Conversely, there are discrepancies about NCoA-2 expression in CRC. Indeed, by immunohistochemistry, Grivas et al[48] found that NCoA-2 expression is significantly higher in carcinoma than in normal colorectal tissues. Moreover, they reported that NCoA-2 overexpression is associated with more advanced disease. Conversely, two other studies suggested that NCoA-2 expression (both mRNA and protein) is downregulated in cancer tissues compared with the adjacent normal mucosa[47,49].
In agreement, NCOA2 knock-down using siRNA in normal colonocytes (NCM460 cells) promotes cell proliferation and reduces apoptosis[49]. Previous studies also reported that NCoA-2 can bind to β-catenin[38,50]. Yu et al[49] confirmed these data by demonstrating that NCoA-2 exerts an inhibitory effect on the WNT signaling pathway. Altogether, these findings suggest a potential tumor-suppressor activity of NCoA-2 in CRC.
NCoA-3, also known as amplified in breast cancer 1 (AIB1) or SRC-3/RAC3/ACTR/TRAM1, is the third member of the p160 family of NR transcriptional coactivators. It was first described in breast adenocarcinoma where it is amplified and strongly expressed[51]. Amplifications in the 20q11-13 region that includes the NCOA3 gene are detected in 10% to 32% of CRC[52,53]. Moreover, the NCoA-3 protein is overexpressed in 35% of human CRC samples[53]. Interestingly, NCoA-3 overexpression does not always correlate with gene amplification, suggesting that it might also be regulated by other molecular mechanisms, such as post-translational modifications[51,54]. Furthermore, NCoA-3 overexpression has been correlated with clinicopathological features, such as advanced clinical stage, lymph node and liver metastases[53,54]. However, NCoA-3 overexpression has been also associated with lower risk of death (43.5% vs 19.3%) and prolonged overall survival[48]. In support of these observations, comparison of normal intestine and CRC cell lines showed that NCoA-3 expression is significantly higher in CRC cell lines. Furthermore, NCOA3 knock-down decreases the proliferation of RKO, HCT116 and CT26 cells and the ability of CT26 cells to form tumors after grafting in BALB/c mice[55]. Few reports have investigated NCoA-3 interactions with components of other pathways that have a critical role in CRC. Xie et al[53] associated NCOA3 molecular abundance with inhibition of the p53 pathway. Moreover, Mo et al[55] demonstrated that NCoA-3 directly interacts with Notch intracellular domain and is recruited to the HES1 promoter to enhance Notch signaling and, subsequently, CRC cell proliferation. Collectively, these findings suggest that NCoA-3 might be a potential target for CRC treatment.
NCoA-6, also referred to as NRC, ASC-2, TRBP, PRIP and RAP250, was originally isolated as a ligand-dependent NR-interacting protein[56]. The NCOA6 gene is amplified and overexpressed in breast, colon and lung cancer[57]. Recently, NCoA-6 has emerged as an important coactivator not only of NRs, but also of a number of other well-known transcription factors involved in CRC, such as c-Jun and p53[58].
PPARGC1A encodes a transcriptional coactivator (PGC1-α) that regulates mitochondrial biogenesis and function[59]. PGC1-α enhances the activity of PPARγ[60] and also of other members of the NR superfamily (e.g., RXR, FXR and RAR) that are involved in the modulation of intestinal cell differentiation and apoptosis[19,61,62]. In the normal intestinal epithelium, PGC1-α is highly expressed in differentiated enterocytes at the surface where cells produce and accumulate reactive oxygen species. Conversely, its expression is reduced in crypts and in colorectal tumors. Indeed, the mRNA levels of PPARGC1A and its target genes are reduced by 70%-90% in colon tumor samples from patients with FAP and from ApcMin/+ mice, compared with the adjacent healthy intestinal mucosa[63].
Furthermore, PGC1-α ectopic expression in CRC cells leads to a reduction of their proliferative rate and to a proapoptotic effect. In agreement, mice that overexpress PGC1-α in the intestinal epithelium are protected against tumors, whereas the opposite is observed in Ppargc1a-/- mice. Altogether, these data suggest that by regulating the intestinal cell fate, PGC1-α could play a role in protecting against CRC formation and mitochondria-mediated apoptosis[63].
CREB-binding protein and p300 are two closely homologous proteins involved in multiple biological processes. They function as coregulators and also as HATs[64,65]. p300 plays a role in many cellular activities, including cell differentiation, growth and DNA repair[66]. A study performed on 27 primary colon adenocarcinoma samples showed that p300 inactivation due to a missense point mutation could be involved in CRC development[67]. In addition, p300 loss in HCT116 cells results in a more aggressive phenotype characterized by increased cell migration and reduced cell adhesion[68]. Finally, low p300 expression is associated with CRC aggressiveness (for instance, lymph node invasiveness)[69]. Comparison of colon adenocarcinoma and normal tissue samples showed that both p300 and CREB-binding protein are overexpressed in tumors. However, while increased CREB-binding protein tumor expression is associated with overall good patient survival, p300 overexpression is associated with poor patient survival[70].
p300 and CREB-binding protein have a key role in the regulation of the WNT/β-catenin signaling pathway[71,72] and several studies connected them to CRC[73]. Specifically, p300-mediated WNT signaling has been associated with embryonic stem cell (ESC) differentiation, while CREB-binding protein-mediated WNT activity promotes cell proliferation[71]. Finally, selectively blocking the association between β-catenin and CREB-binding protein with ICG-001 downregulates WNT transcriptional activity in CRC cells[74,75].
Mediator of RNA polymerase II transcription subunit 1 (also known as mediator complex subunit 1, MED1, or TRAP220) is the main subunit of the TRAP/Mediator complex[76] and a coactivator of PPARγ[77]. MED1 mRNA levels are lower in CRC tissues than in the adjacent normal mucosa[47]. Moreover, absence of MED1 expression in CRC is correlated with lymph node metastasis and with advanced TNM stage[78]. It has been reported that the MED1 gene is hypermethylated in CRC tumors and also in the matched normal mucosa. This indicates that MED1 silencing occurs early in CRC formation and is associated with cancer initiation rather than cancer progression[79].
Nuclear receptor corepressor (N-CoR or N-CoR1) and the highly similar silencing mediator of retinoic and thyroid receptor (N-CoR2 or SMRT) were the first identified NR corepressors, based on their ability to mediate transcriptional repression of TR and RARs[80,81]. Additional studies revealed that N-CoR1 and N-CoR2 mediate the ligand-independent interaction with other NRs. The NR interaction domains located in the C-terminus of N-CoR1 and N-CoR2 correspond to the so-called corepressor NR boxes (CoRNR) and harbor the consensus sequence L/I-X-X-I/V-I or LXXXI/LXXXI/L[82]. Both corepressors exert their function by recruiting various proteins to specific promoters, particularly histone deacetylases 3 (HDAC3), one of the main actors responsible for their repressive activity[83]. Interestingly, HDAC3 is overexpressed in human colorectal adenocarcinomas and in CRC cell lines[84-86]. Moreover, HDAC3 plays a central role in regulating CRC cell proliferation and differentiation, particularly through the regulation of p21[84].
In CRC, N-CoR2 is aberrantly expressed in all tested primary tumors[87]. Moreover, increased IKKα activity in CRC has been correlated with the specific phosphorylation of Ser-2410 in N-CoR2. This phosphorylation event also leads to N-CoR2 cytoplasmic translocation and degradation[88]. IKKα recruitment to chromatin is associated with the transcriptional activation of different Notch target genes (including HES1 and HES5) and with N-CoR2 release from the corresponding promoters[87].
On the other hand, NCOR1 expression level is higher in human CRC tissues than in normal mucosa[47]. IKKα also phosphorylates N-CoR1 and aberrant N-CoR1 cytoplasmic localization is a general feature of CRC[89]. Specifically, N-CoR1 is excluded from the nucleus of 98% of tested tumor samples, whereas it is mainly nuclear in the normal mucosa. These results indicate that N-CoR1 nuclear export might be associated with intestinal tumorigenesis.
This transcription coregulator, also known as receptor-interacting protein of 140 kDa (RIP140), was originally identified in breast cancer as a modulator of ERα activity[90]. Subsequently, it was reported to interact and inhibit other transcription factors, including NRs and E2F transcription factors[91]. 2-Nuclear receptor-interacting protein 1 (NRIP1) exerts its repressive activity via four inhibitory domains that recruit histone deacetylases or C-terminal binding proteins[92]. Several post-translational modifications, such as acetylation and sumoylation, play important roles in controlling NRIP1 subcellular localization and repressive activity[93].
In a recent study, we showed that NRIP1 plays a major role in normal and malignant development of the intestinal epithelium by exerting a negative control on the WNT/β-catenin signaling pathway through regulation of APC transcription[94]. Furthermore, NRIP1 expression (both mRNA and protein) is lower in CRC samples than in the adjacent healthy tissue. Interestingly, NRIP1 is considered a marker of good prognosis in CRC. Overall survival is better in patients with a CRC that strongly expresses NRIP1 than in patients whose tumor shows low NRIP1 expression.
Nevertheless, NRIP1 cross-talk with the WNT/β-catenin signaling pathway is complex because another study using co-immunoprecipitation assays in human hepatocellular MHCC97 cells showed that NRIP1 can also interact directly with β-catenin[95].
We recently identified the transcription coregulator ligand-dependent corepressor (LCoR)[96] as a partner and transcriptional target of NRIP1 (Jalaguier et al[97] submitted for publication). Similar to NRIP1, LCoR was first discovered thanks to its ligand-dependent interaction with ERα, and subsequently was found to interact with many other NRs. The LCOR gene is widely expressed in embryonic and adult tissues. LCoR is expressed also in many human cell lines, and this expression is particularly high in Caco2 cells. Moreover, immunoprecipitation experiments in Caco2 cells showed that the tumor suppressor KLF6 interacts with LCoR[98]. Interestingly, KLF6 is inactivated in CRC, suggesting that it might play an important role in CRC development[99]. As consequence, LCoR interaction with KLF6 could have potential implications in CRC initiation and development.
3-Metastasis-associated protein 1 (MTA1) is a component of the nuclear remodeling and deacetylation (NuRD) complex that is associated with adenosine triphosphate-dependent chromatin remodeling and transcriptional regulation (for reviews, see[100,101]). MTA1 overexpression is closely correlated with an aggressive course in several human carcinoma types. Aberrant MTA1 expression has been observed in CRC[47]. MTA1 mRNA overexpression in CRC has been correlated with deeper invasion through the intestine wall and higher lymph node metastasis rate[102]. Similarly, MTA1 protein expression is significantly higher in moderately and poorly differentiated CRC specimens and liver metastatic tumors compared with normal colon tissues. Moreover, MTA1 overexpression in HCT116 cells enhances cell proliferation, migration and adhesion, while MTA1 silencing inhibits these functions[103].
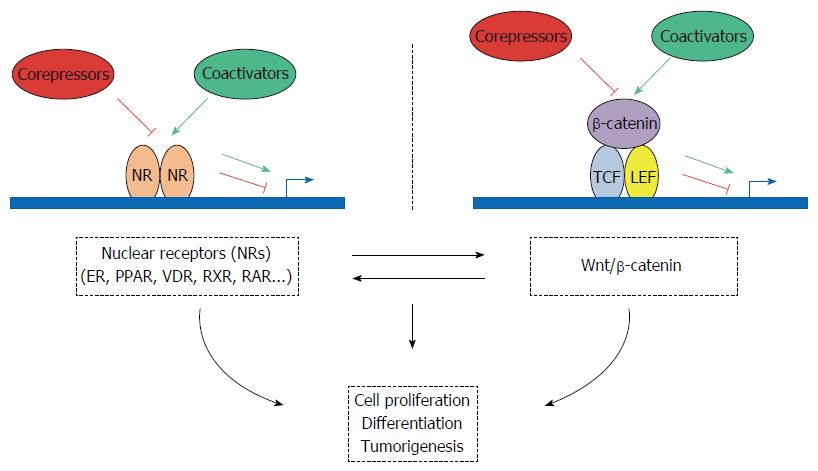
NR coregulators (coactivators and corepressors) represent a family of key regulatory transcription factors that control major steps in gene expression, including not only transcriptional initiation but also elongation, splicing and translation. These molecules are both targets and depositors of a huge number of post-translational modification marks that could play a key role in intestinal pathogenesis. By cross-talking with factors that are part of other signaling pathways, these transcription coregulators are centrally positioned to finely tune major physiopathological processes, such as development, energy storage and utilization, as well as tumor initiation and progression. In this review, we summarized how the expression of the main NR coregulators is dysregulated in CRC (see Table 2 for a synthetic summary). In most of the cases, the molecular mechanisms responsible for the expression deregulation in intestinal tumors are not fully known. One major mechanism could involve variation in gene copy number. As shown in Table 2, this is a relevant explanation in several cases, but other levels of regulation (transcriptional or post-transcriptional) could also be involved. In addition, the multiple qualitative alterations of these genes (i.e., mutations affecting their coding sequence) that have been identified in CRC (Table 3) could play a major role in controlling their biological activity. We also discussed the links between NR coregulators and the WNT/β-catenin signaling pathway that involve, in several cases, a direct interaction with β-catenin (Table 4). Finally, we summarized what is known about the biological relevance of these different cross-talks in intestinal physiopathology (Figure 1 for an overall scheme integrating the different pathways and actors that are involved). Often, their effects have been assessed only using in vitro experimental approaches. Therefore, additional work using transgenic mouse models is required to precisely determine their role in CRC. This will certainly lead to useful information that may help clinicians to improve therapeutic interventions and to develop better prognostic tools for CRC.
| Coregulator | CNV (%) | Expression | Ref. |
| NCOA1 | Gain (5.5) | ND | |
| NCOA2 | Gain (30.1) | ↑ or ↓ | Giannini 2005, Grivas 2009, Yu 2016 |
| NCOA3 | Gain (40.6) | ↑ | Anzick 1997, Aust 2004, Xie 2005 |
| NCOA6 | Gain (42.7) | ↑ | Lee 1999 |
| PPARGC1A | Loss (8.4) | ↓ | D’Errico 2011 |
| EP300 | Loss (19.3) | ↑ | Ishihama 2007 |
| CREBBP | Gain (5.3) | ↑ | Ishihama 2007 |
| MED1 | Gain (11.8) Loss (8.4) | ↓ | Giannini 2005 |
| NCOR1 | Loss (40.4) | ↑ | Fernández-Majada 2007 |
| NCOR2 | Gain (9.8) | ↑ | Giannini 2005, Fernández-Majada 2007 |
| NRIP1 | Loss (17.2) | ↓ | Lapierre 2014 |
| MTA1 | Loss (15.0) | ↑ | Toh 1997 |
| Coregulator | 216 United States patients (COAD-US) | 187 Chinese patients (COCA-CN) | ||
| Patients affected | Mutations | Patients affected | Mutations | |
| NCOA1 | 6 (2.78) | 7 | 9 (4.81) | 14 |
| NCOA2 | 10 (4.63) | 10 | 26 (13.90)1 | 32 |
| NCOA3 | 21 (9.72)1 | 17 | 9 (4.81) | 10 |
| NCOA6 | 19 (8.80)1 | 21 | 8 (4.28) | 10 |
| PPARGC1A | 10 (4.63) | 12 | 5 (2.67) | 6 |
| EP300 | 13 (6.02)1 | 15 | 14 (7.49)1 | 21 |
| CREBBP | 18 (8.33)1 | 20 | 7 (3.74) | 11 |
| MED1 | 3 (1.39) | 4 | 6 (3.21) | 6 |
| NCOR1 | 21 (9.72)1 | 25 | 23 (12.30)1 | 48 |
| NCOR2 | 41 (18.98)1 | 43 | 30 (16.04)1 | 40 |
| NRIP1 | 5 (2.31) | 5 | 6 (3.21) | 10 |
| MTA1 | 11 (5.09)1 | 11 | 12 (6.42)1 | 13 |
| Coregulator | Effect | Assay | Domains on β-catenin | Ref. |
| NCoA-1 | ↑ | GPD CoIP | aa 234-585 | Li 2004, Tong 2015 |
| NCoA-2 | ↓ | GPD | C-terminal part (aa) | Li 2004, Song 2005, Yu 2016 |
| CREBBP | ↑ | Y2H GPD CoIP | AR10 to C-ter | Takemaru 2000 |
| N-CoR1 | GPD | aa 120-683 | Song 2008 | |
| N-CoR2 | GPD | aa 120-683 | Song 2008 | |
| NRIP1 | ↓ | CoIP | Zhang 2014 |
We thank Dr. C Teyssier and S Jalaguier for critical proof-reading of the manuscript. We thank Dr. B Orsetti for extracting and sharing CNV data.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Corrales FJJ S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20512] [Article Influence: 2051.2] [Reference Citation Analysis (20)] |
| 2. | Gonzalez CA, Riboli E. Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer. 2010;46:2555-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] |
| 4. | Deschoolmeester V, Baay M, Specenier P, Lardon F, Vermorken JB. A review of the most promising biomarkers in colorectal cancer: one step closer to targeted therapy. Oncologist. 2010;15:699-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1392] [Article Influence: 87.0] [Reference Citation Analysis (2)] |
| 7. | Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681-8686. [PubMed] |
| 8. | Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3408] [Cited by in RCA: 3555] [Article Influence: 355.5] [Reference Citation Analysis (0)] |
| 9. | Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3740] [Cited by in RCA: 4400] [Article Influence: 338.5] [Reference Citation Analysis (0)] |
| 10. | Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835-839. [PubMed] |
| 11. | Xiao X, Wang P, Chou KC. Recent progresses in identifying nuclear receptors and their families. Curr Top Med Chem. 2013;13:1192-1200. [PubMed] |
| 12. | Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58:685-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 477] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 13. | Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT. Nuclear receptor structure: implications for function. Annu Rev Physiol. 2007;69:201-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311-317. [PubMed] |
| 15. | Berger C, Qian Y, Chen X. The p53-estrogen receptor loop in cancer. Curr Mol Med. 2013;13:1229-1240. [PubMed] |
| 16. | Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm. 2001;62:231-252. [PubMed] |
| 17. | Sas L, Lardon F, Vermeulen PB, Hauspy J, Van Dam P, Pauwels P, Dirix LY, Van Laere SJ. The interaction between ER and NFκB in resistance to endocrine therapy. Breast Cancer Res. 2012;14:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26:898-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Modica S, Gofflot F, Murzilli S, D’Orazio A, Salvatore L, Pellegrini F, Nicolucci A, Tognoni G, Copetti M, Valanzano R. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology. 2010;138:636-648, 648.e1-648.12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | D’Errico I, Moschetta A. Nuclear receptors, intestinal architecture and colon cancer: an intriguing link. Cell Mol Life Sci. 2008;65:1523-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor beta in colonic epithelium. Proc Natl Acad Sci USA. 2006;103:2959-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Kallay E, Pietschmann P, Toyokuni S, Bajna E, Hahn P, Mazzucco K, Bieglmayer C, Kato S, Cross HS. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429-1435. [PubMed] |
| 23. | Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3831] [Cited by in RCA: 4055] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 24. | Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell. 2004;15:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369-387. [PubMed] |
| 26. | Easwaran V, Pishvaian M, Salimuddin , Byers S. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9:1415-1418. [PubMed] |
| 27. | Chesire DR, Isaacs WB. Ligand-dependent inhibition of beta-catenin/TCF signaling by androgen receptor. Oncogene. 2002;21:8453-8469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335-345. [PubMed] |
| 29. | Gupta RA, Tan J, Krause WF, Geraci MW, Willson TM, Dey SK, DuBois RN. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci USA. 2000;97:13275-13280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 311] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 442] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 31. | Peignon G, Durand A, Cacheux W, Ayrault O, Terris B, Laurent-Puig P, Shroyer NF, Van Seuningen I, Honjo T, Perret C. Complex interplay between β-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut. 2011;60:166-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J Biol Chem. 2009;284:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Sirakov M, Boussouar A, Kress E, Frau C, Lone IN, Nadjar J, Angelov D, Plateroti M. The thyroid hormone nuclear receptor TRα1 controls the Notch signaling pathway and cell fate in murine intestine. Development. 2015;142:2764-2774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354-1357. [PubMed] |
| 35. | Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 36. | Zhu Y, Qi C, Calandra C, Rao MS, Reddy JK. Cloning and identification of mouse steroid receptor coactivator-1 (mSRC-1), as a coactivator of peroxisome proliferator-activated receptor gamma. Gene Expr. 1996;6:185-195. [PubMed] |
| 37. | Fonte C, Grenier J, Trousson A, Chauchereau A, Lahuna O, Baulieu EE, Schumacher M, Massaad C. Involvement of {beta}-catenin and unusual behavior of CBP and p300 in glucocorticosteroid signaling in Schwann cells. Proc Natl Acad Sci USA. 2005;102:14260-14265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Li H, Kim JH, Koh SS, Stallcup MR. Synergistic effects of coactivators GRIP1 and beta-catenin on gene activation: cross-talk between androgen receptor and Wnt signaling pathways. J Biol Chem. 2004;279:4212-4220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Tong Z, Li M, Wang W, Mo P, Yu L, Liu K, Ren W, Li W, Zhang H, Xu J. Steroid Receptor Coactivator 1 Promotes Human Hepatocellular Carcinoma Progression by Enhancing Wnt/β-Catenin Signaling. J Biol Chem. 2015;290:18596-18608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Koh SS, Chen D, Lee YH, Stallcup MR. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem. 2001;276:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 268] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Jain S, Pulikuri S, Zhu Y, Qi C, Kanwar YS, Yeldandi AV, Rao MS, Reddy JK. Differential expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) and its coactivators steroid receptor coactivator-1 and PPAR-binding protein PBP in the brown fat, urinary bladder, colon, and breast of the mouse. Am J Pathol. 1998;153:349-354. [PubMed] |
| 42. | Chen C, Lu Y, Liu J, Li L, Zhao N, Lin B. Genome-wide ChIP-seq analysis of TCF4 binding regions in colorectal cancer cells. Int J Clin Exp Med. 2014;7:4253-4259. [PubMed] |
| 43. | Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667-3675. [PubMed] |
| 44. | Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O’Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006;26:6571-6583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Picard F, Géhin M, Annicotte J, Rocchi S, Champy MF, O’Malley BW, Chambon P, Auwerx J. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931-941. [PubMed] |
| 46. | Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 977] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 47. | Giannini R, Cavallini A. Expression analysis of a subset of coregulators and three nuclear receptors in human colorectal carcinoma. Anticancer Res. 2005;25:4287-4292. [PubMed] |
| 48. | Grivas PD, Tzelepi V, Sotiropoulou-Bonikou G, Kefalopoulou Z, Papavassiliou AG, Kalofonos H. Estrogen receptor alpha/beta, AIB1, and TIF2 in colorectal carcinogenesis: do coregulators have prognostic significance? Int J Colorectal Dis. 2009;24:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Yu J, Wu WK, Liang Q, Zhang N, He J, Li X, Zhang X, Xu L, Chan MT, Ng SS. Disruption of NCOA2 by recurrent fusion with LACTB2 in colorectal cancer. Oncogene. 2016;35:187-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Song LN, Gelmann EP. Interaction of beta-catenin and TIF2/GRIP1 in transcriptional activation by the androgen receptor. J Biol Chem. 2005;280:37853-37867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965-968. [PubMed] |
| 52. | Aust DE, Muders M, Köhler A, Schmidt M, Diebold J, Müller C, Löhrs U, Waldman FM, Baretton GB. Prognostic relevance of 20q13 gains in sporadic colorectal cancers: a FISH analysis. Scand J Gastroenterol. 2004;39:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Xie D, Sham JS, Zeng WF, Lin HL, Bi J, Che LH, Hu L, Zeng YX, Guan XY. Correlation of AIB1 overexpression with advanced clinical stage of human colorectal carcinoma. Hum Pathol. 2005;36:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Sakakura C, Hagiwara A, Yasuoka R, Fujita Y, Nakanishi M, Masuda K, Kimura A, Nakamura Y, Inazawa J, Abe T. Amplification and over-expression of the AIB1 nuclear receptor co-activator gene in primary gastric cancers. Int J Cancer. 2000;89:217-223. [PubMed] |
| 55. | Mo P, Zhou Q, Guan L, Wang Y, Wang W, Miao M, Tong Z, Li M, Majaz S, Liu Y. Amplified in breast cancer 1 promotes colorectal cancer progression through enhancing notch signaling. Oncogene. 2015;34:3935-3945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Mahajan MA, Samuels HH. Nuclear hormone receptor coregulator: role in hormone action, metabolism, growth, and development. Endocr Rev. 2005;26:583-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Lee SK, Anzick SL, Choi JE, Bubendorf L, Guan XY, Jung YK, Kallioniemi OP, Kononen J, Trent JM, Azorsa D. A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for ligand-dependent transactivation by nuclear receptors in vivo. J Biol Chem. 1999;274:34283-34293. [PubMed] |
| 58. | Mahajan MA, Murray A, Levy D, Samuels HH. Nuclear receptor coregulator (NRC): mapping of the dimerization domain, activation of p53 and STAT-2, and identification of the activation domain AD2 necessary for nuclear receptor signaling. Mol Endocrinol. 2007;21:1822-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 968] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 60. | Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829-839. [PubMed] |
| 61. | Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589-9594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 62. | Xiao JH, Ghosn C, Hinchman C, Forbes C, Wang J, Snider N, Cordrey A, Zhao Y, Chandraratna RA. Adenomatous polyposis coli (APC)-independent regulation of beta-catenin degradation via a retinoid X receptor-mediated pathway. J Biol Chem. 2003;278:29954-29962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | D’Errico I, Salvatore L, Murzilli S, Lo Sasso G, Latorre D, Martelli N, Egorova AV, Polishuck R, Madeyski-Bengtson K, Lelliott C. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC1alpha) is a metabolic regulator of intestinal epithelial cell fate. Proc Natl Acad Sci USA. 2011;108:6603-6608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 64. | Arany Z, Sellers WR, Livingston DM, Eckner R. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799-800. [PubMed] |
| 65. | Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953-959. [PubMed] |
| 66. | Shikama N, Lee CW, France S, Delavaine L, Lyon J, Krstic-Demonacos M, La Thangue NB. A novel cofactor for p300 that regulates the p53 response. Mol Cell. 1999;4:365-376. [PubMed] |
| 67. | Muraoka M, Konishi M, Kikuchi-Yanoshita R, Tanaka K, Shitara N, Chong JM, Iwama T, Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1996;12:1565-1569. [PubMed] |
| 68. | Iyer NG, Chin SF, Ozdag H, Daigo Y, Hu DE, Cariati M, Brindle K, Aparicio S, Caldas C. p300 regulates p53-dependent apoptosis after DNA damage in colorectal cancer cells by modulation of PUMA/p21 levels. Proc Natl Acad Sci USA. 2004;101:7386-7391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Huh JW, Kim HC, Kim SH, Park YA, Cho YB, Yun SH, Lee WY, Chun HK. Prognostic impact of p300 expression in patients with colorectal cancer. J Surg Oncol. 2013;108:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Ishihama K, Yamakawa M, Semba S, Takeda H, Kawata S, Kimura S, Kimura W. Expression of HDAC1 and CBP/p300 in human colorectal carcinomas. J Clin Pathol. 2007;60:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 2007;104:5668-5673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 72. | Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249-254. [PubMed] |
| 73. | Bordonaro M, Lazarova DL. CREB-binding protein, p300, butyrate, and Wnt signaling in colorectal cancer. World J Gastroenterol. 2015;21:8238-8248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci USA. 2004;101:12682-12687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 723] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 75. | Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619-3631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 261] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 76. | Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG. The TRAP220 component of a thyroid hormone receptor- associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci USA. 1998;95:7939-7944. [PubMed] |
| 77. | Zhu Y, Qi C, Jain S, Rao MS, Reddy JK. Isolation and characterization of PBP, a protein that interacts with peroxisome proliferator-activated receptor. J Biol Chem. 1997;272:25500-25506. [PubMed] |
| 78. | Kwon KA, Yun J, Oh SY, Seo BG, Lee S, Lee JH, Kim SH, Choi HJ, Roh MS, Kim HJ. Clinical Significance of Peroxisome Proliferator-Activated Receptor γ and TRAP220 in Patients with Operable Colorectal Cancer. Cancer Res Treat. 2016;48:198-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Howard JH, Frolov A, Tzeng CW, Stewart A, Midzak A, Majmundar A, Godwin A, Heslin M, Bellacosa A, Arnoletti JP. Epigenetic downregulation of the DNA repair gene MED1/MBD4 in colorectal and ovarian cancer. Cancer Biol Ther. 2009;8:94-100. [PubMed] |
| 80. | Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1491] [Cited by in RCA: 1447] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 81. | Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass CK. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1443] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 82. | Watson PJ, Fairall L, Schwabe JW. Nuclear hormone receptor co-repressors: structure and function. Mol Cell Endocrinol. 2012;348:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 83. | Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45-57. [PubMed] |
| 84. | Spurling CC, Godman CA, Noonan EJ, Rasmussen TP, Rosenberg DW, Giardina C. HDAC3 overexpression and colon cancer cell proliferation and differentiation. Mol Carcinog. 2008;47:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 85. | Wilson AJ, Byun DS, Popova N, Murray LB, L’Italien K, Sowa Y, Arango D, Velcich A, Augenlicht LH, Mariadason JM. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem. 2006;281:13548-13558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 418] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 86. | Weichert W, Röske A, Niesporek S, Noske A, Buckendahl AC, Dietel M, Gekeler V, Boehm M, Beckers T, Denkert C. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res. 2008;14:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 87. | Fernández-Majada V, Aguilera C, Villanueva A, Vilardell F, Robert-Moreno A, Aytés A, Real FX, Capella G, Mayo MW, Espinosa L. Nuclear IKK activity leads to dysregulated notch-dependent gene expression in colorectal cancer. Proc Natl Acad Sci USA. 2007;104:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Hoberg JE, Yeung F, Mayo MW. SMRT derepression by the IkappaB kinase alpha: a prerequisite to NF-kappaB transcription and survival. Mol Cell. 2004;16:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 89. | Fernández-Majada V, Pujadas J, Vilardell F, Capella G, Mayo MW, Bigas A, Espinosa L. Aberrant cytoplasmic localization of N-CoR in colorectal tumors. Cell Cycle. 2007;6:1748-1752. [PubMed] |
| 90. | Cavaillès V, Dauvois S, L’Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 1995;14:3741-3751. [PubMed] |
| 91. | Docquier A, Harmand PO, Fritsch S, Chanrion M, Darbon JM, Cavaillès V. The transcriptional coregulator RIP140 represses E2F1 activity and discriminates breast cancer subtypes. Clin Cancer Res. 2010;16:2959-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Castet A, Boulahtouf A, Versini G, Bonnet S, Augereau P, Vignon F, Khochbin S, Jalaguier S, Cavaillès V. Multiple domains of the Receptor-Interacting Protein 140 contribute to transcription inhibition. Nucleic Acids Res. 2004;32:1957-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 802] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 94. | Lapierre M, Bonnet S, Bascoul-Mollevi C, Ait-Arsa I, Jalaguier S, Del Rio M, Plateroti M, Roepman P, Ychou M, Pannequin J. RIP140 increases APC expression and controls intestinal homeostasis and tumorigenesis. J Clin Invest. 2014;124:1899-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 95. | Zhang D, Wang Y, Dai Y, Wang J, Suo T, Pan H, Liu H, Shen S, Liu H. Downregulation of RIP140 in hepatocellular carcinoma promoted the growth and migration of the cancer cells. Tumour Biol. 2015;36:2077-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Fernandes I, Bastien Y, Wai T, Nygard K, Lin R, Cormier O, Lee HS, Eng F, Bertos NR, Pelletier N. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol Cell. 2003;11:139-150. [PubMed] |
| 97. | Jalaguier S, Teyssier C, Nait Achour T, Lucas A, Bonnet S, Rodriguez C, Elarouci N, Lapierre M, Cavaillès V. Complex regulation of LCoR signaling in breast cancer cells. Oncogene. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 98. | Calderon MR, Verway M, An BS, DiFeo A, Bismar TA, Ann DK, Martignetti JA, Shalom-Barak T, White JH. Ligand-dependent corepressor (LCoR) recruitment by Kruppel-like factor 6 (KLF6) regulates expression of the cyclin-dependent kinase inhibitor CDKN1A gene. J Biol Chem. 2012;287:8662-8674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Reeves HL, Narla G, Ogunbiyi O, Haq AI, Katz A, Benzeno S, Hod E, Harpaz N, Goldberg S, Tal-Kremer S. Kruppel-like factor 6 (KLF6) is a tumor-suppressor gene frequently inactivated in colorectal cancer. Gastroenterology. 2004;126:1090-1103. [PubMed] |
| 100. | Kumar R, Wang RA, Bagheri-Yarmand R. Emerging roles of MTA family members in human cancers. Semin Oncol. 2003;30:30-37. [PubMed] |
| 101. | Sen N, Gui B, Kumar R. Role of MTA1 in cancer progression and metastasis. Cancer Metastasis Rev. 2014;33:879-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 102. | Toh Y, Oki E, Oda S, Tokunaga E, Ohno S, Maehara Y, Nicolson GL, Sugimachi K. Overexpression of the MTA1 gene in gastrointestinal carcinomas: correlation with invasion and metastasis. Int J Cancer. 1997;74:459-463. [PubMed] |
| 103. | Tuncay Cagatay S, Cimen I, Savas B, Banerjee S. MTA-1 expression is associated with metastasis and epithelial to mesenchymal transition in colorectal cancer cells. Tumour Biol. 2013;34:1189-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |