Published online Jun 14, 2017. doi: 10.3748/wjg.v23.i22.4072
Peer-review started: February 11, 2017
First decision: March 3, 2017
Revised: March 16, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: June 14, 2017
Processing time: 124 Days and 14.7 Hours
To assess the efficacy and safety of combined directly acting antivirals (DAAs) for the treatment of Chinese chronic hepatitis C (CHC) patients in a real-world setting.
Hospitalized CHC patients who were treated with DAAs at Peking University First Hospital between January 2015 and December 2016 were enrolled. Samples and clinical data were collected at 0 wk, 2 wk, 4 wk, 8 wk, 12 wk, or 24 wk during DAAs treatment and at 4 wk, 12 wk, and 24 wk after the end of treatment.
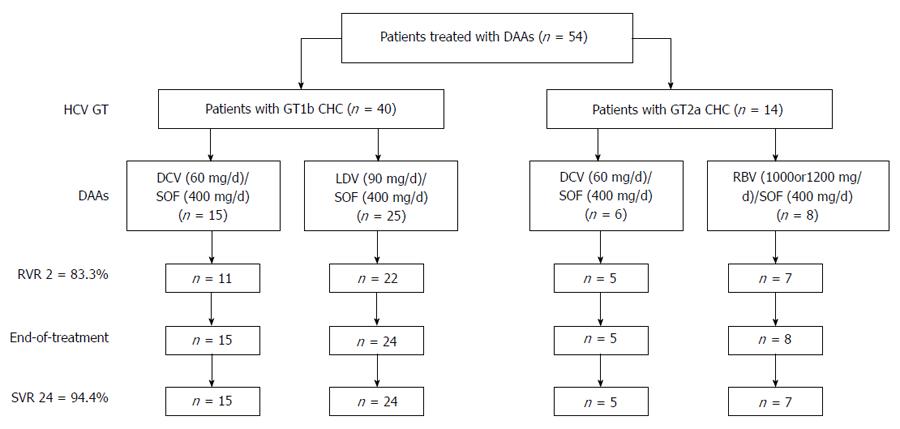
Fifty-four patients who underwent DAAs treatment were included in our study, of whom 83.3% (45/54) achieved rapid virological response at 2 wk after treatment initiation (RVR 2) and 94.4% (51/54) achieved sustained virological response at 24 wk after the end of treatment (SVR 24). Serum creatinine and uric acid levels at the end of treatment were significantly increased compared with baseline levels (83.6 ± 17.9 vs 88.8 ± 19.4, P01 < 0.001; 320.8 ± 76.3 vs 354.5 ± 87.6, P01 < 0.001), and no significant improvements were observed at 24w after the end of treatment (83.6 ± 17.9 vs 86.8 ± 19.1, P02 = 0.039; 320.8 ± 76.3 vs 345.9 ± 89.4, P02 = 0.001). The total frequency of adverse events (AEs) during treatment was 33.3% (18/54), with major AEs being fatigue (16.7%), headache (7.4%), anorexia (7.4%), and insomnia (5.6%).
Though based in a small cohort of patients, the abnormal changes in renal function indices and relative high frequency of AEs during combined DAAs treatment should be taken as a note of caution.
Core tip: Treatment of hepatitis C virus infection has reached a new era with the approval of directly acting antivirals (DAAs), while there had been limited data on the use of DAAs treatment in a real-world setting in China. We explored the changes of hepatorenal function indices before and after DAAs treatment and found that serum creatinine and uric acid levels at the end of treatment were significantly increased compared with baseline levels, and no significant improvements were observed at 24 wk after the end of treatment. This study may serve as a reminder to clinicians to implement close renal function monitoring in patients receiving combined DAAs treatment.
- Citation: Chen JH, Zeng Z, Zhang XX, Zhang Y, Zhang RW, Wang S, Wu CH, Yu M, Liu D, Xi HL, Zhou YX, An YY, Xu XY. Efficacy and safety of combined directly acting antivirals for treatment of Chinese chronic hepatitis C patients in a real-world setting. World J Gastroenterol 2017; 23(22): 4072-4079
- URL: https://www.wjgnet.com/1007-9327/full/v23/i22/4072.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i22.4072
Chronic hepatitis C virus (HCV) infections affect approximately 130-150 million people worldwide and is a major cause of liver cirrhosis and hepatocellular carcinoma[1-3]. Treatment of HCV infection has reached a new era with the approval of first generation directly acting antivirals (DAAs) in 2011 and the subsequent development of interferon (IFN)-free, all-oral DAAs combination regimens[4]. All-oral DAAs combination regimens simplified the treatment and improved compliance of patients who disliked injections or were intolerable to them[5]. Combined DAAs regimens shortened treatment durations from 48 wk to 12 wk or 24 wk; Sofosbuvir (SOF)/Velpatasvir also attained a promising efficacy at 8 wk, and some studies tried to shorten treatment times even further[6]. Similar to cocktail therapies against human immunodeficiency virus, combination therapies that target different stages of the HCV life cycle have been conceived to avoid cross-resistance[7]. Importantly, all-oral combination regimens increased sustained virological response (SVR) rates to more than 90% with fewer contraindications and adverse events (AEs) in patients infected with different HCV genotypes (GT) and in those with different liver conditions, treatment experiences and concomitant diseases[8-14]. Sofosbuvir (SOF)/Velpatasvir combination regimen might even provide complete pan-genotypic treatment for patients with HCV infection[15,16]. Though price decreases for HCV drugs have already been announced for some DAAs, most of them are currently too expensive for governments worldwide to deliver on their promise to cure and eliminate the disease, especially in low- and middle-income countries[17].
China has the greatest number of chronic hepatitis C (CHC) cases worldwide, with an estimated 29.8 million patients infected. GT 1b and GT 2a are the two major HCV subtypes, accounting for 62.78% (95%CI: 59.54%-66.02%) and 17.39% (95%CI: 15.67%-19.11%), respectively[18,19]. The traditional treatment for patients with CHC in China is peginterferon in combination with ribavirin (PegIFNα-2a/RBV, PR), which was found to be associated with lower SVR rates and more AEs[20]. Refractory CHC patients and patients with contraindications and intolerances to the AEs associated with PR treatment try to initiate DAAs treatment.
So far, there have been limited data on the use of combined DAAs treatment in a real-world setting in China. This study aimed to show the efficacy of DAAs for the treatment of Chinese CHC patients and explore the effects of DAAs on hemogram and hepatorenal function indices, and the frequency of AEs during treatment in a real-world setting.
CHC patients who were treated with DAAs while hospitalized at Peking University First Hospital between January 2015 and December 2016 and met the following criteria were enrolled in this study: (1) infected with HCV GT 1b or 2a; (2) negative for hepatitis A virus immunoglobulin (Ig) M, hepatitis B surface antigen, hepatitis E virus IgM and human immunodeficiency virus; (3) no severe heart disease; (4) no active drug use; (5) no severe renal function damage or renal failure (eGFR < 30 mL/min); (6) no pregnancy; (7) appropriate DAAs treatment regimens; and (8) complete clinical information. A total of 16 patients were excluded, including one HBV/HCV co-infected patient, three patients with severe renal function damage, one patient treated with inappropriate DAAs regimens, and 11 patients with incomplete clinical information. All study participants provided informed written consent prior to study enrollment. Ethical approval was given by the Ethics Committee of Peking University People Hospital.
Hematological, biochemical, and urine tests were performed at 0 wk, 2 wk, 4 wk, 8 wk, 12 wk, or 24 wk during DAAs treatment, as well as 4 wk, 12 wk, and 24 wk after the end of treatment at clinical laboratory[21]. White blood cell (WBC) count, red blood cell (RBC) count, hemoglobin concentration (HGB), and blood platelet (PLT) count were used to assess the changes of hemogram; alanine aminotransferase (ALT), aspartate aminotransferase (AST), fibrosis-4 (FIB-4) score, and liver stiffness measurement (LSM) were used to assess the degree of liver inflammation and fibrosis; estimated glomerular filtration rate (eGFR), serum creatinine (Scr), uric acid (UA), and blood urea nitrogen (BUN) were used to assess renal function.
LSM was measured by transient elastography (Fibroscan, Echosens, Paris). Presence of cirrhosis was determined by LSM > 17.5 kPa[22-24]. FIB-4 score was calculated with the equation: FIB-4 = [AGE * AST (U/L)]/[PLT (109/L) * ALT (U/L) ^ (1/2)][25]. The eGFR was calculated with the Modification of Diet in Renal Disease Study equation adjusted for the Chinese population: eGFR = 175 * (serum creatinine)-1.234 * age-0.179 * 0.79 (if female)[26].
HCV RNA quantitation and genotyping were measured at the virus laboratory in department of infectious disease. Serum HCV RNA quantitation was measured using a COBAS Taqman HCV Test kit (Roche Molecular Systems Inc., Pleasanton, CA, United States) according to the manufacturer’s instructions, with COBAS AmpliPrep instrument used for automated specimen processing and COBAS Taqman analyser for automated amplification and detection[27]. HCV genotypes were determined by restriction fragment length polymorphism (RFLP) analysis of the amplified 5′-noncoding genome region[28]. Briefly, HCV RNA was extracted from 140 μL serum samples using a QIAamp viral RNA mini kit (Qiagen, Hilden, Germany). Reverse transcription and polymerase chain reaction (PCR) amplification were performed using BG1 (5’-CTGTGAGGAACTACTGTCTT-3’) and BG2 (5’-AACACTACTCGGCTAG CAGT-3’) as upstream and downstream primers, respectively, for the first round reaction in a reaction system containing 15 μL of 2 × Buffer, 1 μL of BG1 and BG2 each, 10 μL of cDNA, and 3 μL of H2O. The cycling parameters were denaturation at 95 °C for 2 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 60 s, and final extension at 72 °C for 7 min. This was followed by the second round reaction using BG3 (5’-TTCACGCAGAAAGCGTCTAG-3’) and BG4 (5’-GTTGATCCA AGAAAGGACCC-3’) as upstream and downstream primers, respectively, in a reaction system consisting of 15 μL of 2 × Buffer, 1 μL of BG3 and BG4 each, 10 μL of cDNA, and 3 μL of H2O. The cycling parameters were denaturation at 95 °C for 2 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 60 s, and final extension at 72 °C for 7 min. The PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and digested with Hae III at 37 °C for 2 h in a reaction system containing 2 μL of 10 × Buffer, 2.3 μL of Hae III 2.3 μL, 9.7 μL of ddH2O, and 6 μL of PCR production. Subsequently, agarose gel electrophoresis was performed to analyse the RFLP of the digestion products.
Microsoft Excel (Microsoft, Redmond, Washington, United States) was used for data collection and analysis. Data are expressed as mean ± SD or count number. We used Student’s t-test, Fisher’s exact test or χ2 test to calculate the statistical difference in baseline characteristics between different HCV GT infected patients. Repeated measures analysis of variance was used to give comparisons among different groups or different time points and calculate the interaction effect between treating factors and time factors. Mauchly’s test of sphericity was used to judge whether there were relations among the repeatedly measured data. If any (P < 0.05), Greenhouse- Geisser corrected results should be taken; Bonferroni or Fisher’s Least Significant Difference tests (when Epsilon < 0.7, Bonferroni test) were used to do pairwise comparisons of the repeatedly measured data in different measurement times. We carried out statistical analyses with SPSS version 16.0. P < 0.05 was considered statistically significant.
A total of 54 patients who underwent DAAs treatment were enrolled in our study, including 40 HCV GT 1b infected patients and 14 HCV GT 2a infected patients. Their mean age was 55.4 ± 16.6 years. Of the 54 patients included, 29 were male, 21 had experienced PR treatment, and 20 had cirrhosis. The mean score of LSM was 15.9 ± 14.1 (kPa), and the mean PLT count was 147.1 ± 65.1 (109/L). Baseline characteristics for all the 54 CHC patients are shown in Table 1. The distribution of PR treatment experienced patients and cirrhotic patients and all other baseline characteristics did not differ significantly between HCV GT 1b infected patients and HCV GT 2a infected patients (Table 1).
| Characteristic | All (n = 54) | GT1b (n = 40) | GT2a (n = 14) | P(1bvs2a) |
| Age | 55.4 ± 16.6 | 57.2 ± 15.9 | 50.1 ± 18.1 | 0.175 |
| Male/Female | 29/25 | 21/19 | 8/6 | 0.764 |
| HCV RNA log10 (IU/mL) | 6.48 ± 0.97 | 6.63 ± 0.89 | 6.06 ± 1.1 | 0.058 |
| PR(experienced/naive) | 21/33 | 17/23 | 4/10 | 0.358 |
| Non-cirrhotic/cirrhotic | 34/20 | 24/16 | 10/4 | 0.446 |
| LSM (kPa) | 15.9 ± 14.1 | 17.5 ± 15.1 | 11.4 ± 9.8 | 0.162 |
| FIB-4 score | 4.07 ± 4.35 | 4.14 ± 3.53 | 3.87 ± 6.29 | 0.847 |
| ALT (IU/L) | 54.6 ± 36.3 | 57.4 ± 38.7 | 46.5 ± 27.9 | 0.339 |
| AST (IU/L) | 50.8 ± 33.1 | 50.6 ± 26.8 | 46.3 ± 37.1 | 0.555 |
| eGFR(mL/min per 1.73 m2) | 87.1 ± 19.5 | 87.2 ± 20.9 | 86.5 ± 15.7 | 0.908 |
| Scr (μmol/L) | 83.6 ± 17.9 | 83.3 ± 19.5 | 84.4 ± 12.9 | 0.848 |
| UA (μmol/L) | 320.8 ± 76.3 | 315.2 ± 78.5 | 337.4 ± 72.9 | 0.349 |
| BUN (mmol/L) | 5.17 ± 1.50 | 5.21 ± 1.52 | 5.12 ± 1.55 | 0.881 |
| WBC (109/L) | 4.85 ± 1.67 | 4.67 ± 1.65 | 5.35 ± 1.70 | 0.192 |
| RBC (1012/L) | 4.42 ± 0.64 | 4.40 ± 0.67 | 4.49 ± 0.59 | 0.652 |
| HGB (g/L) | 140.8 ± 17.2 | 139.9 ± 17.9 | 143.6 ± 15.6 | 0.492 |
| PLT (109/L) | 147.1 ± 65.1 | 143.0 ± 68.4 | 158.8 ± 55.0 | 0.439 |
Among the 40 HCV GT1b infected patients, 15 were treated with SOF + Daclatasvir (DAC) and 25 were treated with SOF/Ledipasvir (LDV). Among the 14 HCV GT2a infected patients, 6 were treated with SOF + DAC and 8 were treated with SOF + Ribavirin (RBV) (< 75 kg, 1000 mg/d; > 75 kg, 1200 mg/d). All non-cirrhotic patients were treated for 12 wk, HCV GT 1b infected patients with cirrhosis were treated for 24 wk, HCV GT 2a infected patients with cirrhosis were treated with SOF + DAC for 12 wk or SOF + RBV for 20 wk[29].
The majority of patients [83.3% (45/54)] achieved rapid virological response at 2 wk after treatment initiation (RVR 2), while nine patients, including four HCV GT1b infected patients treated with SOF + DAC, three HCV GT1b infected patients treated with SOF/LDV, one HCV GT2a infected patient treated with SOF + DAC, and one HCV GT2a infected patient treated SOF + RBV, had detectable HCV RNA. Of these nine patients, four had experienced PR treatment and five were treatment naïve; four were cirrhotic and five were non-cirrhotic. At the end of treatment, 96.3% (52/54) patients achieved virological response, while one HCV GT1b infected patient treated with SOF/LDV for 12 wk and one HCV GT2a infected patient treated with SOF + DAC for 12 wk still had detectable HCV RNA. SVR rate at 24 wk after the end of treatment (SVR 24) was 94.4% (51/54), and one GT2a patient treated with SOF + RBV for 20 wk relapsed at 12 wk after the end of treatment (Figure 1).
When patients were classified by HCV GT, PR treatment experience, DAAs regimens and liver condition, RVR 2 rates were 73.3% (11/15) in HCV GT 1b infected patients treated with SOF + DAC, 88.0% (22/25) in HCV GT 1b infected patients treated with SOF/LDV, 83.3% (5/6) in HCV GT 2a infected patients treated with SOF + DAC, 87.5% (7/8) in HCV GT 1b infected patients treated with SOF + RBV, 84.8% (28/33) in PR treatment naive patients, 81.0% (17/21) in PR treatment experienced patients, 85.3% (29/34) in non-cirrhotic patients, and 80.0% (16/20) in cirrhotic patients; SVR 24 rates were 97.5% (39/40) in HCV GT 1b infected patients, 85.7% (12/14) in HCV GT 2a infected patients, 93.9% (31/33) in PR treatment naive patients, 95.2% (20/21) in PR treatment experienced patients, 94.1% (32/34) in non-cirrhotic patients, and 95.0% (19/20) in cirrhotic patients.
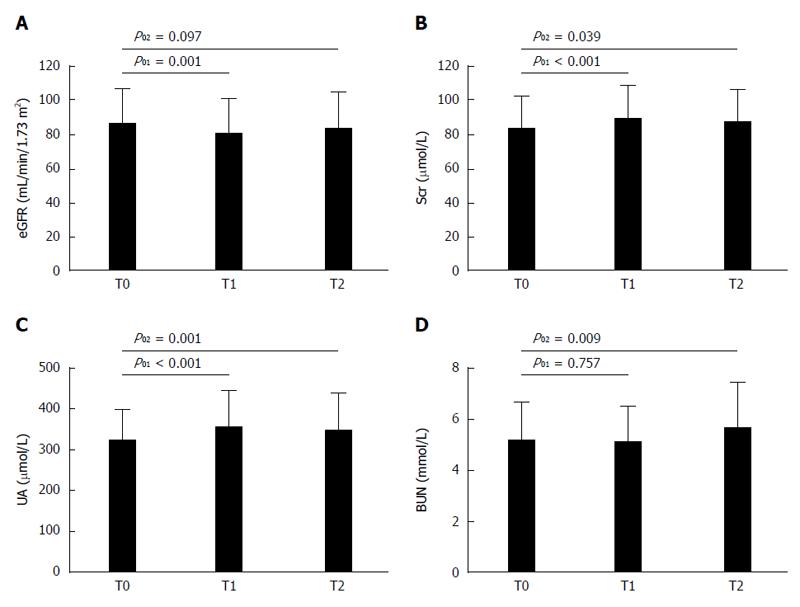
The changes of clinical indices among different observing points at the end of treatment and at 24w after the end of treatment compared with baseline data in 54 included patients are shown in Table 2. For all patients, ALT and AST levels at the end of treatment and at 24 wk after the end of treatment were significantly decreased compared with baseline levels (ALT: 54.6 ± 36.3 vs 20.3 ± 13.3, P01 < 0.001; 54.6 ± 36.3 vs 17.1 ± 6.9, P02 < 0.001. AST: 50.8 ± 33.1 vs 24.4 ± 10.4, P01 < 0.001; 50.8 ± 33.1 vs 22.4 ± 7.0, P02 < 0.001). Post-treatment FIB-4 score exhibited a continued reduction (4.07 ± 4.35 vs 2.94 ± 2.76, P01 < 0.001; 2.94 ± 2.76 vs 2.61 ± 2.21, P12 = 0.003) compared with that at baseline (Table 2). At the end of treatment, eGFR level had a significant decrease (eGFR: 87.1 ± 19.5 vs 81.2 ± 20.0, P01 = 0.001), serum creatinine (Scr) and uric acid (UA) levels were significantly increased compared with baseline levels (Scr: 83.6 ± 17.9 vs 88.8 ± 19.4, P01 < 0.001; UA: 320.8 ± 76.3 vs 354.5 ± 87.6, P01 < 0.001), although no significant improvements were observed at 24 wk after the end of treatment (Scr: 83.6 ± 17.9 vs 86.8 ± 19.1, P02 = 0.039; UA: 320.8 ± 76.3 vs 345.9 ± 89.4, P02 = 0.001). BUN level at the end of treatment had no significant changes compared with baseline level, while an increased BUN level was observed at 24 wk after the end of treatment (5.17 ± 1.50 vs 5.65 ± 1.80, P02 = 0.009) (Figure 2). DAAs regimens and time points had no interactive effects on the changes of hepatorenal function indices, and the interactive effects on changes of RBC and HGB may be caused by RBV (Table 2). Combined DAAs treatment had no significant effect on the WBC count, RBC count, or HGB concentration; however, the PLT count had a remarkable increase at 24 wk after the end of treatment compared with that at baseline (147.1 ± 65.1 vs 158.2 ± 65.9, P02 = 0.008) (Table 2).
| T0 | T1 | T2 | P01 | P12 | P02 | P(DAAs*Time) | |
| FIB-4 score | 4.07 ± 4.35 | 2.94 ± 2.76 | 2.61 ± 2.21 | 0.001 | 0.003 | < 0.001 | 0.399 |
| ALT (IU/L) | 54.6 ± 36.3 | 20.3 ± 13.3 | 17.1 ± 6.9 | < 0.001 | 0.061 | < 0.001 | 0.594 |
| AST (IU/L) | 50.8 ± 33.1 | 24.4 ± 10.4 | 22.4 ± 7.0 | < 0.001 | 0.006 | < 0.001 | 0.733 |
| eGFR(mL/min/1.73 m2) | 87.1 ± 19.5 | 81.2 ± 20.0 | 83.6 ± 21.2 | 0.001 | 0.174 | 0.097 | 0.646 |
| Scr (μmol/L) | 83.6 ± 17.9 | 88.8 ± 19.4 | 86.8 ± 19.1 | < 0.001 | 0.137 | 0.039 | 0.481 |
| UA (μmol/L) | 320.8 ± 76.3 | 354.5 ± 87.6 | 345.9 ± 89.4 | < 0.001 | 0.212 | 0.001 | 0.299 |
| BUN (mmol/L) | 5.17 ± 1.50 | 5.12 ± 1.40 | 5.65 ± 1.80 | 0.757 | 0.003 | 0.009 | 0.858 |
| WBC (109/L) | 4.85 ± 1.67 | 4.91 ± 1.54 | 5.00 ± 1.34 | 0.725 | 0.595 | 0.342 | 0.536 |
| RBC (1012/L) | 4.42 ± 0.64 | 4.43 ± 0.68 | 4.50 ± 0.68 | 0.822 | 0.345 | 0.223 | 0.023 |
| HGB (g/L) | 140.8 ± 17.2 | 139.4 ± 20.9 | 141.1 ± 21.1 | 0.467 | 0.443 | 0.860 | 0.026 |
| PLT (109/L) | 147.1 ± 65.1 | 153.6 ± 67.5 | 158.2 ± 65.9 | 0.053 | 0.117 | 0.008 | 0.540 |
The frequency of AEs during treatment was 33.3% (18/54). The major AEs were fatigue (16.7%), headache (7.4%), anorexia (7.4%), and insomnia (5.6%), and most of them were mild and tolerable. One GT1b patients treated with SOF + DAC discontinued the treatment at 8 wk due to the development of renal area pain (Table 3). The common AEs during traditional PR treatment, like fever, anemia, neutropenia, and thrombocytopenia, rarely occurred during DAAs treatment, and only two patients treated with SOF + RBV were observed with mild anemia.
| Adverse event | n (%) |
| Fatigue | 9 (16.7) |
| Headache | 4 (7.4) |
| Anorexia | 4 (7.4) |
| Insomnia | 3 (5.6) |
| Anemia | 2 (3.7) |
| Pruritus | 1 (1.9) |
| Anxiety | 1 (1.9) |
| Renal area pain | 1 (1.9) |
| Treatment discontinuation | 1 (1.9) |
| Total | 18 (33.3) |
The availability and development of DAAs revolutionized the management of HCV infection. In America, Europe, Japan, and many other countries, DAAs achieved high SVR rates with a low frequency of AEs in clinical trials and real-world cohorts[7], while limited data were available in China. Considering the ethnic, regional, and virological differences, we analyzed the efficacy and safety of combined DAAs for treatment of 54 Chinese CHC patients in a real-world setting.
This study showed a promising SVR rate as those in other countries and areas, while abnormal changes in renal function indices and relative more AEs were unexpected. In this study, 83.3% (45/54) of patients achieved RVR 2 and 94.4% (51/54) of patients achieved SVR 24 which had no significant difference with those reported in previous studies[30-33]. With the application of DAAs, some cases were reported with nephrotoxicity and hepatotoxicity due to DAAs treatment[34,35]. Thus, our study analyzed the changes of hemogram and hepatorenal function indices and the frequency of AEs associated with combined DAAs treatment. After the treatment, liver function indices and FIB-4 score reflecting the liver fibrosis stage had significant improvements. Different with traditional PR treatment, DAAs had no significant effect on hemogram, and along with the improvement of liver function, PLT count at 24 wk after the end of treatment was significantly increased compared with the baseline value. However, the mean Scr and UA levels at the end of treatment had a significant elevation compared with those at baseline, and there was no trend toward improvement at 24 wk after the end of treatment. The specific reasons for changes of renal function indices are unknown, considering that no abnormal changes in renal function indices were found in clinical trials[29,36,37]. The potential drug-drug interactions between combined DAAs regimens and complicated concomitant medications in this real-world cohort may be the major reason. Our study showed relatively more AEs associated with the use of combined DAAs treatment, and the major AEs were fatigue, headache, anorexia, and insomnia. Although most of them were mild and tolerable, more attention should be paid during the treatment.
Though based on a small cohort of patients, the abnormal changes in renal function indices and relative more AEs during treatment should be taken as a note of caution. Clinical physicians should implement close renal function monitoring and attach importance to AEs occurring in patients receiving combined DAAs treatment.
Treatment of hepatitis C virus (HCV) infection has reached a new era with the approval of directly acting antivirals (DAAs). All-oral DAAs combination regimens have achieved high sustained virological response (SVR) rates with minor contraindications and adverse events. China has the greatest number of chronic hepatitis C (CHC) cases worldwide, with an estimated 29.8 million patients infected, while there had been limited data on the use of combined DAAs treatment in a real-world setting in China.
In China, there have been limited data on the use of combined DAAs treatment in a real-world setting. The research hotspot is to show the efficacy of DAAs for treatment of Chinese CHC patients and explore the effects of DAAs on hemogram and hepatorenal function indices, and the frequency of adverse events (AEs) during treatment in a real-world setting.
The changes of clinical indices among different observing points during combined DAAs treatment were analysed. At the end of treatment, eGFR level had a significant decrease, serum creatinine and uric acid levels were significantly increased compared with baseline levels, although no significant improvements were observed at 24 wk after the end of treatment. On the other hand, the current data also showed a high frequency of AEs during combined DAAs treatment (33.3%), and the major AEs were fatigue, headache, anorexia, and insomnia.
The data in this study showed the abnormal changes in renal function indices and relative high frequency of AEs during combined DAAs treatment. This study would remind clinical physicians of implementing close renal function monitoring and focusing on AEs occurring in patients receiving combined DAAs treatment.
DAAs are inhibitors directly acting on different viral targets, including NS3 protease inhibitors, NS5A inhibitors, nucleoside/nucleotide analogues, and non-nucleoside inhibitors of the RNA-dependent RNA polymerase. In 2011, Telaprevir and Boceprevir opened a new area for HCV therapy, while these two NS3/4 protease inhibitors were given in combination with pegylated interferon and ribavirin. Subsequent all-oral DAAs combination regimens have achieved high SVR rates with fewer contraindications and AEs.
The chosen topic is currently one of the hot topics and of great interest to a lot of clinicians. The last few years have witnessed significant progress in HCV therapy by replacing of IFN + ribavirin combined therapy with oral compounds acting directly to inhibit HCV replication (DAAs). DAAs target multiple steps in the HCV life cycle and are currently used in combination to treat HCV infection without need of IFN.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Otsuka M, Said ZNA S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1362] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 2. | Peiffer KH, Sommer L, Susser S, Vermehren J, Herrmann E, Döring M, Dietz J, Perner D, Berkowski C, Zeuzem S. Interferon lambda 4 genotypes and resistance-associated variants in patients infected with hepatitis C virus genotypes 1 and 3. Hepatology. 2016;63:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Chen ZW, Li H, Ren H, Hu P. Global prevalence of pre-existing HCV variants resistant to direct-acting antiviral agents (DAAs): mining the GenBank HCV genome data. Sci Rep. 2016;6:20310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Pawlotsky JM. Hepatitis C Virus Resistance to Direct-Acting Antiviral Drugs in Interferon-Free Regimens. Gastroenterology. 2016;151:70-86. [PubMed] |
| 5. | Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 6. | Everson GT, Towner WJ, Davis MN, Wyles DL, Nahass RG, Thuluvath PJ, Etzkorn K, Hinestrosa F, Tong M, Rabinovitz M. Sofosbuvir With Velpatasvir in Treatment-Naive Noncirrhotic Patients With Genotype 1 to 6 Hepatitis C Virus Infection: A Randomized Trial. Ann Intern Med. 2015;163:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Li G, De Clercq E. Current therapy for chronic hepatitis C: The role of direct-acting antivirals. Antiviral Res. 2017;142:83-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 443] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 9. | Aqel BA, Pungpapong S, Leise M, Werner KT, Chervenak AE, Watt KD, Murphy JL, Ryland K, Keaveny AP, McLemore R. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 in patients with cirrhosis. Hepatology. 2015;62:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, Su F, Berry K. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151:457-471.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 11. | Kawakami Y, Imamura M, Ikeda H, Suzuki M, Arataki K, Moriishi M, Mori N, Kokoroishi K, Katamura Y, Ezaki T. Pharmacokinetics, efficacy and safety of daclatasvir plus asunaprevir in dialysis patients with chronic hepatitis C: pilot study. J Viral Hepat. 2016;23:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Hawkins C, Grant J, Ammerman LR, Palella F, Mclaughlin M, Green R, Mcgregor D, Stosor V. High rates of hepatitis C virus (HCV) cure using direct-acting antivirals in HIV/HCV-coinfected patients: a real-world perspective. J Antimicrob Chemother. 2016;71:2642-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Kanda T, Yasui S, Nakamura M, Suzuki E, Arai M, Haga Y, Sasaki R, Wu S, Nakamoto S, Imazeki F. Daclatasvir plus Asunaprevir Treatment for Real-World HCV Genotype 1-Infected Patients in Japan. Int J Med Sci. 2016;13:418-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Sogni P, Gilbert C, Lacombe K, Piroth L, Rosenthal E, Miailhes P, Gervais A, Esterle L, Chas J, Poizot-Martin I. All-oral Direct-acting Antiviral Regimens in HIV/Hepatitis C Virus-coinfected Patients With Cirrhosis Are Efficient and Safe: Real-life Results From the Prospective ANRS CO13-HEPAVIH Cohort. Clin Infect Dis. 2016;63:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373:2599-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 864] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 16. | Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373:2608-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 649] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 17. | Woode ME, Abu-Zaineh M, Perriëns J, Renaud F, Wiktor S, Moatti JP. Potential market size and impact of hepatitis C treatment in low- and middle-income countries. J Viral Hepat. 2016;23:522-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Han QY, Liu ZW. Current treatment of chronic hepatitis C in China: Dilemma and potential problems. World J Gastroenterol. 2016;22:4615-4618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Chen LM, He M. Hepatitis C Virus in mainland China with an emphasis on genotype and subtype distribution. Virol J. 2017;14:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Nitta S, Asahina Y, Matsuda M, Yamada N, Sugiyama R, Masaki T, Suzuki R, Kato N, Watanabe M, Wakita T. Effects of Resistance-Associated NS5A Mutations in Hepatitis C Virus on Viral Production and Susceptibility to Antiviral Reagents. Sci Rep. 2016;6:34652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Wang LF, Wu CH, Shan Y, Fan XH, Huo N, Lu HY, Xu XY. Prevalence of abnormal glycometabolism in patients with chronic hepatitis C and related risk factors in China. Chin Med J (Engl). 2011;124:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [PubMed] |
| 23. | Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P; FIBROSTIC study group. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 24. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [PubMed] |
| 25. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [PubMed] |
| 26. | Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937-2944. [PubMed] |
| 27. | Zhang R, Shao C, Huo N, Li M, Xu X. Association of IL28B Genotypes and Baseline Serum Interferon-γ-Inducible- Protein-10 Levels with Treatment Response in Hepatitis C Virus Patients in China. Gut Liver. 2016;10:446-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Bai L, Feng ZR, Lu HY, Li WG, Yu M, Xu XY. Prevalence of antinuclear and anti-liver-kidney-microsome type-1 antibodies in patients with chronic hepatitis C in China. Chin Med J (Engl). 2009;122:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Chinese Society of Hepatology, Chinese Medical Association, Wei L; Chinese Society of Infectious Diseases, Chinese Medical Association, Hou JL. [The guideline of prevention and treatment for hepatitis C: a 2015 update]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:906-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 30. | Ji D, Chen GF, Wang C, Wang YD, Shao Q, Li B, Zhao J, You SL, Hu JH, Liu JL. Twelve-week ribavirin-free direct-acting antivirals for treatment-experienced Chinese with HCV genotype 1b infection including cirrhotic patients. Hepatol Int. 2016;10:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Werner CR, Schwarz JM, Egetemeyr DP, Beck R, Malek NP, Lauer UM, Berg CP. Second-generation direct-acting-antiviral hepatitis C virus treatment: Efficacy, safety, and predictors of SVR12. World J Gastroenterol. 2016;22:8050-8059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Jiménez-Pérez M, González-Grande R, España Contreras P, Pinazo Martínez I, de la Cruz Lombardo J, Olmedo Martín R. Treatment of chronic hepatitis C with direct-acting antivirals: The role of resistance. World J Gastroenterol. 2016;22:6573-6581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 33. | Satsangi S, Mehta M, Duseja A, Taneja S, Dhiman RK, Chawla Y. Dual treatment with sofosbuvir plus ribavirin is as effective as triple therapy with pegylated interferon plus sofosbuvir plus ribavirin in predominant genotype 3 patients with chronic hepatitis C. J Gastroenterol Hepatol. 2017;32:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Dyson JK, Hutchinson J, Harrison L, Rotimi O, Tiniakos D, Foster GR, Aldersley MA, McPherson S. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J Hepatol. 2016;64:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Bunnell KL, Vibhakar S, Glowacki RC, Gallagher MA, Osei AM, Huhn G. Nephrotoxicity Associated with Concomitant Use of Ledipasvir-Sofosbuvir and Tenofovir in a Patient with Hepatitis C Virus and Human Immunodeficiency Virus Coinfection. Pharmacotherapy. 2016;36:e148-e153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 910] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 37. | AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 992] [Article Influence: 99.2] [Reference Citation Analysis (0)] |