Published online Jun 14, 2017. doi: 10.3748/wjg.v23.i22.4047
Peer-review started: February 8, 2017
First decision: February 23, 2017
Revised: March 10, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: June 14, 2017
Processing time: 133 Days and 23.2 Hours
To test the hypothesis that K8/K18 variants predispose humans to non-alcoholic fatty liver disease (NAFLD) progression and its metabolic phenotypes.
We selected a total of 373 unrelated adult subjects from our Physical Examination Department, including 200 unrelated NAFLD patients and 173 controls of both genders and different ages. Diagnoses of NAFLD were established according to ultrasonic signs of fatty liver. All subjects were tested for population characteristics, lipid profile, liver tests, as well as glucose tests. Genomic DNA was obtained from peripheral blood with a DNeasy Tissue Kit. K8/K18 coding regions were analyzed, including 15 exons and exon-intron boundaries.
Among 200 NAFLD patients, 10 (5%) heterozygous carriers of keratin variants were identified. There were 5 amino-acid-altering heterozygous variants and 6 non-coding heterozygous variants. One novel amino-acid-altering heterozygous variant (K18 N193S) and three novel non-coding variants were observed (K8 IVS5-9A→G, K8 IVS6+19G→A, K18 T195T). A total of 9 patients had a single variant and 1 patient had compound variants (K18 N193S+K8 IVS3-15C→G). Only one R341H variant was found in the control group (1 of 173, 0.58%). The frequency of keratin variants in NAFLD patients was significantly higher than that in the control group (5% vs 0.58%, P = 0.015). Notably, the keratin variants were significantly associated with insulin resistance (IR) in NAFLD patients (8.86% in NAFLD patients with IR vs 2.5% in NAFLD patients without IR, P = 0.043).
K8/K18 variants are overrepresented in Chinese NAFLD patients and might accelerate liver fat storage through IR.
Core tip: This study presents the first investigation of the association between keratin 8 and 18 (K8/K18) variants and non-alcoholic fatty liver disease (NAFLD) in a Chinese population. We found an increased frequency of variants in NAFLD patients vs controls. We also identified a new amino acid-altering variant of K18. The results demonstrate that keratin variants are overrepresented in Chinese NAFLD patients and might accelerate liver fat storage through insulin resistance.
- Citation: Li R, Liao XH, Ye JZ, Li MR, Wu YQ, Hu X, Zhong BH. Association of keratin 8/18 variants with non-alcoholic fatty liver disease and insulin resistance in Chinese patients: A case-control study. World J Gastroenterol 2017; 23(22): 4047-4053
- URL: https://www.wjgnet.com/1007-9327/full/v23/i22/4047.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i22.4047
Keratin (K) proteins form intermediate filaments (IFs) in epithelial cells, which constitute the cytoskeleton along with actin filaments and microtubules[1,2]. Keratin proteins are primarily expressed in epithelial tissues, hair and skin appendages, and they are classified into relatively acidic type I (K9-K28, K31-K40) and relatively basic type II keratins (K1-K8, K71-K86)[3,4]. Type I and type II keratins exist as paired polymeric filaments that display a tripartite structure containing a conserved α-helical central rod domain flanked by less conserved N-terminal head and C-terminal tail domains[3,5]. For example, K5/K14 and K1/K10 are found in basal and suprabasal keratinocytes, whereas K8/K18 are found in adult hepatocytes and other simple epithelial cells[6]. Moreover, human association studies have identified mutations in keratins that can cause or predispose carriers to a broad range of human diseases, including multiple liver diseases[1,7]. Finally, the importance of K8/K18 in protecting hepatocytes from apoptosis was clearly demonstrated in keratin-related genetically engineered animal models[6,8].
Non-alcoholic fatty liver disease (NAFLD), which is regarded as a hepatic manifestation of metabolic syndrome, has become the most common chronic liver disease worldwide, as it affects 20%-50% of the general population in affluent countries[9,10], and 15% of individuals in China[11]. NAFLD is strongly concomitant with obesity, hypertriglyceridemia, type 2 diabetes mellitus, and insulin resistance (IR)[12]. It is generally accepted that the initiating events in NAFLD depend on the development of obesity and IR in adipose tissue and the liver.
There are accumulating examples of K8/K18 involvement in the glucose-insulin cross-talk, which support the impact of K8/K18 IFs on insulin-dependent glucose metabolism regulation in the liver and its implication in glucose- or insulin-associated diseases[12,13]. Lower fasting glucose levels, increased glucose tolerance and insulin sensitivity, reduced glucose-stimulated insulin secretion and decreased pancreatic insulin content have been shown to occur in K8 knockout mice[14]. The mislocalization of glucose transporter (GLUT) and hexokinase (HK) status may contribute to the modulation of IFs in hepatocytes and hepatoma cells that lack K8/K18[14,15].
Subsequent studies have established that keratin mutation plays an important role in liver diseases and glucose metabolism. Therefore, we hypothesized that K8/K18 variants may contribute to susceptibility to NAFLD. No studies have been reported on the association between K8/K18 mutations and NAFLD. Therefore, we tested our hypothesis by sequencing the K8/K18 coding regions in genomic DNA from 200 NAFLD subjects. Herein, we report our positive findings.
A total of 373 unrelated adult subjects were selected from our Physical Examination Department from January 2011 to December 2012, including 200 unrelated Chinese patients of both genders and different ages (164 males, 36 females; mean age, 40.85 ± 9.9 years) and 173 healthy controls who were matched for sex and age (130 males, 43 females; mean age, 39.55 ± 9.9 years). Diagnoses of NAFLD were performed under standard clinical evaluation conditions by ultrasonography according to the AASLD criteria[16]. Other causes of liver disease were excluded, including increased alcohol intake (> 210/140 g weekly for males/females), viral and autoimmune hepatitis, hereditary hemochromatosis, Wilson’s disease and alpha1-antitrypsin deficiency. Controls were confirmed as healthy based on a medical history, general examinations and laboratory examinations at the same hospital. Each participant underwent an anthropometric assessment, including measurements of weight and stature. Body mass index (BMI) was calculated as weight (kg)/stature (m2). All subjects received blood tests after an overnight 12 h fast, including serum triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), serum uric acid (UA), fasting blood glucose (FBG), fasting insulin (FIN), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). IR was defined by the homeostasis model assessment-IR (HOMA-IR) index. As reported in an epidemiology survey carried out in China, IR was defined as HOMA-IR > 2.69 (i.e., exceeding the 75% percentile of HOMA-IR in normal glucose tolerance subjects)[17,18].
Genomic DNA was obtained from peripheral blood (EDTA anticoagulation) using a DNeasy Tissue Kit (Tiangen, Biotech, Beijing, China). The entire K8/K18 coding regions (15 exons and their adjacent exon-intron boundaries) of the DNA fragments were analyzed, which had been amplified with a Touchdown polymerase chain reaction protocol using Premix PrimeSTAR HS (TaKaRa, Biotechnology, Dalian, China) and previously described primers[19] to obtain a high amplification specificity. Messenger RNA sequences of K8 (NM002273) and K18 (NM000224) were used to localize coding variants, while genomic sequences (hKRT8 [M34482] and hKRT18 [AF179904]) were employed for noncoding variants.
Statistical analyses were performed using SPSS statistical software, version 20.0 for Windows (SPSS Inc., Chicago, IL, United States). Continuous variables are expressed as mean ± SD. For continuous variables, a two-tailed t-test was used for two-group comparisons, whereas Kruskal-Wallis nonparametric one-way analysis of variance was used to compare qualitative variables. K8/K18 variant frequencies in the NAFLD patient and control groups were compared by the two-tailed Fisher exact probability test. P values less than 0.05 were considered statistically significant.
Demographics and clinical information for the NAFLD patients and control groups are summarized in Table 1. A total of 200 patients with NAFLD were included. As expected, most (82%) patients were male. Compared with the control group, NAFLD patients showed higher ALT, AST, FBG, TC, TG, LDL and UA levels and lower levels of HDL.
| Controls (n = 173) | NAFLD patients (n = 200) | P value | |
| Sex (male, %) | 130 (75.14) | 164 (82.00) | 0.107 |
| Age (yr) | 39.55 ± 9.92 | 40.85 ± 9.89 | 0.101 |
| ALT (U/L) | 21.65 ± 12.69 | 43.63 ± 29.16 | < 0.0001 |
| AST (U/L) | 24.74 ± 6.52 | 33.85 ± 12.93 | < 0.0001 |
| FBG (mmol/L) | 4.89 ± 0.62 | 5.40 ± 1.45 | < 0.0001 |
| TC (mmol/L) | 4.71 ± 0.77 | 5.68 ± 1.09 | < 0.0001 |
| TG (mmol/L) | 1.29 ± 0.99 | 2.86 ± 2.14 | < 0.0001 |
| HDL (mmol/L) | 1.22 ± 0.29 | 1.07 ± 0.23 | < 0.0001 |
| LDL (mmol/L) | 2.80 ± 0.61 | 3.32 ± 0.95 | < 0.0001 |
| UA (μmol/L) | 331.33 ± 88.61 | 412.42 ± 96.60 | < 0.0001 |
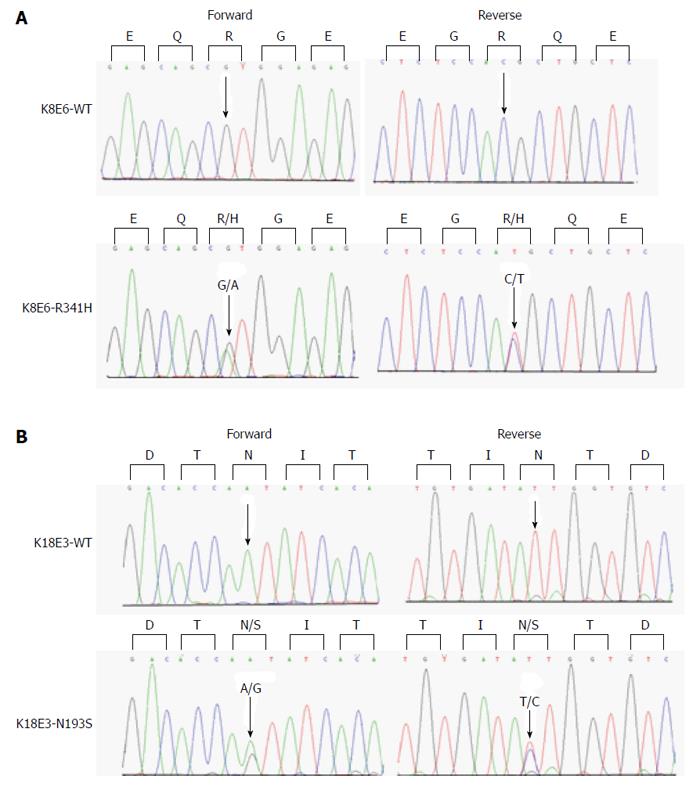
We identified keratin heterozygous variants in 10 (5%) of 200 NAFLD patients, including 5 carriers of amino-acid-altering heterozygous variants and 6 carriers of non-coding heterozygous variants (Table 2). Among 200 NAFLD patients, 2 (1%) carried R341H heterozygous exonic variants (Figure 1A), which is lower than the previously reported frequency. Additionally, one novel amino acid-altering heterozygous variant (K18 N193S) and three novel non-coding variants (K8 IVS5-9A→G, K8 IVS6+19G→A, K18 T195T) were observed (Figure 1B). A total of 9 patients had a single variant and 1 patient had compound variants (K18 N193S+K8 IVS3-15C→G). A previously described silent and common heterozygous K8 L227L variant, which we detected in 43% of patients (not shown), is not included in Table 2 or any subsequent analysis.
| Keratin gene | Variant | NAFLD | ||||
| Nucleotide | Amino acid | Control (n = 173) | Total (n = 200) | NAFLD with IR (n = 79) | NAFLD without IR (n = 121) | |
| K8 | 1022G→A | R341H | 1 | 2 | 2 | 0 |
| 904C→T | R302C | 0 | 1 | 1 | 0 | |
| 1381G→A | V461M | 0 | 1 | 0 | 1 | |
| IVS5-9A→G (new) | 0 | 1 | 1 | 0 | ||
| IVS3-26 C→T | 0 | 1 | 1 | 0 | ||
| K18 | IVS6+19G→A (new) | 0 | 1 | 1 | 0 | |
| IVS6+17C→T | 0 | 1 | 0 | 1 | ||
| IVS3-15C→G | 0 | 11 | 11 | 0 | ||
| 1590C→G | N193S (new) | 0 | 11 | 11 | 0 | |
| 1448A→G | T195T (new) | 0 | 1 | 0 | 1 | |
| Total (%) | 1 (0.58) | 10 (5.00) | 7 (8.86) | 3 (2.48) | ||
We found an increased frequency of variants in NAFLD patients vs controls (5% vs 0.58%, P = 0.015). To explore whether K8/K18 variants could affect the biochemical parameters, we compared non-carriers with variant carriers in all NAFLD patients. We found that there was no significant difference in clinical features, including BMI, liver biochemistry, glucose, lipids or HOMA-IR (Table 3). However, after dividing patients into those with or without IR, keratin variants showed a significant association with IR in NAFLD patients (Table 2; 8.86% in NAFLD patients with IR vs 2.5% in NAFLD patients without IR, P = 0.043).
| Keratin variant carriers | P value | ||
| No (n = 190) | Yes (n = 10) | ||
| Sex (male, %) | 155 (81.58) | 9 (90.00) | 0.436 |
| Age (yr) | 40.75 ± 9.87 | 42.60 ± 10.63 | 0.603 |
| Diastolic pressure (mmHg) | 82.67 ± 10.47 | 85.80 ± 9.38 | 0.330 |
| Systolic pressure (mmHg) | 130.79 ± 14.82 | 134.20 ± 17.07 | 0.550 |
| BMI (kg/m2) | 26.26 ± 2.91 | 26.10 ± 3.09 | 0.877 |
| ALT (U/L) | 35.93 ± 18.35 | 38.40 ± 25.30 | 0.767 |
| AST (U/L) | 34.03 ± 13.08 | 30.40 ± 9.31 | 0.265 |
| TC (mmol/L) | 5.67 ± 1.02 | 6.10 ± 1.69 | 0.453 |
| TG (mmol/L) | 2.85 ± 2.14 | 3.00 ± 2.35 | 0.850 |
| HDL (mmol/L) | 1.09 ± 0.24 | 1.10 ± 0.18 | 0.828 |
| LDL (mmol/L) | 3.31 ± 0.94 | 3.65 ± 1.12 | 0.374 |
| FBG (mmol/L) | 5.38 ± 1.43 | 5.91 ± 1.44 | 0.281 |
| FINS (mmol/L) | 11.17 ± 4.52 | 12.73 ± 6.00 | 0.439 |
| HOMA-IR | 2.64 ± 1.14 | 3.25 ± 1.50 | 0.237 |
| UA (μmol/L) | 414.06 ± 96.89 | 381.50 ± 89.88 | 0.292 |
Previous reports suggested that K8/K18 variants predispose carriers to various types of liver injury, but it is not known whether K8 or K18 variants show an association with NAFLD. For the first time, our present study investigated the association between K8/K18 variants and NAFLD in a Chinese population. We analyzed K8/K18 variants in NAFLD patients and observed the potential association of keratin variants with NAFLD.
Our results demonstrate that keratin variants are overrepresented in NAFLD patients. Moreover, K8 has the most keratin variants, which is consistent with data from patients with chronic hepatitis C, primary biliary cirrhosis and acute liver failure. This finding highlights the importance of K8/K18 gene variants in NAFLD. The frequency of keratin variants presented here is somewhat lower compared with those in patients who suffer from other types of liver disease (e.g., 13.1% and 12.4%[20,21]), as previously reported, but it is close to that observed in an Asian cohort in a United States study[22]. Multiple K8/K18 variants have been verified and R341H represents the most common amino acid-altering K8/K18 variant in Chinese populations. As confirmed herein, the K8 variant R341H also exclusively associates with the intronic K8 IVS7+10delC deletion[19]. We also found a new amino acid-altering variant N193S of K18 in a patient who simultaneously carried an intronic variant IVS3-15C→G. It remains to be determined whether this nucleotide error at one site acts in conjunction with the other.
Hepatic steatosis occurs when insulin signaling is impaired, with the development of IR driven by adipose tissue and the liver, along with the sustained excess delivery of fatty acids to the liver. Here, we observed a correlation of IR with keratin variants. This association suggests that K8/K18 mutations result in increased liver fat, probably through the hepatic IR, in accordance with other studies. For example, early work using K8-null mice found reduced fasting blood glucose levels, increased glucose tolerance and insulin sensitivity, altered insulin vesicle morphology, and reduced pancreatic insulin levels[14]. Similarly, a study conducted in Finland shows that K8/K18 loss in hepatoma cells leads to distinct alterations in HK status, which are associated with a differential modulation of insulin signaling-dependent regulation of glucose-mediated glycogen formation and proliferative capacity[23]. However, differences in K8/K18 IF loss cause the mislocalization of the GLUT 1-4 transport proteins. Additionally, it is important to consider the cellular context, namely, cultured hepatic cells[23] vs in vivo embryonic[24] conditions. This reveals distinctive increases in glucose uptake, glucose-6-phosphate formation, lactate release, and glycogen formation in K8/K18 IF- deficient hepatocytes vs respective IF-containing counterparts. Moreover, compelling evidence suggests that the shape and distribution of mitochondria are modulated by cytoskeletal proteins, including keratins[25]. Together, these findings suggest that hepatocyte keratins have a central role in the systemic regulation of glucose. Although the molecular mechanisms that underlie these metabolic perturbations are unclear, K8/K18 IFs likely modify liver fat content via the insulin signaling pathway[26,27].
This study was limited by the absence of liver biopsies to evaluate the severity of hepatic steatosis and fibrosis stage. The absence of histological evidence may weaken the clinical relevance of these genetic effects, although abdominal ultrasound is generally applied in larger surveys as a noninvasive and convenient tool to diagnose NAFLD[12]. Moreover, cell-based and transgenic mouse studies with genetic K8/K18 mutants or knockouts are needed to verify that keratin variants can cause or predispose carriers to the development of pathologies.
As studies of Asian populations have been limited, we are the first to discuss an association between keratin variants and NAFLD. Our findings provide novel evidence for keratin proteins as modulators in the development of NAFLD and in the pathophysiology of IR. Further studies of K8/K18 mutations as drivers of IR and liver fat accumulation are needed. These observations raise the question of how naturally occurring keratin mutations affect liver fatty in humans, and whether such mutations may similarly affect in vitro models or animals.
Keratins 8 and 18 (K8/K18) protect hepatocytes from various types of injury, and previous reports have suggested that K8/K18 variants predispose carriers to various forms of liver injury. However, it is not known whether K8 or K18 variants have an association with non-alcoholic fatty liver disease (NAFLD). Thus, we tested the hypothesis that K8/K18 variants predispose humans to NAFLD progression and its metabolic phenotypes.
The relationship between keratin variants and liver disease varies in different populations. Therefore, studies enrolling histological evidence and more ethnic groups are required. Further studies are also needed to determine the associations and functional consequences of these variants in NAFLD.
This is the first published study to investigate the relationship between keratin variants and NAFLD. These findings also provide novel evidence for keratin proteins as modulators in the development of NAFLD and in the pathophysiology of insulin resistance.
The association of keratin variants with NAFLD suggests a prominent gene influence on human NAFLD. They should pay more attention to individuals who carries keratin variant, and provide early intervention before disease progression.
Keratins constitute the largest subgroup of intermediate filaments and are expressed primarily in epithelial tissues, hair and skin appendages. NAFLD is the most common cause of liver dysfunction in the Western world. It is a spectrum of liver disease that includes simple steatosis, fatty infiltration plus inflammation, and hepatocellular ballooning degeneration, progressing to fibrosis and ultimately cirrhosis.
This is a very interesting human study that suggests an association between keratin 8/18 variants and NAFLD. Also the authors successfully showed that IR is a potential mediator of this association. These are completely novel viewpoints in NAFLD research. This study is a good start for future research in this field.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Higuera-de la Tijera MF, Ikura Y S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Haines RL, Lane EB. Keratins and disease at a glance. J Cell Sci. 2012;125:3923-3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Pan X, Hobbs RP, Coulombe PA. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr Opin Cell Biol. 2013;25:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol. 2002;14:110-122. [PubMed] |
| 4. | Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006;174:169-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 514] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 5. | Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1059] [Cited by in RCA: 1083] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 6. | Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119:1794-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Strnad P, Paschke S, Jang KH, Ku NO. Keratins: markers and modulators of liver disease. Curr Opin Gastroenterol. 2012;28:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009;119:1784-1793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1620] [Article Influence: 115.7] [Reference Citation Analysis (1)] |
| 10. | Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 11. | Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 12. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 13. | Herrmann H, Strelkov SV, Burkhard P, Aebi U. Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Invest. 2009;119:1772-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Alam CM, Silvander JS, Daniel EN, Tao GZ, Kvarnström SM, Alam P, Omary MB, Hänninen A, Toivola DM. Keratin 8 modulates β-cell stress responses and normoglycaemia. J Cell Sci. 2013;126:5635-5644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Roux A, Gilbert S, Loranger A, Marceau N. Impact of keratin intermediate filaments on insulin-mediated glucose metabolism regulation in the liver and disease association. FASEB J. 2016;30:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1357] [Article Influence: 104.4] [Reference Citation Analysis (4)] |
| 17. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3179] [Article Influence: 353.2] [Reference Citation Analysis (4)] |
| 18. | Zhang L, Chen S, Deng A, Liu X, Liang Y, Shao X, Sun M, Zou H. Association between lipid ratios and insulin resistance in a Chinese population. PLoS One. 2015;10:e0116110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Strnad P, Lienau TC, Tao GZ, Lazzeroni LC, Stickel F, Schuppan D, Omary MB. Keratin variants associate with progression of fibrosis during chronic hepatitis C infection. Hepatology. 2006;43:1354-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Strnad P, Zhou Q, Hanada S, Lazzeroni LC, Zhong BH, So P, Davern TJ, Lee WM, Omary MB. Keratin variants predispose to acute liver failure and adverse outcome: race and ethnic associations. Gastroenterology. 2010;139:828-835, 835.e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Ku NO, Lim JK, Krams SM, Esquivel CO, Keeffe EB, Wright TL, Parry DA, Omary MB. Keratins as susceptibility genes for end-stage liver disease. Gastroenterology. 2005;129:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Usachov V, Urban TJ, Fontana RJ, Gross A, Iyer S, Omary MB, Strnad P. Prevalence of genetic variants of keratins 8 and 18 in patients with drug-induced liver injury. BMC Med. 2015;13:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Mathew J, Loranger A, Gilbert S, Faure R, Marceau N. Keratin 8/18 regulation of glucose metabolism in normal versus cancerous hepatic cells through differential modulation of hexokinase status and insulin signaling. Exp Cell Res. 2013;319:474-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Vijayaraj P, Kröger C, Reuter U, Windoffer R, Leube RE, Magin TM. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J Cell Biol. 2009;187:175-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Anesti V, Scorrano L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta. 2006;1757:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 361] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 27. | Hamer I, Foti M, Emkey R, Cordier-Bussat M, Philippe J, De Meyts P, Maeder C, Kahn CR, Carpentier JL. An arginine to cysteine(252) mutation in insulin receptors from a patient with severe insulin resistance inhibits receptor internalisation but preserves signalling events. Diabetologia. 2002;45:657-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |