Published online Jun 14, 2017. doi: 10.3748/wjg.v23.i22.4039
Peer-review started: February 5, 2017
First decision: February 23, 2017
Revised: March 28, 2017
Accepted: May 9, 2017
Article in press: May 9, 2017
Published online: June 14, 2017
Processing time: 137 Days and 8.8 Hours
To evaluate circulating IL9 in inflammatory bowel disease and disease-associated anemia/cachexia and assess its potential as a mucosal healing marker.
Serum IL9 as well as other cytokines (IL1β, IL6, IL13, IFNγ, TNFα, and VEGF-A) were determined in 293 individuals: 97 patients with Crohn’s disease (CD) and 74 with ulcerative colitis (UC) and in 122 apparently healthy controls. The clinical activity of CD and UC was expressed in terms of the Crohn’s Disease Activity Index (CDAI) and the Mayo Scoring System (MDAI), respectively, and the severity of bowel inflammation in UC patients was assessed using Mayo endoscopic score. Cytokine concentrations were measured by a flow cytometry-based method using Luminex xMAP® technology. High-sensitive C-reactive protein concentrations (hsCRP) were determined in CD and UC patients using the enhanced immunoturbidimetric method.
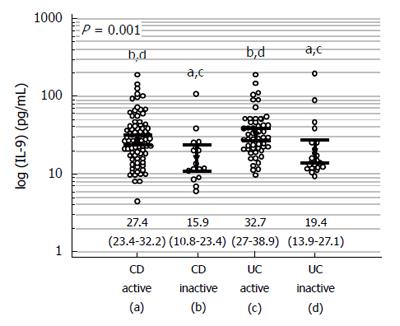
Systemic IL9 was significantly lower in healthy individuals [9 pg/mL (95%CI: 8.2-10)] than in patients with inflammatory bowel disease (IBD): both inactive [14.3 pg/mL (11.9-19.9)] and active [27.6 pg/mL (24.5-32), P < 0.0001]. Cytokine concentrations were significantly higher in active CD [27.4 pg/mL (23.4-32.2)] and in active UC [32.7 pg/mL (27-38.9)] compared to inactive diseases [15.9 pg/mL (10.8-23.4) in CD and 19.4 pg/mL (13.9-27.1) in UC, P = 0.001]. IL9 correlated weakly with CDAI (ρ = 0.32, P = 0.003) and MDAI (ρ = 0.35, P = 0.002) and strongly with endoscopic inflammation in UC (ρ = 0.74, P < 0.0001). As a negative marker of mucosal healing (MH), IL9 had an accuracy superior to hsCRP and IL6 [97% (P < 0.0001), 67% (P = 0.071), and 55% (P = 0.525), respectively]. IL9 was significantly higher in cachectic IBD patients [30.25 pg/mL (24.4-37.5) vs 21.88 pg/mL (18-26.5), P = 0.026] and negatively correlated with hemoglobin concentrations (ρ = -0.27, P < 0.001). Multiple regression showed IL1β and IL13 to be the independent predictors of circulating IL9 in healthy individuals, IFNγ or IL6 in active and inactive UC, respectively, and IL13 and VEGF-A in both active and inactive CD.
The systemic IL9 level is higher in IBD and corresponds with endoscopic inflammation, suggesting its possible application as a negative marker of mucosal healing in UC.
Core tip: Based on a large cohort of patients, our results confirm elevation of IL9 in inflammatory bowel disease (IBD). Additionally, the data demonstrate associations between IL9 and both anemia and wasting syndromes accompanying IBD. Importantly, they show that an elevation in systemic IL9 in ulcerative colitis (UC) corresponds to mucosal inflammation, with IL9 displaying a high level of accuracy as a negative marker of mucosal healing. Also, our results demonstrate IL9 to be more tightly associated with proinflammatory and Th1 cytokines in UC and with angiogenic and Th2 cytokines in Crohn’s disease.
- Citation: Matusiewicz M, Neubauer K, Bednarz-Misa I, Gorska S, Krzystek-Korpacka M. Systemic interleukin-9 in inflammatory bowel disease: Association with mucosal healing in ulcerative colitis. World J Gastroenterol 2017; 23(22): 4039-4046
- URL: https://www.wjgnet.com/1007-9327/full/v23/i22/4039.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i22.4039
Inflammatory bowel disease (IBD) is a group of chronic conditions of the gastrointestinal tract encompassing Crohn’s disease (CD) and ulcerative colitis (UC). The fundamental role of cytokines in the induction and perpetuation of inflammation in IBD is well established. Consequently, cytokines have attracted attention as potential goals of biological therapies, focused either on targeting proinflammatory cytokines and their signaling pathways or on administration of anti-inflammatory cytokines[1].
Recently, a new subtype of helper lymphocytes T has been described and termed Th9 due to their preferential expression of interleukin (IL)-9, a cytokine also found in the repertoire of other T cell subtypes[2,3]. IL9 is a pleiotropic cytokine affecting a variety of cells; yet, its biological activity or physiopathological relevance remains elusive. Nevertheless, the latest findings implicate IL9 in the development of autoimmune diseases[4-7]. Although IBD is not a classic autoimmune disease, IL9 and its receptor have recently been found to contribute to the pathogenesis of UC[8-10]. Inflamed gut biopsies from UC patients have been found to overexpress IL9 at both mRNA and protein levels[9]. In animal models of colitis, IL9 gene expression correlated with the severity of histological inflammation, which could be reduced by IL9-directed antibodies[11]. Evaluation of tissue expression of IL9 gene has been proposed for the monitoring of disease severity in UC. In turn, the cytokine leukocyte expression has been suggested as a systemic inflammatory marker[9].
In recent years the aims of IBD therapy have evolved from the control of symptoms to the control of inflammation as the only action that can in fact change the course of the disease and decrease the risk of complications in terms of therapy intensification, hospitalization and surgery. Evaluation of the activity of IBD remains a challenge in individual patients as well as in designing of clinical trials. Hence, mucosal healing (MH) has become a key end-point of therapy and objective markers of inflammation are intensively searched for. The significance of the optimization of IBD therapy could be even greater as an increasing incidence and prevalence of IBD is observed all over the world[11,12].
Only recently, the elevation of serum IL9 in IBD has been reported and linked with severe prognosis[13]. Supplementing this pioneering research, we aimed to assess circulating cytokines with reference to a large cohort of patients and present these data in the context of other cytokines: proinflammatory (IL1β, IL6, TNFα) and angiogenic (VEGF-A), and of Th1 (IFNγ and TNFα) and Th2 (IL13) subset signatures as well as symptoms accompanying IBD, namely, anemia and cachexia. Circulating IL9, as a serum-based marker, might be a more easily available, less invasive and less expensive indicator of IBD severity and inflammation than tissue and/or leukocyte expression of the IL9 gene. Moreover, if found to follow the pattern described for tissue and leukocyte cytokine, determination of IL9 levels in circulation might be useful as a differential marker in IBD or as a non-invasive MH marker.
Serum IL9 was measured in 293 individuals: 97 patients with CD and 74 with UC and in 122 apparently healthy controls. IBD patients were recruited from the Department of Gastroenterology and Hepatology of Wroclaw Medical University, Poland. Individuals with unclassified colitis or the co-existence of other severe systemic diseases, malignancies, liver diseases, or pregnancies were excluded. The Crohn’s Disease Activity Index (CDAI) was applied for the assessment of CD activity and the Mayo Scoring System (MDAI) for UC activity. The severity of bowel inflammation in UC patients was assessed using Mayo endoscopic score. IBD patients, with a few exceptions, were treated with 5’-aminosalicylate (5’-ASA) derivatives. Cachexia was defined as substantial and involuntary weight loss-higher than 5% of former weight during 3 mo. According to the reference values provided by the Central Hospital Laboratory conducting analyses for our patients, anemia was defined as Hb < 12 g/dL in women and < 13.5 g/dL in men.
Healthy controls were volunteers from among hospital staff or outpatients of the Research, Science, and Educational Center of Dementia Diseases, Scinawa, Poland suffering from headaches or mild cognitive disorders, but otherwise with no significant health history, or from blood donors from the Regional Center for Blood Donation and Therapeutics in Wroclaw, Poland. The following inclusion criteria were applied for the control group: age > 18 years, overall good health condition, and willingness to participate. Exclusion criteria were: pregnancy, active inflammation (based on physical examination and medical history), known severe systemic or dementive disease or depression. The demographic characteristics of the study population are given in Tables 1 and 2.
| Controls | Active IBD | Inactive IBD | P value | |
| n | 122 | 133 | 38 | |
| Age (yr) | 38.5 ± 14.2 | 37.5 ± 13.4 | 37.6 ± 10.5 | 0.825 |
| Gender (F/M) | 55/67 | 63/70 | 16/22 | 0.833 |
| CD active | CD inactive | UC active | UC inactive | P value | |
| N | 81 | 16 | 52 | 22 | |
| Age (yr) | 35.3 ± 12.7 | 35.7 ± 10.5 | 40.9 ± 13.8 | 39 ± 10.5 | 0.081 |
| Gender (F/M) | 42/39 | 5/11 | 21/31 | 11/11 | 0.346 |
| Hb (g/dL) | 12 ± 1.9 | 13.8 ± 2.1 | 12.2 ± 2.4 | 12.8 ± 1.5 | 0.028 |
| PLT (× 103/mm3) | 397 (297-483) | 283 (215-344) | 314 (283-434) | 264 (226-327) | < 0.001 |
| WBC (× 103/mm3) | 7.89 (5.9-10.8) | 6.24 (5.5-7.3) | 7.91 (6.1-9) | 6.2 (5.1-7.6) | 0.046 |
| Protein (g/dL) | 6.97 ± 0.99 | 7.26 ± 0.74 | 6.77 ± 0.88 | 7.23 ± 0.54 | 0.152 |
The study protocol was approved by the Medical Ethics Committee of Wroclaw Medical University and the study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 1983, and informed consent was obtained from all patients.
Blood was drawn following overnight fasting by venipuncture, clotted for 30 min, and centrifuged (15 min, 720 × g). Serum was collected, aliquoted and kept frozen at -80 °C until examination. IL9 as well as IL1β, IL6, IL13, IFNγ, TNFα, and VEGF-A were measured in duplicates by using a flow cytometry-based method utilizing magnetic microspheres conjugated with monoclonal antibodies using the BioPlex 200 platform with HRF (Bio-Rad, United States), incorporating Luminex xMAP® technology, and Bio-Plex Pro™ Human Cytokine, Chemokine, and Growth Factor Magnetic Bead-Based Assays according to the manufacturer’s instructions, except that samples were diluted at a ratio of 1:2 in sample diluent. Standard curves were drawn using 5-PL logistic regression and the data were analyzed using BioPlex Manager 6.0 software. The concentrations of IL9 measured in our study population were within the range determined for human sera by the assay manufacturer[14].
High-sensitive C-reactive protein (hsCRP) was determined using the latex particle-enhanced immunoturbidimetric method with the CRPex-HS CRP test (Good Biotech Corp., Taichung, Taiwan) and a protein multicalibrator (ProDia International, Sharjah, UAE).
Data on hemoglobin and total protein concentrations as well as data on weight loss were retrieved from patients’ medical records.
Data normality was tested using the Kolmogorov-Smirnov test with Lilliefors significance correction and homogeneity of variation using the Levene test. Log-transformation was used if appropriate. Data are presented as medians or means with 95%CI and analyzed using, respectively, the Kruskal-Wallis H test or one-way analysis of variance (ANOVA) with Bonferroni correction for multiple testing and t-test for independent samples. Two-way ANOVA was used to co-examine the influence of MH and cachexia. Logistic regression followed by the Hosmer and Lemeshow goodness of fit test and multiple regression (stepwise method; P < 0.05 as entrance and P > 0.1 as removal criteria) were used to examine IL9 associations. Correlation analysis was conducted using either the Spearman test (ρ) or the Pearson test (r). Frequency analysis was conducted using the χ2 test or Fisher’s exact test. Receiver operating characteristics (ROC) curve analysis was conducted to evaluate IL9 as a disease marker. Marker accuracy was presented as the area under the ROC curve and expressed as a percentage. For an optimal cut-off value, marker sensitivity (sens.) and specificity (spec.) as well as Youden’s J statistic (YI, where J = sensitivity + specificity - 1) were calculated. All calculated probabilities were two-tailed and P-values ≤ 0.05 were considered statistically significant. The analyses were conducted using MedCalc Statistical Software version 16.8.4 (MedCalc Software bvba, Ostend, Belgium; https://http://www.medcalc.org; 2015).
The concentrations of circulating IL-9 were significantly lower in apparently healthy individuals than in patients with IBD; both inactive and active (Figure 1). Patients with active CD and active UC had significantly higher concentrations of IL-9 than patients with inactive diseases, but there was no significant difference between CD and UC (Figure 2).
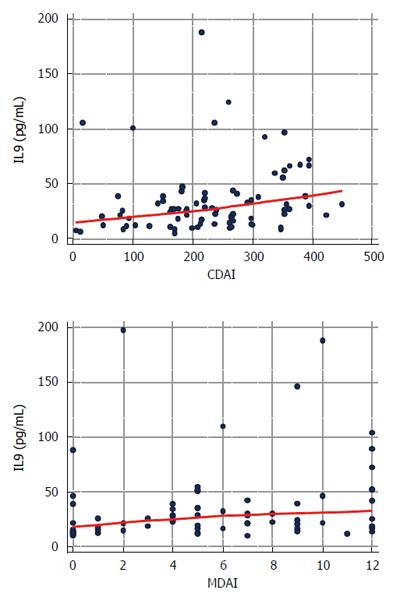
The cytokine concentrations weakly correlated with disease clinical activity scores: ρ = 0.32, P = 0.003 with CDAI and ρ = 0.35, P = 0.002 with MDAI (Figure 3).
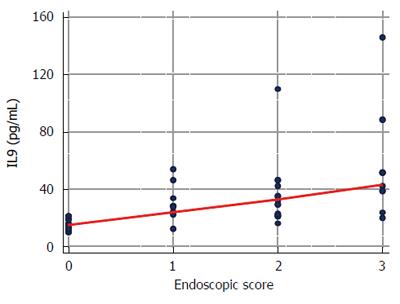
There was a strong positive correlation between IL9 and Mayo endoscopic score: ρ = 0.74, P < 0.0001 (data available for 53 UC patients) (Figure 4). The elevated concentrations of IL9 predicted tissue inflammation on endoscopy (scores 1-3) with an accuracy of 97%. Systemic levels of IL-9 exceeding 20.5 pg/mL were both a sensitive and a specific marker of a lack of mucosal healing (Figure 5).
For comparative purposes, the concentrations of classic markers of systemic inflammation were also evaluated. Circulating IL6 and hsCRP positively correlated with the clinical activity of both CD (ρ = 0.45, P < 0.0001 for IL6 and ρ = 0.45, P = 0.0001 for hsCRP) and UC (ρ = 0.52, P < 0.0001 for IL6 and ρ = 0.67, P < 0.0001 for hsCRP). The associations were stronger than those observed for IL9. However, the association between IL6 and endoscopic findings (ρ = 0.35, P = 0.011) as well as between hsCRP and endoscopic findings (ρ = 0.45, P = 0.002) were less pronounced than that for IL9. As markers of mucosal non-healing, neither hsCRP nor IL6 displayed significant discriminative power (Figure 3).
In logistic regression, log-IL9 was an independent predictor of mucosal non-healing [b = 15.4, P = 0.002; constant-19.2, P = 0.002; goodness of fit (Hosmer and Lemeshow test): χ2 = 2, P = 0.981], correctly classifying 88% of cases, whereas hsCRP and IL6 were not included in the regression model.
IL9 was significantly higher in IBD patients who experienced substantial weight loss than in those who did not: 30.25 pg/mL (24.4-37.5, n = 53) vs 21.88 pg/mL (18-26.5, n = 63), P = 0.026, respectively. Also, IL9 tended to be higher in IBD patients with anemia (29.2 pg/mL (24.8-34.5, n = 91) vs 23.6 pg/mL (20.1-27.6, n = 71), P = 0.069) and was negatively correlated with hemoglobin concentration (ρ = -0.27, P < 0.001).
Cachectic and non-cachectic UC patients differed in their degree of endoscopic inflammation (P = 0.049). When co-examined, both cachexia (P = 0.016) and endoscopic (P < 0.001) inflammation were independently associated with IL9. However, IL9 lost significance as a cachexia predictor when co-examined with classic procachectic cytokines (IL1β, IL6, TNFα), hsCRP and total protein concentration.
In healthy individuals, in univariate analysis, circulating IL9 was positively correlated with inflammatory cytokines (IL1β and IL6), with IL13, a T helper 2-type cytokine, and VEGF-A, a key angiogenic growth factor. In IBD, the correlation pattern was affected by both the disease type and its activity. Circulating IL9 was correlated with inflammatory cytokines in inactive IBD, more pronouncedly in UC than CD. A correlation with IL13 was observed exclusively in CD, and this was more pronounced in patients in remission. Circulating IL9 correlated with VEGF-A in all IBD patients, but the association was more pronounced in inactive CD (Table 3).
| CD active | CD inactive | UC active | UC inactive | Controls | |
| IL1β | NS | 0.58, P = 0.020 | 0.42, P = 0.002 | 0.76, P < 0.0001 | 0.51, P < 0.00011 |
| IL6 | NS | 0.58, P = 0.018 | 0.38, P = 0.005 | 0.77, P < 0.00011 | 0.33, P < 0.001 |
| IL13 | 0.48, P < 0.00011 | 0.62, P = 0.0101 | NS | NS | 0.46, P < 0.00011 |
| IFNγ | 0.29, P = 0.008 | NS | 0.51, P = 0.00011 | 0.74, P = 0.0001 | NS |
| TNFα | NS | NS | NS | 0.65, P = 0.001 | 0.19, P = 0.045 |
| VEGFA | 0.37, P < 0.0011 | 0.77, P < 0.0011 | 0.38, P = 0.006 | 0.46, P = 0.033 | 0.36, P = 0.0001 |
Multiple regression showed IL1β and IL13 to be the independent predictors of circulating IL9 in healthy individuals, IFNγ or IL6 in active and inactive UC, respectively, and IL13 and VEGF-A in both active and inactive CD (Table 2).
The recent discovery of Th9 cells has rekindled interest in IL9 and their targeting has emerged as a potential therapeutic option in UC[15,16]. In turn, IL9 gene expression in bowel tissue and leukocytes has been proposed as a marker of local and systemic inflammation, respectively[10]. The strict association between IL9 and local inflammation is of particular relevance in the light of mucosal healing becoming a key treatment goal in IBD, a new measure of IBD activity, an outcome predictor, and an endpoint in clinical trials[17,18]. However, evaluation of tissue-based markers is invasive, expensive, and time-consuming, not easily accessible and related to some risks for patients. Such an evaluation may also be unavailable for CD patients with lesions situated in the small intestine. Clinical indices of IBD activity correlate with endoscopic findings rather poorly and as such do not allow for effective treatment modification. Therefore, surrogate markers of mucosal healing are needed[18].
Defendenti et al[13] were the first to report on an elevation of circulating IL9 in IBD and link this finding to severe prognosis. Using a more sensitive, fluorescence-based assay for cytokine determination, our research corroborates their findings on a larger set of patients, but focuses on IL9 as a possible MH marker. Although non-invasive and easier to assess, it is not clear to what extent serum-based markers can accurately reflect local immune response. Indeed, we observed IL6 and CRP to positively correlate with IBD clinical activity rather than Mayo endoscopic score. In contrast, IL9 predominantly mirrored the endoscopic activity of UC and was only weakly correlated with a clinical one. As a marker of mucosal non-healing (defined as scores other than 0 on the Mayo Clinic endoscopy scoring system), systemic IL9 is highly accurate with near perfect sensitivity and specificity. Our findings are consistent with the pathogenic role attributed to IL9 in wound healing[9,10]. In animal models of colitis, Il9 expression correlated with the severity of histological inflammation, which could be reduced with antibodies against IL9[10]. Functionally, IL9 altered the expression of tight junction proteins, inducing a notable bacteria translocation[10]. It also perpetuated inflammatory response via up-regulation of IL8, facilitating leukocyte trafficking and survival[9].
Unlike the cytokine tissue expression, preferential in UC[9,10], circulating IL9 did not differ between CD and UC in either Defendenti et al[13] or our cohorts. However, we observed a divergent association pattern with inflammatory and angiogenic indices that might translate into functional differences in IL9 between both conditions. An elevation in circulating IL9 was related to systemic inflammation in UC rather than CD, as evidenced by stronger correlations with proinflammatory cytokines. IL9, more so in CD, was correlated with VEGF-A, which might imply an association between IL9 and IBD angiogenesis. Correspondingly, in atopic dermatitis, IL9 and VEGF-A mRNA expressions were positively correlated and IL9 induced VEGF-A expression in cultured keratinocytes[19]. In IBD, IL9 might also be indirectly associated with angiogenesis by being a growth factor for mast cells, the source of VEGF-A, FGF2, and IL8[20].
IL9 in CD was also tightly correlated with IL13, an important Th2 cytokine. Traditionally, CD has been referred to as a Th1 condition and UC as a Th2 disease. However, this classic paradigm has recently been challenged, as the cytokines considered specific signatures for Th1 and Th2 subsets display diverse and often opposing activities[21]. Moreover, the discovery of the Th17 subset has further changed our understanding of IBD pathogenesis. Analysis of IL9 correlation patterns in CD and UC exemplifies the complexity of cytokine interactions. IL9 correlation with IL13 is in line with their co-expression by Th2 lymphocytes and the role of IL9 in maintaining IL13 production by innate lymphoid cells[22,23]. However, since this association was observed exclusively in our CD cohort, encompassing patients with the disease located in the small intestine, this may reflect the effect of IL9 on Paneth cells. Paneth cells play a critical role in resistance against enteric bacterial pathogens and in the maintenance of the normal composition of the gut microbiota[24,25]. IL9 induces their hyperplasia via up-regulation of IL13 expression[26]. Interestingly, both at a systemic level in the current study and at an mRNA level in inflamed bowel tissue[9], IL9 positively correlated with IFNγ. This association was particularly pronounced in UC patients, although IFNγ serves as a subset specific signature for Th1 cells and has been reported to inhibit IL9 production (reviewed in[3]).
IBD can ultimately lead to malnutrition, which, unaddressed, might lead to unfavorable outcomes[27,28]. In the face of the overweight/obesity epidemic, BMI has lost some of its credibility as a marker of malnutrition[29]. Accordingly, the vast majority of our cachectic patients had normal BMI (70%) and some (7%) remained overweight. Biochemical markers of poor nutritional status might facilitate prompt classification of IBD patients for dietary intervention. However, the application of traditional markers such as CRP and albumins has recently been criticized. These represent inflammation, which is an etiologic factor in cachexia, and are the main reason for reduced visceral protein levels[29]. The rationale for evaluating IL9 as a potential marker of cachexia was provided by Gerlach et al[10], who demonstrated that IL9-deficient mice responded to oxazolone challenge with less pronounced weight loss than wild type animals expressing IL9. We validated IL9’s association with weight loss in a clinical setting. However, this seems to be mediated by IL9’s correlation with classic procachectic cytokines, hampering IL9’s suitability as a cachexia marker.
Our results confirm IL9 elevation in IBD in a large cohort of patients and demonstrate IL9’s association with anemia and wasting syndromes accompanying IBD. Importantly, the data show that an elevation in systemic IL9 in UC corresponds with mucosal inflammation, with IL9 displaying a high level of accuracy as a negative marker of mucosal healing. Also, our results demonstrate IL9 to be more tightly associated with proinflammatory and Th1 cytokines in UC and with angiogenic and Th2 cytokines in CD.
Recently, a new subtype of helper lymphocytes T that overexpress cytokine IL9, termed Th9, has been described and involved in the pathogenesis of ulcerative colitis. Targeting IL9 signaling has emerged as a potential new therapeutic option in ulcerative colitis and evaluation of IL9 gene expression in bowel tissue and leukocytes has been proposed as a marker of respectively local and systemic inflammation. These findings were followed by an observation on the elevation of systemic IL9 in inflammatory bowel disease (IBD) patients, regardless the type of the disease, and linked with poor prognosis.
In the last years the aims of the IBD therapy evolved from the control of symptoms to control of inflammation, the only mode of action capable of altering the disease course. As such, mucosal healing become a key treatment goal in IBD, a new measure of IBD activity, an outcome predictor, and an endpoint in clinical trials and non-invasive methods of its evaluation are intensively searched for. IBD leads to malnutrition, worsening patient’s quality of life, increasing the disease severity or risk of relapse, negatively affecting patient’s response to treatment, and facilitating the development of systemic manifestations of the disease. Markers of poor nutritional status might facilitate prompt classification of IBD patients for dietary intervention. However, the suitability of traditional ones like BMI, C-reactive protein concentrations or albumin concentrations has recently been questioned.
The authors confirm findings of previous study showing an elevation of systemic IL9 in IBD in a large cohort of patients and expand it on a link between cytokine elevation and local inflammation in ulcerative colitis and cachexia and anemia of chronic diseases. The authors also observed that although there is no difference in the degree of IL9 elevation between two main types of IBD, there are dissimilarities in the pattern of interplay between IL9 and other cytokines manifested by more pronounced association with proinflammatory and Th1 cytokines in ulcerative colitis and with angiogenic and Th2 cytokines in Crohn’s disease.
IL9 measurement might be considered, as an adjunct to endoscopy, for non-invasive evaluation of mucosal healing in patients with ulcerative colitis.
The present study adds relevant news to the current literature and could be of practical interest for clinicians experienced in IBD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Can G, da Silva Figueredo CM, Katsanos KH, Matowicka-Karna J, Testa A S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1977] [Article Influence: 179.7] [Reference Citation Analysis (1)] |
| 2. | Stassen M, Schmitt E, Bopp T. From interleukin-9 to T helper 9 cells. Ann N Y Acad Sci. 2012;1247:56-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Pan HF, Leng RX, Li XP, Zheng SG, Ye DQ. Targeting T-helper 9 cells and interleukin-9 in autoimmune diseases. Cytokine Growth Factor Rev. 2013;24:515-522. [PubMed] |
| 5. | Hughes-Austin JM, Deane KD, Derber LA, Kolfenbach JR, Zerbe GO, Sokolove J, Lahey LJ, Weisman MH, Buckner JH, Mikuls TR. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA). Ann Rheum Dis. 2013;72:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Kokkonen H, Söderström I, Rocklöv J, Hallmans G, Lejon K, Rantapää Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Ciccia F, Guggino G, Rizzo A, Manzo A, Vitolo B, La Manna MP, Giardina G, Sireci G, Dieli F, Montecucco CM. Potential involvement of IL-9 and Th9 cells in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford). 2015;54:2264-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Weigmann B, Neurath MF. Th9 cells in inflammatory bowel diseases. Semin Immunopathol. 2017;39:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Nalleweg N, Chiriac MT, Podstawa E, Lehmann C, Rau TT, Atreya R, Krauss E, Hundorfean G, Fichtner-Feigl S, Hartmann A. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut. 2015;64:743-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, Lehr HA, Wirtz S, Vieth M, Waisman A. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol. 2014;15:676-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 312] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 11. | Papay P, Ignjatovic A, Karmiris K, Amarante H, Milheller P, Feagan B, D’Haens G, Marteau P, Reinisch W, Sturm A. Optimising monitoring in the management of Crohn’s disease: a physician’s perspective. J Crohns Colitis. 2013;7:653-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 13. | Defendenti C, Sarzi-Puttini P, Saibeni S, Bollani S, Bruno S, Almasio PL, Declich P, Atzeni F. Significance of serum Il-9 levels in inflammatory bowel disease. Int J Immunopathol Pharmacol. 2015;28:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Chapman P, Reyes C, Gupta V. Normal Physiological Levels of Human Cytokines Using Bio-Plex Pro™ Cytokine Assays. Bio-Plex Pro™ suspension array system tech note 6029. (assessed on 26thJan 2017). Available from: http://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6029.pdf. |
| 15. | Hufford MM, Kaplan MH. A gut reaction to IL-9. Nat Immunol. 2014;15:599-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Gerlach K, McKenzie AN, Neurath MF, Weigmann B. IL-9 regulates intestinal barrier function in experimental T cell-mediated colitis. Tissue Barriers. 2015;3:e983777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Dave M, Loftus EV. Mucosal healing in inflammatory bowel disease-a true paradigm of success? Gastroenterol Hepatol (N Y). 2012;8:29-38. [PubMed] |
| 18. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 664] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 19. | Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, Yu J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol. 2014;175:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim Biophys Acta. 2012;1822:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | de Mattos BR, Garcia MP, Nogueira JB, Paiatto LN, Albuquerque CG, Souza CL, Fernandes LG, Tamashiro WM, Simioni PU. Inflammatory Bowel Disease: An Overview of Immune Mechanisms and Biological Treatments. Mediators Inflamm. 2015;2015:493012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 22. | Doherty TA. At the bench: understanding group 2 innate lymphoid cells in disease. J Leukoc Biol. 2015;97:455-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang HE, Locksley RM. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol. 2016;9:275-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 24. | Fernandez MI, Regnault B, Mulet C, Tanguy M, Jay P, Sansonetti PJ, Pédron T. Maturation of paneth cells induces the refractory state of newborn mice to Shigella infection. J Immunol. 2008;180:4924-4930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 835] [Cited by in RCA: 911] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 26. | Steenwinckel V, Louahed J, Lemaire MM, Sommereyns C, Warnier G, McKenzie A, Brombacher F, Van Snick J, Renauld JC. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009;182:4737-4743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Halmos EP, Gibson PR. Dietary management of IBD--insights and advice. Nat Rev Gastroenterol Hepatol. 2015;12:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Hebuterne X, Filippi J, Schneider SM. Nutrition in adult patients with inflammatory bowel disease. Curr Drug Targets. 2014;15:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 2015;34:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1171] [Article Influence: 117.1] [Reference Citation Analysis (0)] |