Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3907
Peer-review started: September 18, 2016
First decision: October 10, 2016
Revised: October 27, 2016
Accepted: December 8, 2016
Article in press: December 8, 2016
Published online: June 7, 2017
Processing time: 263 Days and 10.6 Hours
To detect the expression of pleiotrophin (PTN) and N-syndecan in pancreatic cancer and analyze their association with tumor progression and perineural invasion (PNI).
An orthotopic mouse model of pancreatic cancer was created by injecting tumor cells subcapsularly in a root region of the pancreas beneath the spleen. Pancreatic cancer tissues were taken from 36 mice that survived for more than 90 d. PTN and N-syndecan proteins were detected by immunohistochemistry and analyzed for their correlation with pathological features, PNI, and prognosis.
The expression rates of PTN and N-syndecan proteins were 66.7% and 61.1%, respectively, in cancer tissue. PTN and N-syndecan expression was associated with PNI (P = 0.019 and P = 0.032, respectively). High PTN expression was closely associated with large bloody ascites (P = 0.009), liver metastasis (P = 0.035), and decreased survival time (P = 0.022). N-syndecan expression was significantly associated with tumor size (P = 0.025), but not with survival time (P = 0.539).
High PTN and N-syndecan expression was closely associated with metastasis and poor prognosis, suggesting that they may promote tumor progression and PNI in the orthotopic mouse model of pancreatic cancer.
Core tip: Perineural invasion (PNI) is a primary cause of local recurrence and poor survival in patients with pancreatic cancer. However, the exact mechanism of PNI remains unclear. Pleiotrophin (PTN) and its receptor, N-syndecan, may play an important role in tumor growth and PNI of pancreatic cancer. In a previous study, we found that high expression of PTN and N-syndecan may contribute to increased PNI and poor prognosis in patients with pancreatic cancer. In this study, we further elucidated the function of PTN and N-syndecan using an orthotopic mouse model of pancreatic cancer. We demonstrated that PTN and N-syndecan promoted tumor progression and PNI.
- Citation: Yao J, Zhang LL, Huang XM, Li WY, Gao SG. Pleiotrophin and N-syndecan promote perineural invasion and tumor progression in an orthotopic mouse model of pancreatic cancer. World J Gastroenterol 2017; 23(21): 3907-3914
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3907.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3907
Pancreatic cancer is a deadly cancer. It is the fourth leading cause of cancer-related deaths[1], with less than 5% of patients surviving 5 years or more. Moreover, as high as 60% of the patients who have undergone surgical treatments present with local or regional recurrence[2]. The poor prognosis of pancreatic cancer is linked with local recurrence, lymph node metastasis, liver metastasis, peritoneal dissemination, and Perineural invasion (PNI)[3,4]. PNI is a prominent characteristic of pancreatic cancer, which is found in nearly 100% of pancreatic cancer cases upon targeted histopathologic inspection of surgical specimens[5]. Recent studies have demonstrated that pancreatic cancer exhibits high invasive and metastatic incidences to the nerves within the pancreas and the peripheral nerve plexus[6,7], which is a critical predictor of local postoperative recurrence. Postoperative recurrence is a cause of postoperative failure in pancreatic cancer. PNI is characterized by cancer cells which invade the space surrounding the nerves, called the perineural space. Pancreatic cancer has one of the highest incidences of PNI (70%-100%) compared with other cancer types, which correlates with poor prognosis and decreased survival[8,9].
A family of proteins consisting of neurotrophic factors is of research interest, because recent studies have demonstrated their involvement in neural invasion[10,11]. Pleiotrophin (PTN) is a type of neurotrophic factor found in the mouse, rat, and human. PTN proteins across these different organisms are highly homologous with midkine, another member of the PTN family of proteins[12,13]. PTN and midkine are overexpressed in various human cancers, where they promote neuritic outgrowth, angiogenesis, expansion, and metastasis of tumor cells[14-18]. In normal pancreatic tissues, PTN is not expressed, but it is highly expressed in pancreatic cancer tissues. In addition, high expression of PTN correlates with pancreatic cancer progression[19,20]. N-syndecan is an essential component for neurite outgrowth and it is a strong receptor for PTN[21]. In in vitro studies, anti-N-syndecan antibodies inhibited PTN-induced neurite outgrowth[22]. N-syndecan is primarily expressed in neural tissues and is found localized in neuritis[23]; however, its localization in the neurites remains unknown during progression of pancreatic cancer. Therefore, PTN is expected to play an important role in the progression of pancreatic cancer and its neural invasion.
In our previous study, we compared expression patterns of PTN and N-syndecan by immunohistochemistry and determined whether their expression was associated with clinicopathological features, PNI, or overall survival (OS) in patients with pancreatic cancer. We found that high expression of PTN and its receptor may contribute to increased PNI and subsequently poor prognosis in patients. In this study, we further studied the functional role of PTN and N-syndecan in an orthotopic mouse model of pancreatic cancer.
Fifty male athymic nude mice (BALB/c background) were purchased from the Shanghai Experimental Animal Center (Shanghai, China). Mice were housed and maintained in laminar flow cabinets under specific pathogen-free conditions. All 50 nude mice were used in accordance with institutional guidelines when they were 6 wk old.
Human pancreatic cancer cell line (MiaPaCa-2) was obtained from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). MiaPaCa-2 cells were cultured in an atmosphere of 5% CO2 and 95% air at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) or Roswell Park Memorial Institute (RPMI) medium (Gibco, United States) containing 10% or 5% fetal bovine serum (Hyclone, United States), as required. All cells were mycoplasma-free and mouse antibody production (MAP) tested.
For in vivo injection, cells were harvested from culture flasks after trypsinizing for 2 to 3 min. Cells were then transferred to serum-free Hanks’ balanced salt solution (HBSS). Only single-cell suspensions of greater than 90% viability (trypan blue exclusion) were used for injection. Male nude mice were anesthetized with methoxyflurane. A small left abdominal flank incision was made and the spleen was exteriorized. Tumor cells (1 × 106/40 μL HBSS) were injected subcapsularly in a root region of the pancreas beneath the spleen. A 30-gauge needle, a 1-mL disposable syringe, and a calibrated, pushbutton-controlled dispensing device were used to inject the tumor cell suspension (Hamilton Syringe Co, Reno, NV, United States). A successful subcapsular intrapancreatic injection of tumor cells was identified by the appearance of a fluid bleb without intraperitoneal leakage. In cases of leakage, a cotton swab was held for 1 min over the site of injection. One layer of the abdominal wound was closed with wound clips (Autoclip; Clay Adams, Parsippany, NJ, United States). The animals tolerated the surgical procedure well and no anesthesia-related deaths occurred.
PTN and N-syndecan proteins were detected by immunohistochemistry using a standardized streptavidin-peroxidase (SP) method. Sections (4 μm) from paraffin-embedded tissue were used for immunohistochemistry. Sections were deparaffinized in xylene followed by a graded series of ethanol (100%, 95%, 80%), and rehydrated in phosphate-buffered solution (pH 7.5). All samples were incubated with a 3% H2O2 in methanol solution for 12 min at room temperature to block endogenous peroxidases. Sections were then washed three times with PBS and then incubated for 20 min at room temperature in a protein-blocking solution. The primary antibodies were diluted in protein blocking solution to the desired concentration and applied to the sections overnight at 48 °C. The sections were then rinsed in PBS and incubated for 10 min in protein-blocking solution before adding peroxidase-conjugated secondary antibody. After incubation in secondary antibody for 1 h at room temperature, the samples were washed and incubated with stable diaminobenzidine (DAB, Research Genetics, Huntsville, AL, United States). Staining was monitored under a bright-field microscope, and the reaction was stopped with distilled water. Finally, the sections were counterstained with hematoxylin, rinsed with water, dehydrated, cleared and cover-slipped. The following primary antibodies were applied: anti-PTN (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, United States) and anti-N-syndecan (1:100 dilution; Boster, Wuhan, China). Negative controls for immunostaining included non-immune goat or rabbit serum. Breast carcinoma and neurogliocytoma were used as positive controls for PTN and N-syndecan, respectively. PTN and N-syndecan expression was evaluated by calculating the percentage of positively stained cells (> 10% was considered positive).
PNI was assessed in all pancreatic cancer samples by two independent observers that were blinded to the samples. Any differences were resolved by a joint review and consultation with a third observer. For each sample, approximately 10 tissue sections from different tumor locations were analyzed. PNI was defined as positive if the infiltration of cancer cells into the perineurium or neural fasciculus was detected at the leading point, as previously reported[24]. The degree of PNI was defined microscopically as follows: 0 (PNI was difficult to find, with ≤ one occurrence per slide), 1 (PNI was easy to find, with two to four occurrences per slide), and 2 (PNI was easy to find, with more than four occurrences per slide or intraneural invasion).
Univariate analysis was performed using the χ2 test. Survival rates were calculated by the Kaplan-Meier method, and differences were examined using the log-rank test. Factors found to be significant were then chosen for a stepwise Cox multivariate proportional hazard model to determine their prognostic value. These analyses were performed using SPSS 16.0 for Windows (SPSS, Chicago, IL, United States). P-values less than 0.05 were considered statistically significant.
An orthotopic mouse model of pancreatic cancer was generated to further study the role of PTN and N-syndecan in pancreatic cancer. A total of 50 mice were involved in the study, in which 14 survived for less than 90 d, and 36 survived for more than 90 d. Thirty-six mice with better OS were used for further studies since these mice did not develop PNI associated with pancreatic cancer.
Bloody ascites was observed in almost all abdominal cavities of nude mice that died, of which six had large bloody ascites. Pancreatic tumors had an irregular shape and showed adhesion with the surrounding organs such as the liver, intestine, and spleen. Metastases to the spleen, peritoneum, and liver were also observed. These tumors generally had necrotic centers and abundant blood vessels.
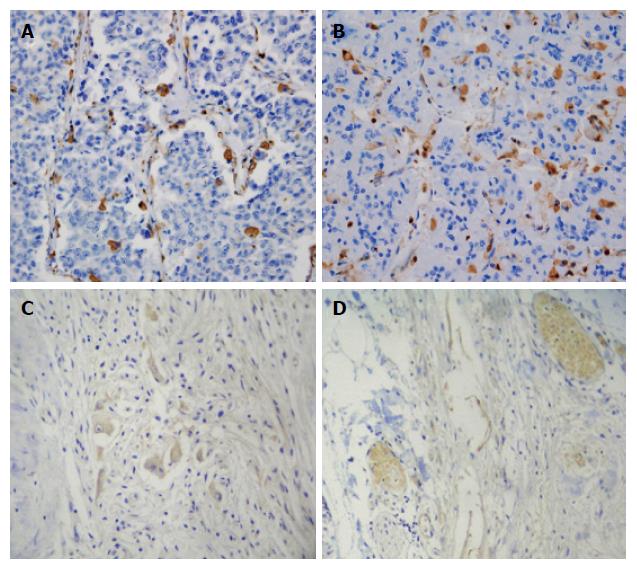
PTN protein was expressed in the cytoplasm of tumor cells. It was predominantly localized to invasive carcinoma cells (Figure 1A and B). No PTN expression was observed in normal pancreatic cells. Interestingly, moderate PTN staining was found in cells in the adjacent tissues. PTN expression was also found in desmoplastic stromal cells within the pancreatic cancer mass in PTN-positive tumors. No N-syndecan protein staining was observed in the perineurium of nerves in normal pancreatic tissues. N-syndecan protein staining was also not observed in acinar and ductal cells. Pancreatic tumor cells were devoid of any N-syndecan (Figure 1C). In tissues around the pancreatic tumor mass, N-syndecan protein staining was not found in the perineurium of nerves. However, in pancreatic tumor tissues, strong N-syndecan expression was noted in the perineurium of pancreatic nerves (Figure 1D). In total, the expression rate of PTN was 66.7% (24/36) in pancreatic cancer tissues and 22.2% (8/36) in tissues around the tumor mass. The expression rate of N-syndecan in the perineurium of pancreatic nerves was 61.1% (22/36).
Table 1 summarizes the association between PTN protein and its receptor expression and anatomical parameters in our mouse model. PTN expression was observed in 37.5% of mice with minimal bloody ascites (3/8), 68.2% of those with detectable bloody ascites (15/22), and 100% of those with large bloody ascites (6/6) (P = 0.009). High PTN expression was associated with liver metastasis (P = 0.035), and N-syndecan expression was associated with tumor size (P = 0.025). A trend between PTN expression and tumor size seemed likely, but it was not statistically significant (P = 0.064). PTN and N-syndecan expression was not associated with lymph node metastasis (P > 0.05).
| Factor | PTN positive cases | N-syndecan positive cases |
| Peritoneal dissemination | ||
| Negative (n = 14) | 71.4 (10) | 57.1 (8) |
| Positive (n = 22) | 63.6 (14) | 63.6 (14) |
| Histological grade | ||
| I (n = 4) | 50.0 (2) | 25.0 (1) |
| II (n = 19) | 73.7 (14) | 68.4 (13) |
| III (n = 13) | 61.5 (8) | 61.5 (8) |
| Tumor size | ||
| ≤ 1 cm (n = 8) | 50.0 (4) | 25.0 (2)a |
| > 1 cm but ≤ 2 cm (n = 21) | 61.9 (13) | 66.7 (14)a |
| > 5 cm (n = 7) | 100.0 (7) | 85.7 (6)a |
| Bloody ascites | ||
| V ≤ 1 mL (n = 8) | 37.5 (3)b | 50.0 (4) |
| 1 cm < V ≤ 2 mL (n = 22) | 68.2 (15)b | 59.1 (13) |
| V > 2 mL (n = 6) | 100.0 (6)b | 83.3 (5) |
| Liver metastases | ||
| Negative (n = 14) | 35.7 (5)a | 42.8 (6) |
| Positive (n = 22) | 86.4 (19)a | 72.7 (16) |
| Lymph node metastasis | ||
| Negative (n = 20) | 50.0 (10) | 50.0 (10) |
| Positive (n = 16) | 87.5 (14) | 75.0 (12) |
Tumors with positive PTN protein expression had significantly higher expression of N-syndecan (P = 0.048) (Table 2). A significant association was found between high PTN expression and PNI (P = 0.019), and between N-syndecan expression and PNI (P = 0.032). The expression levels of PTN and N-syndecan were significantly higher in mice with PNI than in those without (Table 3).
| Protein | PTN expression | P value | |
| Positive | Negative | ||
| N-syndecan (+) (n = 22) | 17 | 5 | 0.048 |
| N-syndecan (-) (n = 14) | 7 | 7 | |
| Protein | PIN | P value | ||
| 0 | 1 | 2 | ||
| PTN (+) (n = 24) | 3 | 10 | 11 | 0.019 |
| PTN (-) (n = 12) | 6 | 4 | 2 | |
| N-syndecan (+) (n = 22) | 2 | 8 | 12 | 0.032 |
| N-syndecan (-) (n = 14) | 6 | 5 | 3 | |
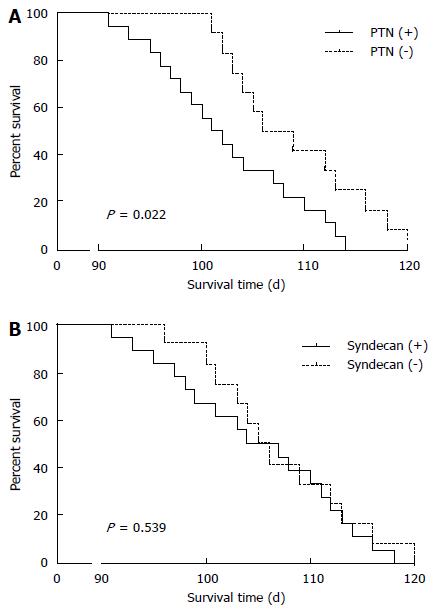
To determine the prognostic value of PTN protein and N-syndecan in our mouse model, we analyzed the cumulative survival of 36 nude mice. The median survival time of nude mice that were PTN negative (n = 12) was 109 d, whereas the median survival time of PTN positive mice (n = 24) was 98 d (P = 0.022) (Figure 2A). In addition, nude mice that were N-syndecan negative (n = 14) had a higher median survival time than N-syndecan positive ones (n = 22); however, the difference was not statistically significant (P > 0.05) (Figure 2B).
Multivariate analysis showed that tumor mass size (P = 0.038) and bloody ascites (P = 0.011) were independent prognostic factors for OS in our mouse model. PTN protein expression or N-syndecan protein expression was not an independent prognostic factor for OS (P > 0.05). Additionally, we found that PNI and other clinical parameters were not independent prognostic factors in our study.
Pancreatic cancer is an aggressive malignant disease with an insidious onset and is associated with poor prognosis[25-27]. The 5-year survival rate of pancreatic cancer remains poor despite the continued improvements and optimization of treatment strategies in recent years. PNI is a notable biological characteristic of pancreatic cancer[28]. In this study, we determined the significance of PTN expression in an orthotopic mouse model of pancreatic cancer to better define its functional role and potential as a novel therapeutic target.
PTN is localized in the cytoplasm of pancreatic cancer cells and is expressed in necrotic tumor cells. The results from our present study showed that high PTN expression significantly correlates with bloody ascites, liver metastasis, and PNI. Univariate analysis showed that there was a positive correlation between the intensity of PTN expression and lymph node metastasis. This may be due to some small metastatic lymph nodes in nude mice that were undetectable. Interestingly, positive PTN expression is also observed in normal tissue around the tumor. This could be due to the up-regulation of PTN in normal cells around the tumor, resulting in the binding and uptake of PTN that was generated by the tumor cells.
A histopathologic characteristic of pancreatic cancer is the extending of PNI into the pancreatic nerve plexus[9,29]. It has been reported that the binding of PTN with N-syndecan in pancreatic nerves promotes the development of neurites[30]. Malignant pancreatic cancer cells penetrate and damage the perineurium of pancreatic nerves. Neuronal damage disrupts neural homeostasis. A study by Yao et al[30] proposed a potential repair of the pancreatic nerves by the growth of additional neurons generated by neurons and Schwann cells. Nevertheless, more PTN positive cancer cells could invade the injured site because of the increased levels of N-syndecan. Finally, higher levels of PTN and N-syndecan can result in continued nerve injury, which may promote cancer invasion and proliferation into the neural structures. PTN and N-syndecan work collaboratively as neurite development-promotion factors to promote the development of PNI[31]. In this study, we found that the protein levels of PTN and N-syndecan were markedly increased in pancreatic cancer in comparison with normal pancreatic tissues. N-syndecan was not present in pancreatic cancer cells and mainly localized in the perineurium of pancreatic nerves. PTN was highly expressed in pancreatic cancer cells. Therefore, these observations could provide some explanations for the migration of pancreatic cancer cells to the pancreatic nerves. In our study, high PTN and N-syndecan expression was significantly associated with PNI (P = 0.019 and P = 0.032, respectively). PTN and N-syndecan expression levels in pancreatic cancers were significantly higher in nude mice with PNI than in those without. These results suggested that the PTN-N-syndecan pathway could play an important role in the process of PNI.
In this study, we assessed whether high PTN expression is associated with prognosis in pancreatic cancer. Negative PTN expression correlated with longer survival times (109 d vs 98 d, respectively). Previous studies have shown that PNI is associated with poor prognosis[4,32,33] and increased mortality risk[29,34-36]. However, some studies have reported a lack of association between PNI and prognosis in pancreatic cancers[37-40]. In this study, PNI was not an independent prognostic factor.
In summary, PTN is overexpressed in the orthotopic mouse model of pancreatic cancer and is significantly associated with bloody ascites, tumor size, PNI, and poor prognosis. These observations indicate that PTN may be a biomarker for advanced pancreatic tumor. Moreover, N-syndecan is frequently overexpressed in pancreatic tumor. The co-expression of PTN and N-syndecan proteins suggests their functional coordination in the tumor PNI and association with poor prognosis in pancreatic tumor.
Perineural invasion (PNI) is a primary cause of local recurrence and poor survival in patients with pancreatic cancer. However, the exact mechanism of PNI remains unclear. Pleiotrophin (PTN) is a neurite growth-promoting factor. It exhibits several tumorigenic properties. PTN and N-syndecan are likely critical for tumor growth and PNI of pancreatic tumor.
A histopathologic characteristic of pancreatic cancer is the perineural invasion extending into the pancreatic nerve plexus. In a previous study, we found that high expression of PTN and N-syndecan may contribute to increased PNI and poor prognosis in patients with pancreatic cancer. In this study, we further elucidated the function of PTN and N-syndecan using an orthotopic mouse model of pancreatic cancer. PTN and N-syndecan proteins were detected by immunohistochemistry and analyzed for their correlation with prognosis, pathological features, and perineural invasion.
In recent years, studies in pancreatic tumor have largely focused on biological characterization, especially neural invasion. In this study, we further elucidated the function of PTN and N-syndecan using an orthotopic mouse model of pancreatic cancer. These studies demonstrated that PTN and N-syndecan promoted tumor progression and PNI in our mouse model of pancreatic cancer.
The study indicates that PTN and N-syndecan appear to be attractive gene therapy targets for inhibiting neural invasion in pancreatic tumor.
PTN is a neurite growth promoting factor. N-syndecan protein shows high binding for PTN. PTN and N-syndecan promote neurite outgrowth. PNI extending into the pancreatic nerve plexus is a unique histopathologic feature of pancreatic cancer. The perineural invasion of pancreatic cancer results in metastasis, local recurrence, and poor prognosis, giving rise to challenges associated with the diagnosis and treatment of pancreatic cancer.
In the current manuscript, the authors unravel the role of PTN, a heparin-binding growth factor and its receptor, and N-syndecan in the occurrence of PNI, a hallmark of pancreatic cancer progression and aggressiveness. The study is well designed, data interpretations are very insightful and accurate, and the conclusions are precisely compelling.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ali I, Lyakhovich A, Schuller HM, Su CC, Syed V, Weyemi U S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9957] [Article Influence: 995.7] [Reference Citation Analysis (0)] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21363] [Article Influence: 2136.3] [Reference Citation Analysis (3)] |
| 3. | Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 336] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 5. | Demir IE, Friess H, Ceyhan GO. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front Physiol. 2012;3:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Demir IE, Friess H, Ceyhan GO. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 7. | Demir IE, Ceyhan GO, Liebl F, D’Haese JG, Maak M, Friess H. Neural invasion in pancreatic cancer: the past, present and future. Cancers (Basel). 2010;2:1513-1527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Ceyhan GO, Demir IE, Altintas B, Rauch U, Thiel G, Müller MW, Giese NA, Friess H, Schäfer KH. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun. 2008;374:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Hirai I, Kimura W, Ozawa K, Kudo S, Suto K, Kuzu H, Fuse A. Perineural invasion in pancreatic cancer. Pancreas. 2002;24:15-25. [PubMed] |
| 10. | Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, Büchler MW, Friess H. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Veit C, Genze F, Menke A, Hoeffert S, Gress TM, Gierschik P, Giehl K. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64:5291-5300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359-371. [PubMed] |
| 13. | Yao J, Li WY, Gao SG. The advances of Midkine with peripheral invasion in pancreatic cancer. Am J Cancer Res. 2015;5:2912-2917. [PubMed] |
| 14. | Bao X, Mikami T, Yamada S, Faissner A, Muramatsu T, Sugahara K. Heparin-binding growth factor, pleiotrophin, mediates neuritogenic activity of embryonic pig brain-derived chondroitin sulfate/dermatan sulfate hybrid chains. J Biol Chem. 2005;280:9180-9191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Hida H, Jung CG, Wu CZ, Kim HJ, Kodama Y, Masuda T, Nishino H. Pleiotrophin exhibits a trophic effect on survival of dopaminergic neurons in vitro. Eur J Neurosci. 2003;17:2127-2134. [PubMed] |
| 16. | Papadimitriou E, Mikelis C, Lampropoulou E, Koutsioumpa M, Theochari K, Tsirmoula S, Theodoropoulou C, Lamprou M, Sfaelou E, Vourtsis D. Roles of pleiotrophin in tumor growth and angiogenesis. Eur Cytokine Netw. 2009;20:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Sakamoto K, Kadomatsu K. Midkine in the pathology of cancer, neural disease, and inflammation. Pathol Int. 2012;62:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Yao J, Li WY, Li SG, Feng XS, Gao SG. Midkine promotes perineural invasion in human pancreatic cancer. World J Gastroenterol. 2014;20:3018-3024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Weber D, Klomp HJ, Czubayko F, Wellstein A, Juhl H. Pleiotrophin can be rate-limiting for pancreatic cancer cell growth. Cancer Res. 2000;60:5284-5288. [PubMed] |
| 20. | Klomp HJ, Zernial O, Flachmann S, Wellstein A, Juhl H. Significance of the expression of the growth factor pleiotrophin in pancreatic cancer patients. Clin Cancer Res. 2002;8:823-827. [PubMed] |
| 21. | Kinnunen A, Kinnunen T, Kaksonen M, Nolo R, Panula P, Rauvala H. N-syndecan and HB-GAM (heparin-binding growth-associated molecule) associate with early axonal tracts in the rat brain. Eur J Neurosci. 1998;10:635-648. [PubMed] |
| 22. | Raulo E, Chernousov MA, Carey DJ, Nolo R, Rauvala H. Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3). J Biol Chem. 1994;269:12999-13004. [PubMed] |
| 23. | Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB, Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J Biol Chem. 1998;273:10702-10708. [PubMed] |
| 24. | Zhu Z, Friess H, diMola FF, Zimmermann A, Graber HU, Korc M, Büchler MW. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17:2419-2428. [PubMed] |
| 25. | Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, Tsunoda T, Suzuki T, Monden M, Saitoh Y. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Nakao A, Harada A, Nonami T, Kaneko T, Takagi H. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12:357-361. [PubMed] |
| 27. | Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007;35:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Shimada K, Nara S, Esaki M, Sakamoto Y, Kosuge T, Hiraoka N. Intrapancreatic nerve invasion as a predictor for recurrence after pancreaticoduodenectomy in patients with invasive ductal carcinoma of the pancreas. Pancreas. 2011;40:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Takahashi T, Ishikura H, Motohara T, Okushiba S, Dohke M, Katoh H. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol. 1997;65:164-170. [PubMed] |
| 30. | Yao J, Ma Q, Wang L, Zhang M. Pleiotrophin expression in human pancreatic cancer and its correlation with clinicopathological features, perineural invasion, and prognosis. Dig Dis Sci. 2009;54:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Yao J, Hu XF, Feng XS, Gao SG. Pleiotrophin promotes perineural invasion in pancreatic cancer. World J Gastroenterol. 2013;19:6555-6558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379-3391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 859] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 33. | Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 864] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 34. | Miyazaki I. [Perineural invasion and surgical treatment of the pancreas head cancer]. Nihon Geka Gakkai Zasshi. 1997;98:646-648. [PubMed] |
| 35. | Sudo T, Murakami Y, Uemura K, Hayashidani Y, Hashimoto Y, Ohge H, Shimamoto F, Sueda T. Prognostic impact of perineural invasion following pancreatoduodenectomy with lymphadenectomy for ampullary carcinoma. Dig Dis Sci. 2008;53:2281-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Mitsunaga S, Hasebe T, Kinoshita T, Konishi M, Takahashi S, Gotohda N, Nakagohri T, Ochiai A. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol. 2007;31:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Perini MV, Montagnini AL, Jukemura J, Penteado S, Abdo EE, Patzina R, Cecconello I, Cunha JE. Clinical and pathologic prognostic factors for curative resection for pancreatic cancer. HPB (Oxford). 2008;10:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Mössner J. What’s new in therapy of pancreatic cancer? Dig Dis. 2010;28:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Meduri F, Diana F, Merenda R, Losacco L, Zuin A, Cecchetto A, Gerunda GE, Neri D, Zangrandi F, Maffei-Faccioli A. Pancreatic cancer and retroperitoneal neural tissue invasion. Its implication for survival following radical surgery. Zentralbl Pathol. 1994;140:277-279. [PubMed] |