Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3883
Peer-review started: February 8, 2017
First decision: March 3, 2017
Revised: March 20, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: June 7, 2017
Processing time: 129 Days and 13.4 Hours
To investigated the prognostic value of the neutrophil-lymphocyte ratio (NLR) in patients with acute pancreatitis and determined an optimal cut-off value for the prediction of adverse outcomes in these patients.
We retrospectively analyzed 490 patients with acute pancreatitis diagnosed between March 2007 and December 2012. NLRs were calculated at admission and 24, 48, and 72 h after admission. Patients were grouped according to acute pancreatitis severity and organ failure occurrence, and a comparative analysis was performed to compare the NLR between groups.
Among the 490 patients, 70 had severe acute pancreatitis with 31 experiencing organ failure. The severe acute pancreatitis group had a significantly higher NLR than the mild acute pancreatitis group on all 4 d (median, 6.14, 6.71, 5.70, and 4.00 vs 4.74, 4.47, 3.20, and 3.30, respectively, P < 0.05). The organ failure group had a significantly higher NLR than the group without organ failure on all 4 d (median, 7.09, 6.72, 6.27, and 6.24 vs 4.85, 4.49, 3.35, and 2.34, respectively, P < 0.05). The optimal cut-off value for baseline NLR was 4.76 in predicting severity and 4.88 in predicting organ failure in acute pancreatitis.
Elevated baseline NLR correlates with severe acute pancreatitis and organ failure.
Core tip: This is a retrospective study to demonstrate the usefulness of the neutrophil-lymphocyte ratio in predicting the manifestation of severe acute pancreatitis and organ failure in the early stages in patients with acute pancreatitis. The neutrophil-lymphocyte ratio is cost-effective and easy to use, utilizing tests already routinely performed. This study found the neutrophil-lymphocyte ratio to be a reliable predictor of adverse outcomes in patients with acute pancreatitis and established the optimal cut-off value of the neutrophil-lymphocyte ratio for predicting these outcomes.
- Citation: Jeon TJ, Park JY. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J Gastroenterol 2017; 23(21): 3883-3889
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3883.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3883
Acute pancreatitis is an acute inflammatory process of the pancreas that starts with local acinar cell injury with variable involvement of other regional tissues or remote organ systems[1]. Although the majority of acute pancreatitis cases are mild and self-limiting, severe cases can be accompanied by complications such as necrosis or organ failure in approximately 25% of patients[2-5]. In such severe acute pancreatitis (SAP), high mortality rates of up to 50% have been reported[4]. The severity of acute pancreatitis is related to extrapancreatic organ failure secondary to the patient’s systemic inflammatory response, and a poor prognosis of SAP is thought to be the result of uncontrolled systemic inflammatory response syndrome or multi-organ dysfunction syndrome[2,4,6,7]. White blood cell (WBC) counts and C-reactive protein (CRP) levels are non-specific markers of systemic inflammation that can be measured using routine serum hematological tests. In addition, the WBC count is correlated with poor prognosis as a compositional element of Ranson’s criteria, Glasgow score, Acute Physiology and Chronic Health Evaluation-II (APACHE-II), and Bedside Index of Severity in Acute Pancreatitis (BISAP), which are the prognostic scoring systems of acute pancreatitis[4,8]. However, the total WBC count can fluctuate based on various physiological and pathological conditions including hydration status, stress, and pregnancy in addition to how the blood specimen is handled[8]. The neutrophil-lymphocyte ratio (NLR) has been identified as a more reliable predictor of adverse outcomes in several benign and malignant diseases, such as coronary heart disease, esophageal cancer, colorectal cancer, and hepatocellular carcinoma, when compared with the WBC count[9-14]. Neutrophils and lymphocytes reflect the immune response better than the total WBC count[9-11]. In particular, studies have demonstrated the correlation between peripheral lymphocytopenia and the severity of acute pancreatitis[15,16]. In addition, one study established the superiority of the NLR over the total WBC counts in predicting the severity of acute pancreatitis[8].
The aim of this study was to demonstrate the usefulness of the NLR in predicting the manifestation of SAP and organ failure in the early stages in patients with acute pancreatitis. In addition, this study examined the optimal cut-off value of the NLR in predicting adverse outcomes in patients with acute pancreatitis.
We retrospectively analyzed the clinical, laboratory, and radiological data of patients diagnosed with acute pancreatitis and hospitalized at Sanggye Paik Hospital in Seoul, South Korea, between March 2007 and December 2012. Up to 72 h of data were analyzed during the course of hospitalization for each patient. Patients with missing baseline laboratory or vital signs data (as they were transferred from a different hospital) were excluded from further analysis. According to the protocol of our department, all the hospitalized patients with organ failure or fever were routinely administered a broad-spectrum antibiotic intravenously from the first day to at least 72 h of hospitalization. This study was approved by the Institutional Review Board.
Patients were diagnosed with acute pancreatitis if more than 2 of the following conditions were satisfied: (1) abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain often radiating to the back); (2) serum amylase and/or lipase level at least 3 times greater than the upper limit of the normal value; and (3) characteristic manifestation of acute pancreatitis on contrast-enhanced computed tomography, magnetic resonance imaging, or transabdominal ultrasonography[6]. Acute pancreatitis was categorized into mild acute pancreatitis (MAP), moderately severe acute pancreatitis, and severe acute pancreatitis (SAP) in accordance with the revised Atlanta classification[6]. For the purpose of analysis, we grouped the moderately severe pancreatitis cases with the SAP cases[6]. Consequently, SAP was considered to be the presence of transient (less than 48 h) or persistent (over 48 h) organ failure and/or local or systemic complications. Local complications included an acute peripancreatic fluid collection, a pancreatic pseudocyst, an acute necrotic collection, and a walled-off necrosis. Exacerbation of pre-existing co-morbidities, such as coronary artery disease or chronic lung disease, precipitated by the acute pancreatitis was defined as a systemic complication. Organ failure included shock (systolic blood pressure < 90 mmHg), pulmonary insufficiency (arterial PO2 < 60 mmHg at room air or the need for mechanical ventilation), or renal failure (serum creatinine level > 2 mg/dL after rehydration or hemodialysis)[2,3]. The NLR was computed by calculating the ratio of the absolute neutrophil and lymphocyte counts, and the analysis was conducted using the NLR values on the day of hospitalization (baseline, NLR 0) and at 24 (NLR 1), 48 (NLR 2), and 72 h (NLR 3) after hospitalization.
Descriptive data are presented as median and interquartile ranges for continuous variables. The Student’s t-test or Mann-Whitney U test was used for the analysis of continuous data for comparison between the SAP and MAP patients. The χ2 test or Fishers’ exact test was used for categorical data analysis. The NLR was treated as a continuous variable, and severity of acute pancreatitis and organ failure distribution were treated as categorical variables. Correlation of the NLR with the severity of acute pancreatitis or the organ failure distribution was determined by using the Spearman’s rank correlation test. The optimal cut-off value of NLR was computed by using the trade-off between sensitivity and specificity on the receiver-operating characteristic (ROC) curves, and the accuracy of prediction of the NLR was estimated using the area under the receiver-operating curve (AUC). Statistical significance was defined as a P value < 0.05.
During the period of the study, 531 patients were admitted with acute pancreatitis. Among these, 41 patients with unavailable baseline data were excluded, and a total of 490 patients were enrolled in this study. Of these 490 patients, 70 were diagnosed with SAP.
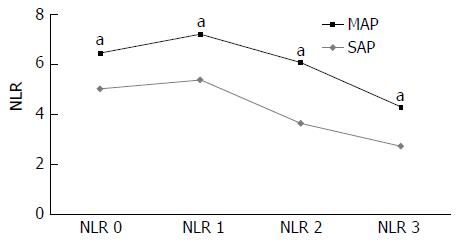
Clinical characteristics and initial laboratory findings of all patients are summarized in Table 1. The patients with SAP demonstrated higher CRP levels and lower total calcium and albumin levels compared to those in patients with MAP at the time of hospitalization. There was no significant difference in the total WBC count upon admission between the MAP and SAP groups. The differences in the NLR between the MAP and SAP groups are shown in Table 2. Although there was no significant difference in the baseline total WBC count between the 2 groups, the baseline NLR was 6.14 in the SAP group, which was significantly higher than that in the MAP group at 4.74 (median, P = 0.013). The NLRs after 24, 48, and 72 h of hospitalization in the SAP group were significantly higher compared to those in the MAP group (median, 6.71, 5.70, and 4.00 vs 4.47, 3.20, and 3.30, respectively, P < 0.05). The sequential changes in the NLR are illustrated in Figure 1. Although the MAP group demonstrated the highest NLR on admission with a gradual decrease after 24 h of hospitalization, the SAP group demonstrated the highest NLR after 24 h of hospitalization with a gradual decrease thereafter, and the NLR after 48 h remained higher than the highest score in the MAP group, indicating ongoing inflammation.
| All (n = 490) | MAP (n = 420) | SAP (n = 70) | P value | |
| Age (yr) | 52.36 ± 16.98 | 52.34 ± 17.15 | 52.46 ± 16.01 | 0.958 |
| Sex: male (%) | 352 (71.8) | 297 (70.7) | 55 (78.6) | 0.176 |
| Etiology, n (%) | 0.839 | |||
| Gallstones | 136 (27.8) | 119 (28.3) | 17 (24.3) | |
| Alcohol | 250 (51.0) | 212 (50.5) | 38 (54.3) | |
| Hypertriglyceridemia | 8 (1.6) | 6 (1.4) | 2 (2.9) | |
| Idiopathic | 84 (17.1) | 73 (17.4) | 11 (15.7) | |
| Cancer | 12 (2.4) | 10 (2.4) | 2 (2.9) | |
| Laboratory (at admission) | ||||
| WBC (× 109/L) | 11.90 ± 5.61 | 11.80 ± 5.68 | 12.53 ± 5.14 | 0.315 |
| CRP (mg/dL) | 6.55 ± 8.10 | 5.90 ± 7.32 | 10.32 ± 11.03 | 0.001 |
| Hct (%) | 41.51 ± 5.78 | 41.61 ± 5.81 | 40.89 ± 5.52 | 0.330 |
| BUN (mg/dL) | 14.91 ± 9.51 | 14.76 ± 9.63 | 15.84 ± 8.76 | 0.382 |
| Calcium (mg/dL) | 8.50 ± 0.85 | 8.60 ± 0.78 | 7.90 ± 0.99 | 0.001 |
| Albumin (g/dL) | 3.90 ± 0.63 | 3.94 ± 0.62 | 3.71 ± 0.63 | 0.006 |
| Glucose (mg/dL) | 155.25 ± 98.25 | 153.45 ± 100.41 | 165.54 ± 84.76 | 0.343 |
| LDH (U) | 523.36 ± 480.75 | 518.47 ± 492.08 | 553.15 ± 406.77 | 0.585 |
| BMI (kg/m2) | 23.85 ± 4.12 | 23.93 ± 4.19 | 23.42 ± 3.72 | 0.368 |
| Hospital stay (d) | 9.81 ± 7.15 | 9.21 ± 6.34 | 13.36 ± 10.11 | 0.001 |
| All (n = 490) | MAP (n = 420) | SAP (n = 70) | P value | |
| NLR 0 | 4.95 (6.15) | 4.74 (5.79) | 6.14 (6.64) | 0.010 |
| NLR 1 | 4.64 (6.28) | 4.47 (5.82) | 6.71 (7.71) | 0.002 |
| NLR 2 | 3.39 (4.50) | 3.20 (4.15) | 5.70 (6.75) | 0.001 |
| NLR 3 | 2.45 (3.47) | 3.30 (3.26) | 4.00 (5.90) | 0.001 |
We examined the differences in the NLRs by dividing the acute pancreatitis patients into those with and without organ failure (Table 3). All of the NLR 0, NLR 1, NLR 2, and NLR 3 measurements were significantly higher in the group of patients with organ failure than in the group of patients without organ failure (median, 4.85, 4.49, 3.35, and 2.34 vs 7.09, 6.72, 6.27, and 6.24, respectively, P < 0.05). Both the groups showed the highest NLR value at admission followed by a gradual decrease thereafter.
| All (n = 490) | Organ failure (-)(n = 459) | Organ failure (+)(n = 31) | P value | |
| NLR 0 | 4.95 (6.15) | 4.85 (6.09) | 7.09 (7.87) | 0.03 |
| NLR 1 | 4.64 (6.28) | 4.49 (6.08) | 6.72 (12.37) | 0.003 |
| NLR 2 | 3.39 (4.50) | 3.35 (4.29) | 6.27 (12.19) | 0.002 |
| NLR 3 | 2.45 (3.47) | 2.34 (3.35) | 6.24 (8.37) | 0.001 |
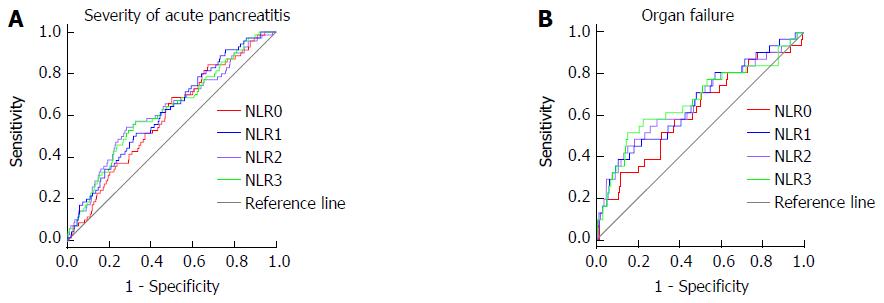
The ROCs for the NLR of each day predicting SAP and organ failure are shown in Figure 2. The ROC yielded an AUC of 0.59 (95%CI: 0.52-0.66) for baseline NLR in predicting disease severity and 0.62 (95%CI: 0.51-0.72) in predicting development of organ failure. The AUC for NLR 1 was higher than the baseline NLR and similar to NLR 2 and NLR 3 both for predicting disease severity and development of organ failure. On the basis of the highest sensitivity and specificity values generated from the ROC, we generated the optimal cut-off values of baseline NLR and NLR 1 for predicting the development of SAP and organ failure. This demonstrated an optimal baseline NLR of 4.76 and NLR 1 of 5.18 for SAP and a baseline NLR of 5.03 and NLR 1 of 4.81 for organ failure (Table 4).
| Severity | Organ failure | Necrosis | Local Cx | ICU adm | Mortality | |
| NLR 0 | 0.59a (0.52-0.66) | 0.62a (0.51-0.72) | 0.52 (0.44-0.60) | 0.54 (0.48-0.60) | 0.58 (0.47-0.70) | 0.44 (0.21-0.67) |
| NLR 1 | 0.62a (0.55-0.69) | 0.66a (0.55-0.77) | 0.60a (0.52-0.68) | 0.57 (0.51-0.63) | 0.73a (0.63-0.83) | 0.62 (0.38-0.85) |
| NLR 2 | 0.62a (0.55-0.70) | 0.66a (0.55-0.78) | 0.61a (0.53-0.69) | 0.59a (0.53-0.66) | 0.74a (0.64-0.84) | 0.60 (0.30-0.85) |
| NLR 3 | 0.62a (0.55-0.70) | 0.67a (0.56-0.79) | 0.59a (0.51-0.66) | 0.60a (0.54-0.67) | 0.73a (0.63-0.83) | 0.62 (0.39-0.85) |
In this study, we evaluated the usefulness of the NLR as an early predictive marker for development of SAP and organ failure in acute pancreatitis. Although the total WBC count is a compositional element of Ranson’s criteria, Glasgow score, APACHE-II, and BISAP (scoring systems for evaluation of the prognosis of acute pancreatitis), the total WBC count itself, unlike CRP or blood urea nitrogen, is not assessed as an independent marker for the prediction of the prognosis of acute pancreatitis[4,8]. However, the NLR was identified by several existing studies as an index that reflects the prognosis of various benign inflammatory or malignant diseases[9-14]. This study demonstrated that the NLR was elevated in patients presenting with acute pancreatitis and that NLR can allow providers to classify patients according to disease severity and the presence of organ failure. In particular, evaluating the severity of acute pancreatitis at the initial stage of manifestation is critical to improve the patient’s prognosis[2,3]. Therefore, there is a need for a simple indicator that can easily predict the patient’s prognosis within 24 h of the manifestation of the disease[2,3]. Prognostic scoring systems such as the Ranson’s criteria, APACHE-II, and sequential organ failure assessment (SOFA) score have limitations in actual application since they are complex and contain data not routinely ordered or collected during hospitalization at the current time[2,4]. However, the NLR is a simple test that is inexpensive, routinely performed during the initial evaluation of patients, not affected by the volume status of the patient, and can be repeated easily[4,8]. In particular, since neutrophilia and lymphopenia are indexes of systemic inflammation and physiological stress, they can better reflect complications such as necrosis or organ failure[4,8,15,16]. The neutrophil, as a major cell associated with the active inflammation response, is the main initiator of tissue destruction caused by several inflammatory cytokines such as interleukin 1 and interleukin 6[4,8]. Therefore, neutrophilia generated by the acute and severe pancreatic tissue damage and inflammation in SAP increases the NLR[4,8]. On the other hand, there was a decreased number of peripheral total and lymphocyte subsets measured within 48 h after admission in patients with acute pancreatitis[4,8]. As evidenced by existing studies, patients with SAP had a significantly lower number of lymphocytes in comparison with patients having the mild form of the disease, thereby further increasing the NLR. It is probably due to an impaired lymphocyte proliferative response to mitogens in acute pancreatitis patients[4,8,15,16]. In the literature review, elevation of the NLR in early phase of SAP was estimated to be abnormalities in cellular immunity due to early apoptosis of lymphocytes and delay of neutrophil apoptosis[16].
This study demonstrated that the NLR values from the time of admission to 72 h after admission are significantly different depending on disease severity and existence of organ failure. In particular, we aimed to identify the standards for early evaluation of SAP and organ failure by computing the cut-off value of baseline NLR. The cut-off value of baseline NLR presented in this study for predicting SAP is 4.76, which is similar to the NLR of 4.7 presented by Azab et al[8]. In addition, this displayed a sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy (63.6, 56.7, 21.2, 89.5, and 57.7, respectively) similar to those for the cut-off value presented by Suppiah et al[4]. However, when compared with the results of other studies performed by the author, the NLR had a lower accuracy for predicting adverse outcomes in acute pancreatitis compared with other prognostic scoring systems of acute pancreatitis (BISAP, Ranson’s criteria, APACHE-II, and CT severity index) (AUC; 0.80, 0.74, 0.80, and 0.67 vs 0.59 in SAP; 0.93, 0.84, 0.95, and 0.57 vs 0.62 in organ failure, respectively)[3]. In addition, the NLR cannot be asserted to have superior predictability in comparison to other scoring systems with the sensitivity of APACHE-II, Imrie, Ranson, SOFA score, and Pancreatitis Outcome Prediction score ranging from 60% to 90% in numerous studies[2,5,17]. This implies that although a high NLR can predict adverse outcomes of acute pancreatitis, its accuracy is lower than the accuracy of other currently used scoring systems. However, further studies are needed for more accurate evaluation since a comparative analysis between the NLR and other scoring systems was not carried out in this study.
Similar to other published studies by Azab and Suppiah[4,8], this study investigated the predicting value of the NLR for development of organ failure. In our study, baseline NLR predicted the development of organ failure with a slightly higher accuracy than it predicted SAP with an AUC of 0.62 and 0.59, respectively. This is because the NLR represents the patients’ inflammatory response, and organ failure occurs because of a systemic inflammatory response. Assuming that organ failure reflects systemic inflammation in acute pancreatitis, the ability of the sequential changes of the NLR to predict development of organ failure might be affected by antibiotic use (Table 5).
| Cut-off value | P value | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| SAP | NLR 0 = 4.76 | 0.013 | 68.6% | 50.0% | 18.6% | 90.5% | 52.7% |
| NLR 1 = 5.18 | 0.002 | 62.9% | 55.5% | 18.7% | 89.6% | 46.9% | |
| Organ failure | NLR 0 = 5.03 | 0.032 | 64.3% | 53.1% | 8.3% | 95.6% | 49.4% |
| NLR 1 = 4.81 | 0.003 | 62.9% | 55.5% | 8.7% | 96.0% | 49.2% |
Although there may be differences in the normal range of the NLR based on race or gender[8], we computed the NLR in accordance with the normal reference range presented by the diagnostic laboratory of our unit since there was no alternative reference range. Computation of the highest possible NLR value by dividing the highest measured neutrophil count by the lowest measured lymphocyte count resulted in the value of 3.75. The median baseline NLR of all the patients enrolled in this study was 4.95, and the NLR of the MAP patients was 4.74, thereby confirming that the NLR of the acute pancreatitis patient is higher than that of a healthy person, irrespective of the severity of the disease.
The majority of existing studies that evaluated the usefulness of the NLR as a prognostic marker used a cut-off value of NLR of ≥ 5 to predict adverse outcomes of several benign or malignant diseases[9-11]. This finding is similar to the values of 4.76 for predicting SAP and 5.03 for predicting organ failure presented in this study. As the result of analysis of prognostic accuracy of the NLR when the cut-off value of initial NLR is set at ≥ 5 by using the data obtained from this study, both SAP and organ failure demonstrated sensitivity, specificity, PPV, NPV, and accuracy that were similar to those for the cut-off value presented in this study (64.3%, 52.9%, 18.5%, 89.9%, and 54.5% in predicting SAP, respectively, and 64.5%, 51.4%, 8.2%, 95.5%, and 52.2% in predicting organ failure, respectively).
In this study, unlike the study by Suppiah et al[4], the cut-off values of baseline NLR and NLR 1 were computed because the initial assessment within 24 h of admission is considered the most important in the treatment of acute pancreatitis[2,3]. The greatest benefit of the NLR that differentiates it from other prognostic markers is the rapid confirmation of the results in an emergency examination. In this study, the MAP group demonstrated continuous reduction in the NLR over time to 3.20 after 48 h of hospitalization, which is a lower value than the normal highest possible NLR of 3.75 that we have determined. This can be explained by the fact that MAP patients generally recover with 48-72 h of hospital treatment[1,2]. On the contrary, the NLR was maintained at higher than the normal levels after 72 h of hospitalization in the SAP group.
The NLR is a simple, inexpensive, and easy to carry out prognostic tool to predict adverse outcomes in patients with acute pancreatitis. Although the accuracy of the NLR for predicting adverse outcomes in patients with acute pancreatitis is not higher than the other currently used prognostic scoring systems, the NLR can be used to classify patients according to disease severity or presence of organ failure. In addition, sequential changes in the NLR over time seem to reflect the progress and therapeutic response of acute pancreatitis. Additional studies would be required to compare the prognostic value of the NLR to other currently used scoring systems in patients with acute pancreatitis.
Several prognostic scoring systems have been developed to predict severe acute pancreatitis. However, they are complex and some laboratory data are not obtained immediately. The neutrophil-lymphocyte ratio (NLR) can be obtained easily and is usually included in routine orders. This study investigated clinical usefulness of the NLR to predict severe acute pancreatitis at an early stage.
The NLR has been studied as a more reliable predictor of adverse outcomes in several benign and malignant diseases, such as coronary heart disease, esophageal cancer, colorectal cancer, and hepatocellular carcinoma. There have been few studies in acute pancreatitis.
This study demonstrated that the NLR values from the time of admission to 72 h after admission are significantly different depending on disease severity and existence of organ failure. In particular, the aimed to identify the standards for early evaluation of severe acute pancreatitis and organ failure by computing the cut-off value of baseline NLR.
The NLR is the simple, easy, and inexpensive method to predict the severe acute pancreatitis and organ failure. Further large randomized controlled study would be required.
The NLR was computed by calculating the ratio of the absolute neutrophil and lymphocyte counts, and the analysis was conducted using the NLR values on the day of hospitalization (baseline, NLR 0) and at 24 (NLR 1), 48 (NLR 2), and 72 h (NLR 3) after hospitalization.
The paper adds some interesting information to the discussion of study about the prognostic value of the NLR in patients with acute pancreatitis and it an optimal cut-off value for the prediction of adverse outcomes in these patients. The proposed test is cheap and easy to use, using tests routinely performed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Manenti A, Sliwinska-Mosson MSM S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 518] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 2. | Papachristou GI, Muddana V, Yadav D, O’Connell M, Sanders MK, Slivka A, Whitcomb DC. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435-441; quiz 442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Park JY, Jeon TJ, Ha TH, Hwang JT, Sinn DH, Oh TH, Shin WC, Choi WC. Bedside index for severity in acute pancreatitis: comparison with other scoring systems in predicting severity and organ failure. Hepatobiliary Pancreat Dis Int. 2013;12:645-650. [PubMed] |
| 4. | Suppiah A, Malde D, Arab T, Hamed M, Allgar V, Smith AM, Morris-Stiff G. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: identification of an optimal NLR. J Gastrointest Surg. 2013;17:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Harrison DA, D’Amico G, Singer M. The Pancreatitis Outcome Prediction (POP) Score: a new prognostic index for patients with severe acute pancreatitis. Crit Care Med. 2007;35:1703-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4328] [Article Influence: 360.7] [Reference Citation Analysis (45)] |
| 7. | Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 561] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 8. | Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, Lesser M, Widmann WD. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 335] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 10. | Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA. Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362-3369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 11. | Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, Jeffrey RR, Buchan KG, El-Shafei H, Hillis GS. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 843] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 14. | Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 15. | Pezzilli R, Billi P, Beltrandi E, Casadei Maldini M, Mancini R. Impaired lymphocyte proliferation in human acute pancreatitis. Digestion. 1997;58:431-436. [PubMed] |
| 16. | Takeyama Y, Takas K, Ueda T, Hori Y, Goshima M, Kuroda Y. Peripheral lymphocyte reduction in severe acute pancreatitis is caused by apoptotic cell death. J Gastrointest Surg. 2000;4:379-387. [PubMed] |
| 17. | Mofidi R, Patil PV, Suttie SA, Parks RW. Risk assessment in acute pancreatitis. Br J Surg. 2009;96:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |