Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3832
Peer-review started: November 28, 2016
First decision: December 28, 2016
Revised: February 21, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: June 7, 2017
Processing time: 192 Days and 20.7 Hours
To investigate the levels, ratios, and clinical significance of T helper 17 (Th17) cells and regulatory T (Treg) cells in the peripheral blood of patients with autoimmune liver disease (AILD).
Forty-two AILD patients were included in the experimental group (group E), and 11 healthy subjects were recruited as the control group (group C). Flow cytometry was performed to determine the percentages of Th17 and Treg cells in peripheral blood lymphocytes. Furthermore, a range of biochemical indices was measured simultaneously in the blood of group E patients.
The percentage of Th17 cells and the Th17/Treg ratio were higher in group E than in group C (P < 0.01), whereas the percentage of Tregs was lower in the group E patients (P < 0.05). Patients in group E who were admitted with AILD in the active stage showed significantly higher Th17 percentages and Th17/Treg ratios than those measured in patients with AILD in remission (P < 0.05). In addition, among patients with AILD in the active stage, individuals that remained unhealed after hospitalization showed significantly higher baseline values of the Th17 percentage and the Th17/Treg ratio than those detected in patients who improved after treatment (P < 0.05). The results suggested that imbalance in the Th17/Treg ratio plays an important role in the pathogenesis and development of AILD.
A high Th17/Treg ratio appears to predict poor short-term prognosis in patients with AILD in the active stage.
Core tip: This study investigated the levels, ratios, and clinical significance of T helper 17 (Th17) cells and regulatory T (Treg) cells in the peripheral blood of patients with autoimmune liver disease (AILD). The results suggested that an imbalance in the Th17/Treg ratio plays an important role in the pathogenesis and development of AILD. A high Th17/Treg ratio appears to predict poor short-term prognosis in patients with AILD in the active stage.
- Citation: Feng TT, Zou T, Wang X, Zhao WF, Qin AL. Clinical significance of changes in the Th17/Treg ratio in autoimmune liver disease. World J Gastroenterol 2017; 23(21): 3832-3838
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3832.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3832
Autoimmune liver disease (AILD) is a class of chronic diseases characterized by immunopathological damage to the liver. Although the causes of AILD have not been established clearly, it is thought that autoimmune diseases develop because the body’s immune system targets its own autoantigens[1]. AILD includes autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), and overlap syndrome (OS). T lymphocyte infiltration in the liver tissue is one of the characteristic features of AILD, suggesting that the presence of certain T lymphocyte subsets has an important relationship with the occurrence and development of AILD[2,3]. In this study, the percentages of T helper 17 (Th17) and regulatory T (Treg) cells were measured in AILD patients in the active stage of the disease (before treatment) and during the remission stage. In addition, biochemical indices of liver function were determined in the peripheral blood of these patients to explore the significance of Th17 and Treg abnormalities in AILD pathogenesis and to assess the possibility of short-term clinical prognosis of AILD patients based on Th17 and Treg parameters during the active stage of the disease.
Forty-two AILD patients who were admitted and diagnosed clinically in the First Affiliated Hospital of the Soochow University between June 2012 and June 2015 were selected, including 29 cases of AIH and 13 cases of PBC. AILD patients were referred to collectively as group E (experimental). Among these 42 cases, 31 cases were in the active stage (subgroup EA). In 19 patients with AILD in the active stage, liver function was restored within 3 mo of hospitalization, whereas in the 12 other cases, liver function did not return to normal or appeared to be aggravated within 3 mo of hospitalization. The remaining 11 selected patients had AILD in the remission stage (subgroup ER), and their serum transaminase and bilirubin were maintained at normal levels for at least six months following previous treatment. In addition, 11 healthy subjects were selected as the control group (group C).
AIH was diagnosed according to the clinical scoring criteria for AIH as revised by the EASL clinical practice guidelines:Autoimmune hepatitis in 2015[4]. The diagnostic criteria for PBC followed the comment on biochemical response to ursodeoxycholic acid and long-termprognosis in primary biliary cirrhosis in 2009[5].
To detect Th17 cells, heparin-anticoagulated blood samples were first washed with Roswell Park Memorial Institute (RPMI)-1640 culture medium and then placed into a culture medium containing phorbol esters, ionomycin, and brefeldin (Invitrogen, Carlsbad, CA, United States) for a 5-h incubation at 37 °C. Next, the cells were labeled with CD3 and CD8 monoclonal antibodies (Beckman Coulter, Brea, CA, United States) and incubated in the dark for 20 min. After washing with phosphate-buffered saline (PBS) and fixation, the cell membranes were broken and incubated in the dark for 15 min with a monoclonal antibody against interleukin (IL)-17 (eBioscience, San Diego, CA, United States) or with IgG1κ (eBioscience) for homogenic control marking while mixing gently. After washing with PBS, the mixture was centrifuged at 200 × g for 5 min and the supernatant was discarded. The cell pellet was resuspended in 200 μL of PBS and analyzed for the presence of CD3+CD8- IL-17-producing lymphocytes, i.e., Th17 cells.
To detect Treg cells, heparin-anticoagulated blood samples were labeled with CD4 and CD25 monoclonal antibodies (Beckman Coulter) and incubated in the dark for 20 min. After washing in PBS and fixation, the cell membranes were broken and incubated for 30 min with an enzyme-labeled anti-FOXP3 antibody or control anti-FOXP3 antibody. The mixture was washed with PBS, centrifuged, and the supernatant was discarded. The cell pellet was resuspended in 200 μL of PBS and Treg cells were detected and analyzed as CD4+CD25+FOXP3+ cells.
The following markers of liver function were detected according to the manufacturer’s instructions included with the respective kits: total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transferase (γ-GT), and globulin (GLB).
SPSS 20.0 statistical software was used for the statistical analyses (SPSS, Inc., Chicago, IL, United States). Normally distributed data are presented as the mean ± SD, whereas non-normally distributed data are presented as medians with the interquartile range (25th-75th percentile). Groups with normally distributed data were compared using Student’s t-test. Data from the groups with non-normally distributed data were compared using the Mann-Whitney U-test. More than two groups were compared using the Kruskal-Wallis H-test. Comparisons of group counts were carried out using the χ2 test. In all cases, differences between groups were considered significant when P < 0.05.
Among the 42 enrolled AILD patients in group E, 31 patients were in the active stage of the disease. These patients had abnormal liver function and tested positive for the presence of autoantibodies. The mean age of this patient subgroup EA was 55.03 ± 13.45 years (range 25-79 years). The other 11 patients were in the remission stage and had exhibited normal liver function for at least 6 mo after previous treatment. Subgroup ER patients also had abnormal liver function and tested positive for autoantibodies. The mean age of patients in the subgroup ER was 55.72 ± 8.95 years (range 42-66 years). The biochemical parameters of the patients from these two subgroups are shown in Table 1. The control group comprised 11 healthy individuals with a mean age of 46.64 ± 8.71 years (range 28-59 years). There were no statistically significant differences in the age and gender proportions between the control group and two subgroups of group E (P > 0.05 for all comparisons). Details regarding abnormal liver function in the patients from subgroups EA and ER are presented in Table 1.
| Liver function | Subgroup EA (n = 31) | Subgroup ER (n = 11) | P value |
| TBIL (μmol/L) | 133.10 (55.00, 200.20) | 17.60 (12.10, 24.90) | < 0.001 |
| DBIL (μmol/L) | 87.20 (38.80, 151.10) | 7.60 (6.20, 15.80) | < 0.001 |
| ALT (U/L) | 116.00 (47.00, 314.00) | 28.00 (10.00, 56.00) | < 0.001 |
| AST (U/L) | 103.00 (76.00, 343.00) | 37.00 (30.00, 51.00) | < 0.001 |
| ALP (U/L) | 128.00 (113.00, 173.00) | 74.00 (51.00, 110.00) | 0.001 |
| γ-GT (U/L) | 160.50 (99.75, 281.00) | 34.00 (16.00, 44.00) | < 0.001 |
| GLB (g/L) | 29.69 ± 5.70 | 28.89 ± 4.35 | 0.678 |
In 12 AILD patients from subgroup EA, liver function was not restored during hospitalization and, in some cases, appeared to be aggravated after treatment. Worsening of symptoms was caused by co-morbid pulmonary infections or liver failure. Six of these patients were diagnosed with AIH and another six patients had PBC. Their mean age was 59.41 ± 11.62 years (range 42-77 years).
Nineteen patients from subgroup EA, including 14 cases of AIH and five cases of PBC, improved after treatment. Their mean age was 52.26 ± 14.08 years (range 25-79 years). There were no significant differences in the gender and disease type distributions, or in the mean age between these subsets (improved and unhealed) of group EA patients (P > 0.05). The results of liver function analysis upon admission revealed that ALT and AST values in the treatment-resistant patients were significantly lower (P < 0.05) than those in group EA patients who improved during hospitalization. The parameters of liver function recorded upon admission in all patient groups are shown in Table 2.
| Liver function | Unhealed group (n = 12) | Improved group (n = 19) | P value |
| TBIL (μmol/L) | 166.75 (112.70, 244.30) | 103.10 (46.90, 175.20) | 0.114 |
| DBIL (μmol/L) | 122.0 (80.20, 122.00) | 70.30 (28.80, 121.00) | 0.059 |
| ALT (U/L) | 57.50 (44.00, 106.50) | 195.00 (104.00, 724.00) | 0.002 |
| AST (U/L) | 86.00 (53.50, 94.75) | 151.00 (103.00, 513.00) | 0.001 |
| ALP (U/L) | 153.00 (115.00, 405.00) | 121.50 (95.50, 151.25) | 0.089 |
| γGGT (U/L) | 259.00 (144.50, 370.00) | 133.00 (69.75, 253.50) | 0.072 |
| GLB (g/L) | 30.16 ± 6.27 | 29.48 ± 5.42 | 0.756 |
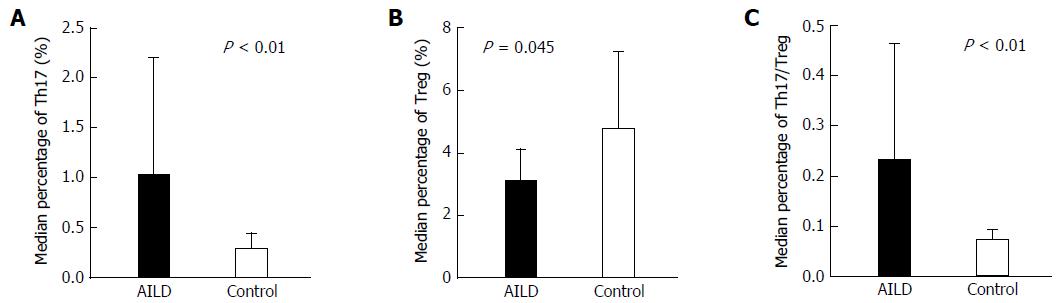
The median Th17 percentages in groups E and C were 0.73% (0.22%, 2.31%) and 0.23% (0.05%, 0.70%), respectively, and this difference was significant (Z = -3.664, P < 0.01). The mean Treg percentages in groups E and C were 3.04% ± 1.10% and 4.75% ± 2.45%, respectively, and this difference was also significant (t = -2.255, P = 0.045). The median values of the Th17/Treg ratio in groups E and C were 0.23 (0.14, 0.46) and 0.07 (0.03, 0.09), respectively, and this difference was significant (Z = -4.014, P < 0.01) (Figure 1), indicating that the percentage of Th17 cells in peripheral blood lymphocytes in group E patients was significantly higher than that in the healthy controls. However, because the Treg percentage in group E patients was slightly lower than that in group C individuals, the Th17/Treg ratio was significantly increased in AILD patients (Figure 1).
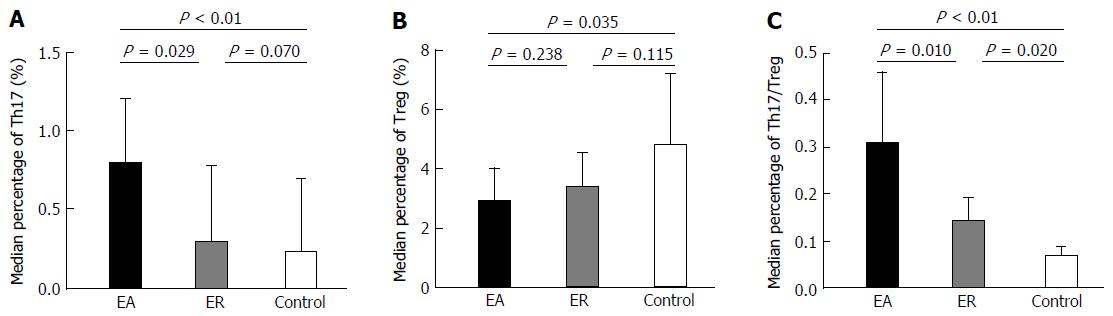
Th17 cell percentages in subgroups EA and ER were 0.79% (0.48%, 1.20%) and 0.29% (0.22%, 0.78%), respectively (Table 3) and this difference was significant (Z = -2.189, P = 0.029; Figure 2). In addition, the difference between Th17 cell percentages in subgroup EA and group C was significant (Z = -3.992, P < 0.001), whereas the difference between these values in subgroup ER and group C was not significant (Z = -1.810, P = 0.070).
The relative levels of Treg cells in subgroups EA and ER were 2.91% ± 1.08% and 3.38% ± 1.16%, respectively (Table 3) and, as in the case of Th17 cells, these values were significantly different (t = -1.197, P = 0.238; Figure 2). The difference between Treg frequencies in subgroup EA and group C was also statistically significant (t = -2.396, P = 0.035), whereas the difference between these parameters in subgroup ER and group C was not significant (t = -1.667, P = 0.115).
The Th17/Treg ratios in subgroups EA and ER were 0.31 (0.18, 0.46) and 0.14 (0.07, 0.19), respectively (Table 3), and this difference was significant (Z = -2.560, P = 0.010; Figure 2). The difference between Th17/Treg ratios in subgroup EA and group C was also significant (Z = -4.220, P < 0.001), as was the difference between these parameters in subgroup ER and group C (Z = -2.332, P = 0.020).
These results suggested that because the percentage of Th17 cells in subgroup EA was significantly higher than that in group C, whereas the percentage of Treg cells was significantly lower, the Th17/Treg ratio in subgroup EA patients was significantly higher than that in healthy controls. However, when AILD patients remitted after treatment, the percentage of Th17 cells and the Th17/Treg ratio in the peripheral blood were reduced significantly, although the Th17/Treg ratio in subgroup ER was still significantly higher than that in group C.
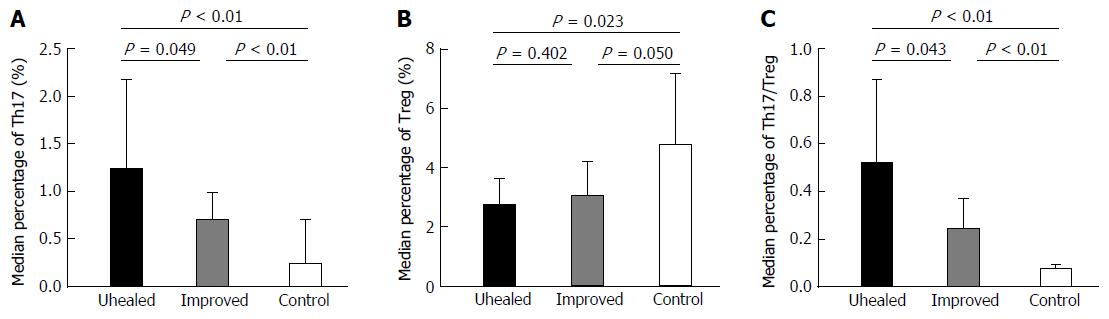
The median values of the Th17 percentage in unhealed and improved patients from subgroup EA were 1.22% (0.56%, 2.18%) and 0.69% (0.42%, 0.99%), respectively.
The level of Th17 cells in the unhealed group was significantly higher than that in improved subgroup EA patients (Z = -1.968, P < 0.05) and healthy controls (Z = -3.512, P < 0.05). The mean values of the Treg percentage in unhealed and improved subgroup EA patients were similar (2.71% ± 0.91% and 3.04% ± 1.17%, respectively). However, only the former value was significantly lower than that in group C (t = -2.595, P < 0.05). The median Th17/Treg ratios values in unhealed and improved patients from subgroup EA were 0.52 (0.20, 0.87) and 0.24 (0.18, 0.37), respectively. This ratio in the unhealed group was significantly higher than that in the other two groups (Z-values of -2.028 and -3.877, respectively, P < 0.05 in both cases; Figure 3).
AILD is an autoimmune disease characterized by selective immunopathological damage to the liver. AIH is considered an autoimmune pathological process triggered in genetically susceptible individuals by infectious agents or drugs. At the molecular level, AILD onset is related to the production of autoantibodies and the presence of certain main histocompatibility complex alleles responsible for aberrant presentations of autoantigenic targets to T cell receptors. Anti-mitochondrial antibodies are important in PBC pathogenesis and can be detected in the serum of at least 95% of PBC patients. The corresponding antigens can be found on the apical membrane of biliary epithelial cells of PBC patients. Although AIH and PBC are two different autoimmune liver disorders, they have several characteristics in common. Both disorders are accompanied by the presence of one or more autoantibodies in the serum. Clinical manifestations of AIH and PBC are similar and are often co-morbid with symptoms of other autoimmune diseases. Females are more susceptible to AIH and PBC than males[6].
AIH exhibits high heterogeneity and its pathogenesis is not fully understood. However, it is still considered to be a corticosteroid-sensitive status[7]. Standard treatment results in remission in 60%-80% of patients; however, in 20%-40% of cases, the patient’s condition fails to improve. Continuing autoimmune activity causes the onset or aggravation of cirrhosis, eventually leading to complications, liver transplantation, and often death. The autoimmune mechanisms of PBC has resulted in a variety of immunosuppressive drugs being evaluated; however, most either had no effect or exhibited high toxicity. Currently, the standard treatment is ursodeoxycholic acid (UDCA), which was shown to delay the progression of PBC in a number of randomized controlled studies[8]. However, liver biochemical indices are not restored to the normal range in all patients treated with UDCA. Moreover, decompensated liver disease may still occur in patients with cirrhosis or those who have progressed to cirrhosis while being treated with UDCA, and thus liver transplantation remains necessary[7].
Patients in subgroups EA and ER in this study were not significantly different in terms of gender, disease type, or age. Furthermore, no significant differences were noted after a 3-mo treatment between unhealed and improved patients in subgroup EA in terms of gender, age, disease type, and several biochemical parameters, such as TBIL, DBIL, ALP, γ-GT, and GLB. However, patients in the improved group exhibited significantly higher ALT and AST at admission compared with those in the unhealed group, indicating that in the active stage of AILD, there is a positive correlation between the inflammation state of the liver and the sensitivity of patients to hormonal or UDCA therapy.
Th17 cells are induced to differentiate and survive by IL-23. Th17 cells mainly secret cytokines, such as IL-17, IL-21, IL-22, IL-6, and tumor necrosis factor (TNF)-α. IL-17 can induce secretion of other inflammatory cytokines, such as IL-6 and TNF-α, and chemokines MCP-1 and MCP-2, resulting in the local infiltration of inflammatory cells and inflammatory damage to tissues and organs[9]. Th17-deficiency can prevent or alleviate the onset of autoimmune diseases such as autoimmune encephalomyelitis[10]. Additionally, IL-17 is involved in the proliferation, maturation, and chemotaxis of neutrophils and dendritic cells. In this study, the percentage of Th17 in peripheral blood lymphocytes of AILD patients was significantly higher than that in the control group subjects. This finding was consistent with findings in patients from China and other countries[11,12]. This higher level of Th17 cells in AILD might be triggered by infection or drugs that initiate the patient’s autoimmune liver cell damage. In response, the body secretes a large amount of inflammatory cytokines that induce the differentiation of Th17 cells, which, in turn, exacerbate liver inflammation. In most studies, the increased level of Th17 cells in AIH patients is related to the degree of liver inflammation and fibrosis. Harada et al[13] found that infiltration of Th17 cells into the impaired bile duct of PBC patients can aggravate chronic cholangitis. In the present study, the percentage of Th17 cells in the peripheral blood of AILD patients was higher than that in the control group subjects[14]. Moreover, these significantly higher levels of Th17 cells were observed in several subsets of AILD patients, such as in both unhealed and improved patients with active-stage AILD. This suggested that Th17 cell proliferation in AILD patients is increased, which promotes liver inflammation. Notably, the percentage of Th17 cells in the unhealed subset was significantly higher than that in the improved patients; which suggested that the high level of Th17 cells not only reflects AILD pathogenesis, but also can indicates poor short-term prognosis.
Treg cells are a class of T lymphocytes that play an important role in maintaining the immune homeostasis by maintaining tolerance against self-antigens and preventing autoimmune reactions. Expression of markers such as CD4, CD25, and FOXP3, in combination with downregulated expression of CD127, can be used to identify Treg cells. Treg cells exert their immunosuppressive effects through the following four basic mechanisms: (1) triggering apoptosis of target cells; (2) downregulating the co-stimulatory molecules CD80 and CD86, thereby preventing antigen-presenting cells from initiating an adaptive immune response[14]; (3) damaging metabolic pathways; and (4) secreting anti-inflammatory cytokines, such as transforming growth factor (TGF)-α, IL-10, or IL-35. In this study, the percentage of Treg cells in AILD patients was significantly lower than that in the control group. This difference was more pronounced in patients admitted with AILD in the active stage than in subjects with AILD in remission. After treatment, patients in the remission stage showed partly recovered levels of Treg cells, consistent with previously published findings[15]. This suggested that the disturbance in the number of Treg cells is related to the onset of AILD and that the Treg cell population can be restored partially after glucocorticoid treatment. Sasaki et al[16] found a significantly higher number of FOXP3+ cells in the liver of PBC patients than in the control group, indicating that Treg cells can aggregate at the inflamed site, reducing their level in the peripheral blood. In this study, the level of Treg cells in AILD patients was significantly lower in the peripheral blood compared with the fraction in control subjects. The difference was more obvious in patients with AILD in the active stage, whereas the Treg cell percentage in patients that healed after treatment was significantly lower than that in the control group. Therefore, Treg cells might play a protective role in the occurrence and development of AILD by restoring immune homeostasis and remitting tissue inflammation.
It has been confirmed that under normal circumstances, TGF-α induces naïve T cells to differentiate into FOXP3+ Treg cells, thus maintaining immune tolerance and preventing the occurrence of autoimmune diseases. When the body is infected or invaded by pathogens, the secretion of IL-6, IL-21, or other inflammatory cytokines increases; these molecules co-act with TGF-α to inhibit the differentiation of Treg cells, while promoting the differentiation of Th17 cells simultaneously. Therefore, the Th17/Treg ratio imbalance appears to mediate inflammation. Fletcher et al[17] confirmed that CD39+ Treg cells could inhibit the production of IL-17 by Th17 cells, indicating that Treg and Th17 cells can office@igandan.comsuppress each other mutually. The percentage of Treg cells in the peripheral blood of AILD patients is lower than that in healthy subjects, and thus their inhibitory effect on Th17 cells is weaker. The latter circumstance might increase the percentage of Th17 cells in the peripheral blood, which is consistent with our results. In this study, the increase in the percentage of Th17 cells and the decrease in the percentage of Treg cells were significant in patients admitted with AILD in the active stage, whereas in patients with AILD in remission, these parameters did not differ from those in healthy control subjects. Nonetheless, the Th17/Treg ratio in patients with AILD in the remission stage was significantly higher than that in the control group. We concluded that after treatment, the production of inflammatory cytokines and, as a result, Th17 cell differentiation, in these patients was diminished. Consequently, the number of Treg cells was restored to some extent and the generation of Th17 cells was suppressed, although some Th17/Treg imbalance persisted. When the autoimmune response is re-triggered by certain factors, AILD may might re-enter the active stage from the remission stage. The Th17/Treg ratio in patients remaining unhealed was significantly higher than that in patients whose condition improved. A particularly high Th17/Treg ratio in the unhealed AILD patients might reflect the fact that these patients were still in a state of immune disorder, and the Th17/Treg imbalance likely explains their poor short-term prognosis.
In summary, an imbalanced Th17/Treg ratio might induce the onset of AILD and promotes disordered immune function in AILD patients. The Th17/Treg imbalance can be improved by pharmacological treatment; thus, therapeutics that can rectify this imbalance might be promising to cure AILD.
Autoimmune liver disease (AILD) is a class of chronic diseases characterized by immunopathological damage to the liver. Although the causes of AILD have not been established clearly; generally, it is thought that autoimmune diseases develop because the body’s immune system targets its own autoantigens.
In this study, the percentages of T helper 17 (Th17) and regulatory T (Treg) cells were measured in AILD patients in the active stage of the disease (before treatment) and during the remission stage. In addition, biochemical indices of liver function were determined in the peripheral blood of these patients to explore the significance of Th17 and Treg abnormalities in AILD pathogenesis and to assess the possibility of short-term clinical prognosis of AILD patients based on Th17 and Treg parameters during the active stage of the disease.
The Th17/Treg imbalance can be improved by pharmacological treatment; thus, therapeutics that can rectify this imbalance might be promising to cure AILD.
Although the role of T regs is well known, it is important that such works be published. AILDs are uncommon diseases; therefore, it is necessary that scientists from different centers report their research so that the therapeutic results improve in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kim YB, Rodrigues AT S- Editor: Qi Y L- Editor: Stewart G E- Editor: Zhang FF
| 1. | Liberal R, Vergani D, Mieli-Vergani G. Paediatric Autoimmune Liver Disease. Dig Dis. 2015;33 Suppl 2:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Liberal R, Grant CR, Ma Y, Csizmadia E, Jiang ZG, Heneghan MA, Yee EU, Mieli-Vergani G, Vergani D, Robson SC. CD39 mediated regulation of Th17-cell effector function is impaired in juvenile autoimmune liver disease. J Autoimmun. 2016;72:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Mitra S, Anand S, Das A, Thapa B, Chawla YK, Minz RW. A molecular marker of disease activity in autoimmune liver diseases with histopathological correlation; FoXp3/RORγt ratio. APMIS. 2015;123:935-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Lohse AW, Chazouilleres O, Dalekos G, Drenth J, Heneghan M, Hofer H, Lammert F, Lenzi M. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 847] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 5. | Walker LJ, Newton J, Jones DE, Bassendine MF. Comment on biochemical response to ursodeoxycholic acid and long-termprognosis in primary biliary cirrhosis. Hepatology. 2009;49:337-338; author reply 338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | McFarlane IG, Krawitt EL. Treatment of autoimmune hepatitis. Medical management of liver diseases. New York: Marcel Dekker Inc 1999; . |
| 7. | Liberal R, Vergani D, Mieli-Vergani G. Update on Autoimmune Hepatitis. J Clin Transl Hepatol. 2015;3:42-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Poupon RE, Lindor KD, Parés A, Chazouillères O, Poupon R, Heathcote EJ. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39:12-16. [PubMed] |
| 9. | Chan CW, Gunsar F, Feudjo M, Rigamonti C, Vlachogiannakos J, Carpenter JR, Burroughs AK. Long-term ursodeoxycholic acid therapy for primary biliary cirrhosis: a follow-up to 12 years. Aliment Pharmacol Ther. 2005;21:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1754] [Cited by in RCA: 1817] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 11. | Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566-573. [PubMed] |
| 12. | Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X, Qiu D, Wei J, Liu Y, Shen L. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS One. 2011;6:e18909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1406] [Cited by in RCA: 1313] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 15. | Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 304] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Sasaki M, Ikeda H, Sawada S, Sato Y, Nakanuma Y. Naturally-occurring regulatory T cells are increased in inflamed portal tracts with cholangiopathy in primary biliary cirrhosis. J Clin Pathol. 2007;60:1102-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KH. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602-7610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 390] [Article Influence: 24.4] [Reference Citation Analysis (0)] |